GLASS What is glass History of glass How

























- Slides: 26

GLASS What is glass? History of glass. How is glass made? Uses of glass.

What is Glass? • “An inorganic product of fusion which has cooled to a rigid condition without crystallizing” • Uniform amorphous solid – will only have ONE refractive index – have atoms randomly arranged throughout the sample • No specific m. p. • Softens over a temperature range

What is Glass? • • • Glass combines some properties of crystals and some of liquids but glass is distinctly different from both. • Glass is rigid like a crystal but the molecules that make up glass are arranged randomly like liquids. In general glass is formed by melting crystalline substances and then cooling the liquid before the molecules can form a crystal. Properties of glass include: Mechanically Strong – Glass has great inherent strength and is weakened only by surface imperfections Hard surface – Glass resists scratches and abrasions Elastic – Glass “gives” under stress – up to a breaking point – but rebounds exactly to its original shape Chemical corrosion-resistant – Glass is affected by few chemicals. It resists most industrial and food acids. Thermal shock-resistant – Glass withstands intense heat or cold as well as sudden temperature changes. Heat-absorbent – Glass retains heat, rather than conducts it. Glass absorbs heat better than metal. Optical Properties – Glass reflects, bends, transmits and absorbs light with great accuracy.

History of Glass • May date to 5000 BC when sailors, carrying soda ash as cargo on their ships, used the blocks of soda ash on which to rest their cooking vessels. The heat produced soda glass. • Bottles were used in Egypt and Babylon as early as 1500 BC. • Glassblowing began around 250 BC, within 150 years glass replaced metals as status symbols in the Roman culture.

History of Glass • Flat glass was produced in the 13 th century, but it was not until the early 1800’s that flat glass could be produced at reasonable cost for everyday use in windows. • In 1883 Pittsburgh Plate Glass Company became the first successful manufacturer of plate glass in the United States. • Until the early 20 th century, glass was made by hand in America, making glass products a luxury. In 1903, Michael Owens invented the first automated glass bottleblowing machine.

The Composition of Glass • Because glass is used in so many different ways, there is no one chemical composition for each glass sample. • There are thousands of different glass compositions BUT only 3 categories of substances in ALL glass – Formers – Fluxes – Stabalizers

The Composition of Glass • The former makes up the bulk of the glass. – silicon dioxide, Si. O 2, in the form of sand – most common. – Other possible formers include B 2 O 3 and P 2 O 5. • The fluxes change the temperature at which the formers melt during the manufacturing of glass. – sodium carbonate, Na 2 CO 3, and potassium carbonate, K 2 CO 3. •

The Composition of Glass • The stabilizers strengthen the glass and make it resistant to water. – Calcium carbonate, Ca. CO 3, most frequently used

What’s in Glass? • Network Components-Formers: Si. O 2, B 2 O 3, P 2 O 5, Ge. O 2, V 2 O 5, As 2 O 3, Sb 2 O 5 • Fluxes–Softeners [lowers melting point]: Na 2 O, K 2 O, Li. O, Al 2 O 3, B 2 O 3, Cs 2 O • Stabilizers–Chemical/Corrosion Resistance: Ca. O, Mg. O, Al 2 O 3, Pb. O, Sr. O, Ba. O, Zn. O, Zr. O What do they all have in common? !

The Composition of Glass • The raw materials for making glass are all oxides. • The composition of any sample of glass can be given in terms of the percent of each oxide used to make it. • There are six basic types of glass based on composition

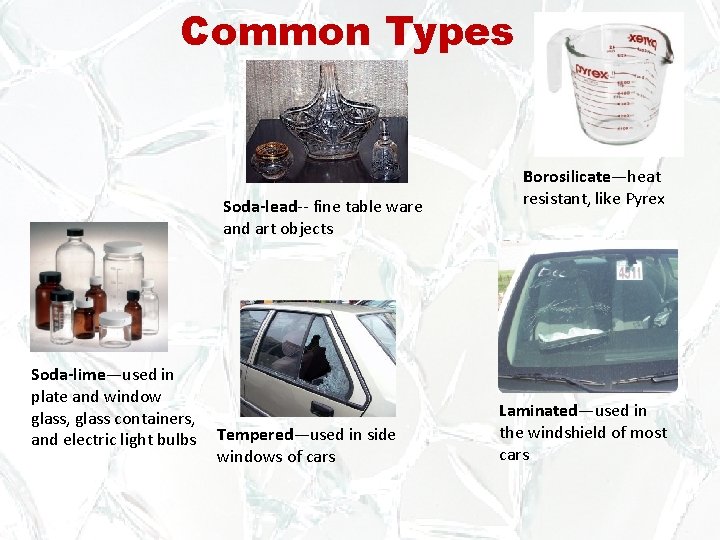
Common Types Soda-lead-- fine table ware and art objects Soda-lime—used in plate and window glass, glass containers, and electric light bulbs Tempered—used in side windows of cars Borosilicate—heat resistant, like Pyrex Laminated—used in the windshield of most cars

Common Types § Soda-lime—used in plate and window glass, glass containers, and electric light bulbs § Soda-lead—fine table ware and art objects § Borosilicate—heat resistant, like Pyrex § Tempered—used in side windows of cars § Laminated—used in the windshield of most cars Kendall/Hunt Publishing Company 12

Soda-Lime Glass Si. O 2 + Na 2 O / K 2 O + Ca. O / Al 2 O 3 / Mg. O The most common (90% of glass made) least expensive form of glass It usually contains 60 -75% silica, 12 -18% soda, 5 -12% lime • Resistance to high temperatures and sudden changes of temperature are not good • Resistance to corrosive chemicals is only fair. • •

Lead Glass • High percentage of lead oxide Pb. O (between 20% and 80% of the batch) • It is relatively soft, and its refractive index gives a brilliance that may be exploited by cutting • Somewhat more expensive than soda-lime glass • Favored for electrical applications because of its excellent electrical insulating properties. • This glass will not withstand high temperatures or sudden changes in temperature.

Borosilicate Glass • B 2 O 3 (replaces Na 2 O) • Any silicate glass having at least 5% of boric oxide in its composition. • High resistance to temperature change and chemical corrosion. • Cost is moderate when measured against its usefulness.

Tempered Glass • Tempered(Safety glass) – treatment involves heating the glass so that it begins to soften (about 620 C) and then rapidly cooling it. – Produces a glass which, if broken, breaks into small pieces without sharp edges

Laminated Glass • Laminated glass – glass is made up of a sandwich of two or more sheets of glass (or plastic), bonded together by a flexible, normally transparent material. – if cracked or broken, the flexible material is designed to hold the glass fragments in place

Ways Glass is Made • Rolled Glass – 'Ordinary' sheet glass – made by passing the molten glass through rollers – process gives an almost flat finish but with distortions. – glass is used in glazing greenhouses and garden sheds

Ways Glass is Made • Float glass (plate) – molten glass is 'floated' onto a bed of molten tin – produces a glass which is flat and distortion free – process imparts a fluorescence to the glass [UV light]

Ways Glass is Made • Tempered(Safety glass) • Heating: – the glass begins a heat treatment process in which it travels through a tempering oven – The oven heats the glass to a temperature of more than 600 degrees Celsius. • Cooling: – The glass then undergoes a high-pressure cooling procedure called "quenching. " – High-pressure air blasts the surface of the glass for 5 seconds. – Quenching cools the outer surfaces of the glass much more quickly than the center. which gives tempered glass its strength.

Video Of Tempered Glass

Ways Glass is Made • Laminated glass • Two layers of glass with a thickness of about 0. 12 inches (3 mm) are placed on either side of the PVB layer which is 0. 015 inches (. 38 mm) thick. • Rollers press the layers together to expel any air pockets. • The glass is then heated to 158 degrees Fahrenheit (70 degrees C) in a pressurized oil bath to permanently bond all the layers together. • Additional layers of glass and PVB will increase the strength of laminated glass.

Video Of Laminated Glass

Other Glass Bullet Proof Glass • A thicker version of laminated glass • Armored cars have a thickness of 2. 8 -3 inches • Armored Hummers (war) have thickness of 4 -5 inches • VERY HEAVY

Other Glass Plexiglas • Not glass at all, but a synthetic thermoplastic polymer • Chemical name is polymethyl methacrylate (PMMA) • Sold as Plexiglas, Acrylite and Lucite. • Less dense than glass, does not shatter, is easily shaped, transmits visible light readily and does not block UV radiation.

Additives and Color • Color can be added to glass as a result of impurities in the substances used to make the glass. • Color can also be added by dissolving one or more metal oxides, by dispersing a colloid throughout the mix or by suspending pigments to create opaque areas. Oxide – Color Iron - green, brown Manganese – amethyst Gold – red Lead - yellow Oxide - Color Antimony - white Cobalt - deep blue Copper - light blue
 What is the glass escalator
What is the glass escalator True or false hot glass looks the same as cold glass.
True or false hot glass looks the same as cold glass. Does hot glass look like cold glass
Does hot glass look like cold glass Also history physical
Also history physical Surface runoff meaning
Surface runoff meaning The looking glass self examples
The looking glass self examples Lady in the looking glass summary
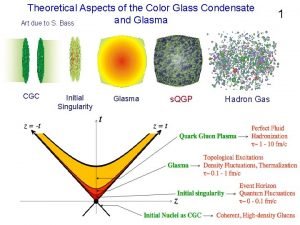
Lady in the looking glass summary Colour glass condensate
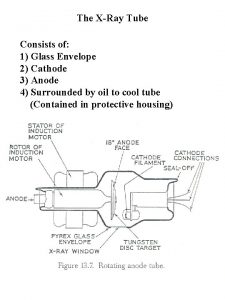
Colour glass condensate Glass envelope x ray tube
Glass envelope x ray tube Example of resocialization
Example of resocialization The glass roses alden nowlan
The glass roses alden nowlan The glass menagerie themes
The glass menagerie themes The glass menagerie by tennessee williams summary
The glass menagerie by tennessee williams summary The glass menagerie background
The glass menagerie background Glass house & philip johnson analysis
Glass house & philip johnson analysis Lp_solve
Lp_solve Glass av kvarg
Glass av kvarg Martini glass story structure
Martini glass story structure Nature morte still life
Nature morte still life Volar splint ortho glass
Volar splint ortho glass Looking glass self examples
Looking glass self examples Thermochromic smart glass
Thermochromic smart glass Schott borofloat 33
Schott borofloat 33 Satire is a sort of glass
Satire is a sort of glass Shattered glass
Shattered glass What is the answer
What is the answer Kolitong instrument drawing
Kolitong instrument drawing