GLASS IONOMER CEMENT Background Developed in attempt to

GLASS IONOMER CEMENT

Background • Developed in attempt to combine the favorable properties of silicate cement and polycarboxylate cement. • Was introduced in 1972 by Wilson and Kent. • The first introduced GIC was opaque, limited shade selections, mixing and handling problems and technique sensitivity.
![Advantages : • Aesthetic [glass content] • Anticariogenic [fluoride content] Advantages : • Aesthetic [glass content] • Anticariogenic [fluoride content]](http://slidetodoc.com/presentation_image_h/87959b6fc79195a8a0e89dc1de423d03/image-3.jpg)
Advantages : • Aesthetic [glass content] • Anticariogenic [fluoride content]

Disadvantages • Early clinical failure. • Highly irritant to the pulp due to. - Its high acidity. - Arsenic impurity content. • • • Highly soluble in oral fluids. Inadequate mechanical properties. Discoloration by time. Radiolucent. No adhesive bond between the tooth & restoration.

classifications According to Wilson and Mc. Lean • Type I – luting cement • Type II - Restorative cement restorative aesthetics restorative re-inforced According to Mc. Lean • Glass Ionomer cement (traditional) • Resin modified GIC • Poly acid modified composite resins

According to application • Type I – Luting cement • Type II – Restorative cement • Type III – Lining cement • Type IV – FISSURE SEALANT • Type V – ORTHODONTIC CEMENT • Type VI – CORE BUILD UP CEMENT

Newer classification I TRADITIONAL GI • TYPE I - LUTING CEMENT • TYPE II - RESTORATIVE CEMENT • TYPE III - LINERS AND BASES II METAL MODIFIED GI • MIRACLE MIX • CERMET CEMENT

III. Light Cure GI HEMA added to liquid IV. Hybrid Glass Ionomer /Resin Modified GI composite resin in which fillers are substituted with glass ionomer particles. pre-cured glasses blended into composites
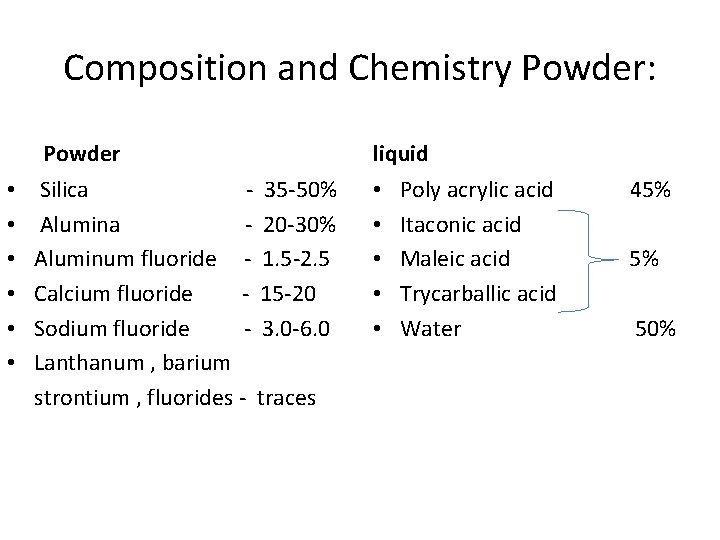
Composition and Chemistry Powder: Powder • • • Silica - 35 -50% Alumina - 20 -30% Aluminum fluoride - 1. 5 -2. 5 Calcium fluoride - 15 -20 Sodium fluoride - 3. 0 -6. 0 Lanthanum , barium strontium , fluorides - traces liquid • • • Poly acrylic acid Itaconic acid Maleic acid Trycarballic acid Water 45% 5% 50%

Modifications in Powder modifications were to improve upon the existing property in manipulation and the area of clinical use. • Dried Poly Acrylic Acids-anhydrous Gic • Silver Tin Alloy-(miracle Mix) • Silver Palladium/Titanium(cermet Cement) • BISgma , TEGDMA , HEMA

Powder • Fluoro-aluminosilicate glass prepared with fluoride fluxes. • Type II (restorative material) is generally coarser than that of type I (Luting agent). • Glass is thermally treated to form the powder, the quality of the setting reaction can be partially controlled by thermal handling. • INCREASED melting temp. more rapid setting rate

Liquid • The major component of the liquid is water. • 35 -65% aqueous solution of various polyacids. • Most common polyacids, polyacrylic acid or copolymers of acrylic, Maleic acid or acrylic and Itatonic acid. • Itatonic acid tends to reduce the viscosity, Tartaric acid in small amount improves working and setting characteristics.

• Some manufacturers freeze or vacuum dry the polyacids and copolymers, to incorporate them with the cement powder rather than the liquid. • this was an attempt to ensure the accuracy of the P/L ratio, and avoid the thickening of the liquid with storage. • Hydrous Cement • Anhydrous Cements • Semihydrous cements

Setting Reaction • A typical acid base reaction Metal + Acid = Salts (exothermic) • Powder+liquid acid soluble glass reacts with the polyacids releasing Al, Ca, Na, F ions. Ca and Al polysalts are formed. • The salts Hydrate to form a gel matrix, while the unreacted glass particles act as fillers surrounded by the gel.

• Set cement consist of unreacted glass surrounded by silica gel bonded together by a matrix of hydrated Ca and Al-polysalts. • Fluoride ions are not integral part of the matrix formation, they are available for clinical release without compromising the structure of the cement.

Stages of Setting Reaction I. Decomposition: acid attack and release of metal ions from glass. II. Migration: ions move into the aqueous phase. at this stage the cements has a shiny glossy appearance.

III. Gelation: Ca-poly-acrylate primary components. cement at this stage starts to become rigid and has an opaque appearance(because of the large difference in the refractive index between the glass particle & the matrix). clinical set, polishing and finishing postponed 24 hrs. At this stage the cement has to be protected from dryness and moisture.

IV. Post-set hardening: ions become bound to polyacid chains, Alpolyacrylate becomes dominant component. V. Maturation: increase cross-linking gives greater physical properties, the cement becomes resistant to acid attack and desiccation. increase translucency. at this stage the cement can be finished & polished.
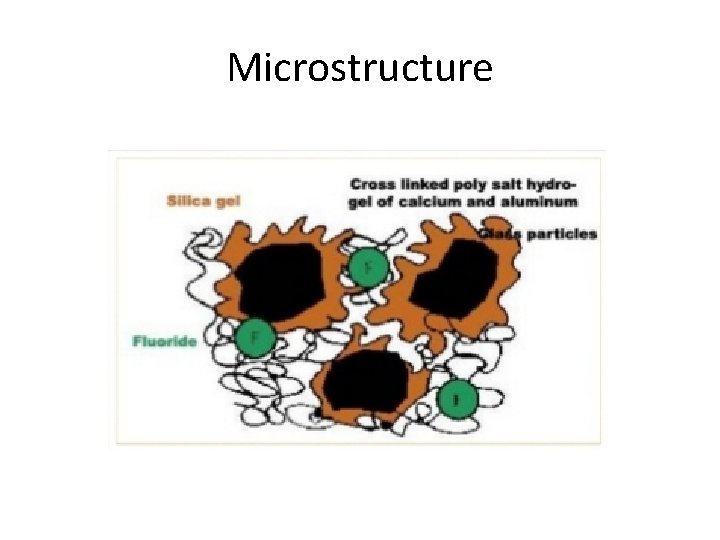
Microstructure

Role of Water • Water plays a crucial role in the setting reaction of glass-ionomer cement. • It act as a reaction medium. • Facilitate the formation of the hydration of the salts and enable the cross-linking to progress properly.

• Excess water: contamination will dilute the metal ions in soluble form and result in increased opacity and decreased strength and hardness of the final cement. • Water loss during setting: desiccation of the hydrogel disrupts the cement structure during maturation resulting in crazing and cracking of the final cement.

Manipulation: The P/L ratio of GIC is critical. P/L ratio is 3: 1 by weight. Must be mixed within 45 sec. the resulting mix must have a surface gloss or it will not adhere. • Pre-capsulated form is available. • •

• Care of the liquid: In order to preserve the amount of water inside the liquid 1)Dispense the liquid just before mixing 2)Reseal the bottle immediately 3)Discard the last third of the liquid Use cool glass slab(not below the dew point in order not to change the water content)
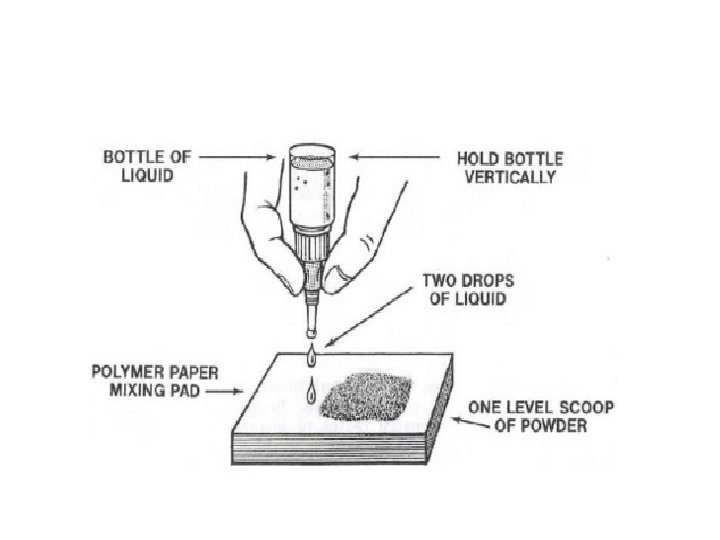

• Mixing over large area (for heat dissipation) with plastic spatula • Isolation of the cavity • Application of calcium hydroxide liner • Conditioning of cavity wall • Bulk insertion of the G. I. • Coating of the G. I. surface with cavity varnish • Finishing &polishing

Properties Biological Properties: • G. I. is mildly irritant to the pulp due to: Mild exothermic setting reaction Mild acidic irritation of polycarboxylic acid Rapid rise to neutrality within 24 hrs. • Difficult penetration of the large acid molecules inside the dentinal tubules. • Anti-cariogenic property due to Fluoride release from G. I. to tooth • Recharging ability - It is recommended in patients with high caries index.

Mechanical Properties: • Compressive strength: fairly high up to 200 MPa. • Flexural strength: fairly low 5 to 40 MPa. • Shear strength: fairly low 3 to 5 MPa. • Coefficient of thermal expansion similar to tooth structure. • Dimensional changes: shrinks on setting, expand with water sorption.

• The strength and hardness are lower than those of the silicate cements. • low toughness and less wear resistance when compared with resin composite. • The strength of G. I. depends on: 1)Powder/Liquid 2)Protection of G. I. against dryness & moisture in the initial set stage During setting very sensitive to water but once it sets it is characterized with a very low solubility, the lowest of the available dental cements.

Esthetic & Optical Properties • Tooth colored restoration • acceptable esthetic but not as good as the resin composite. • G. I. is radio-opaue by the addition of barium to the glass powder.

Bonding TO Tooth Structure • GIC share the adhesive potential of the polycarboxylate cement. • They appear to bond primarily to the inorganic component (Ca) of the tooth st. by initial hydrogen bonding, forming a metal ion bridges. • Good isolation to avoid contamination and moisture, smear layer should be removed and the cement should be used when its in its glossy stage.

Clinical Uses for GIC • Type I cement: luting agent or liner The film thickness is 20µm. • Type II cement: Class V erosion/caries Temporary rest (caries control) Class II, IV(dentin margins) The film thickness is 45µm. • Type III cement: Pit & Fissure sealant. The film thickness is 25 -35µm • Type IV cement: for core build up in high stress bearing areas. The film thickness is 45µm or more • Type V cement: for liner & bases

Modifications PURPOSE: • 1 )To improve the mechanical properties • 2 )To decrease the moisture sensitivity • The modification is done either to the powder or to the liquid.

TYPES OF MODIFICATIONS: • 1)METAL G. I. MIXTURE (MIRACLE MIX): G. I. powder +Amalgam alloy powder It was not successful • 2) CERMET: Precious metal e. g. silver is sintered to the G. I.

MIRACLE MIX • Introduced By Simmons In 1983 • Prepared By Incorporation Of Silver Tin Alloy Intoglass Ionomer Powder • Grey Or Blackish Colour To Cement Was Aesthetically Un Acceptable

Cermet • Introduced By Mc. LEAN AND GASSER IN 1985 • glass and metal powder are sinterred at high temperature which will be made to react with poly acrylic acid to form improved glass ionomer cement • Cermet can be used as: 1)Core build up 2)Deciduous posterior restorations Properties of cermet : 1)Higher mechanical properties 2)More opaque 3)Less fluoride release

Resin-modified GIC • Glass ionomer cement in which the acid-base setting reaction has been supplemented by a polymerization reaction of added resin components. the following criteria must be fulfilled: • the acid base reaction must be critical to the setting reaction. • must contain fluoroaluminosilicate glass, polymeric carboxylic acid and water.

• A hydrophilic monomer as HEMA is grafted to the aqueous polycarboxylic acid copolymer liquid 1)The monomer should be hydrophilic to avoid separation of the resin from the liquid. 2)Initiator activator system for light & or chemical curing of HEMA should be included.

Setting reaction: • supplied as powder&liquid) 1)Acid base reaction 2)Light polymerization of HEMA 3)Chemical polymerization of HEMA • Hybrid ionomer can be used as: 1)Lining under composite restorations 2)Anterior restorations Class III & V

ADVANTAGES • • Stronger Nearly Insoluble Fluoride Release Good Radio Opacity Easy Manipulation Satisfactory Wear Resistance Better Aesthetics

Compomer ( Polyacid Modified Composite Resin): • It is a modified C. R. ( C. R. +G. I. ) • Composition: 1)Dimethacrylate oligomer with two carboxylic groups. 2)Ion leachable glass filler. 3)Partially silanated to allow for bonding of filler to matrix&leaching out of fluoride from the filler.

Setting reaction supplied as one paste • 1)Light polymerization of the oligomer(major) 2)Acid base reaction(minor; it occurs only inside the patient`s mouth) • Properties: 1)High translucency as C. R. 2)Improved mechanical properties as C. R. 3)Chemical bonding to tooth but less than G. I.

GIOMER • It is again a mixture of C. R. + G. I, but with surface or fully pre-reacted glass ionomer. Setting reaction: (supplied as one paste) • light polymerization of the oligomer • Properties: Its properties are intermediate between C. R. & G. I.
- Slides: 42