Give examples of 3 acids and 3 alkalis
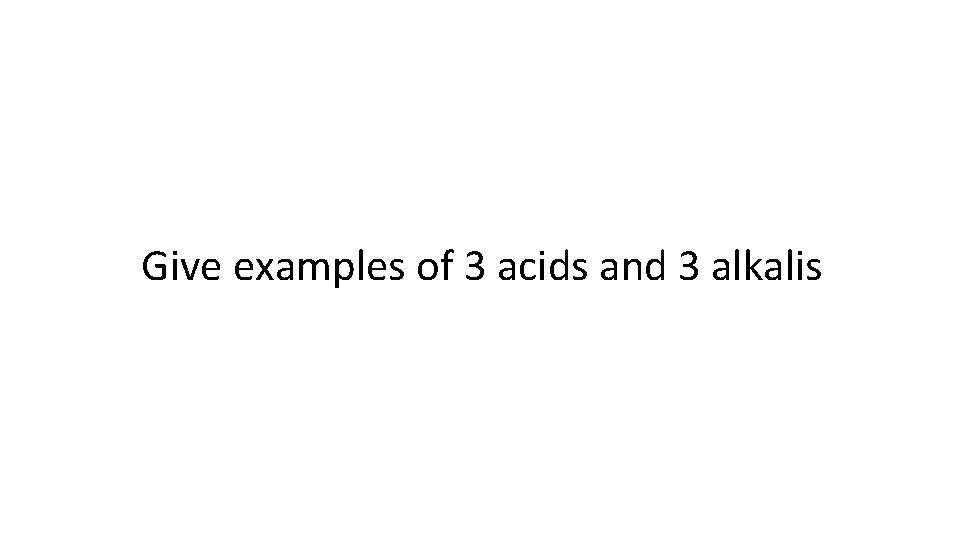































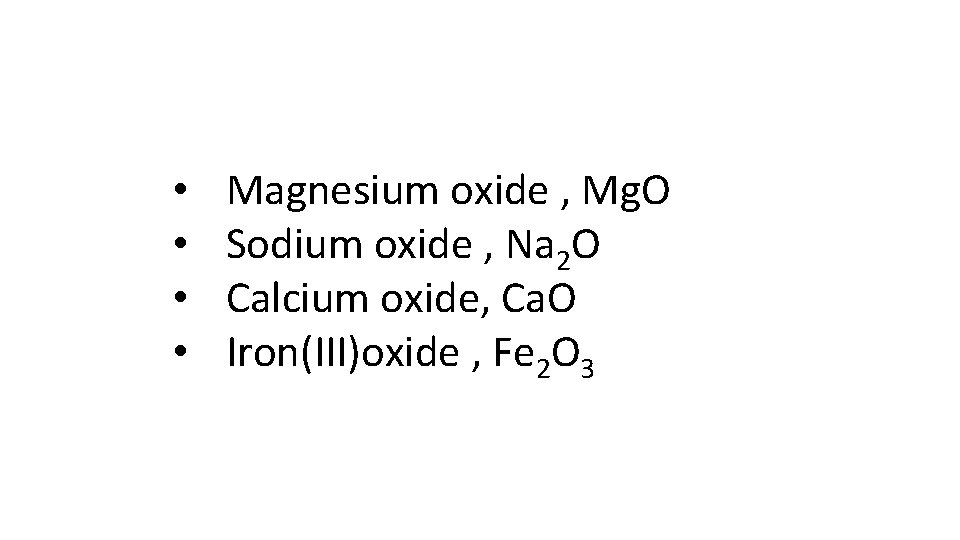






- Slides: 52
Give examples of 3 acids and 3 alkalis

Acids: Hydrochloric , HCl Sulfuric acid , H 2 SO 4 Nitric acid, HNO 3 Alkali: Sodium hydroxide, Na. OH Potassium hydroxide, KOH Ammonia solution, NH 3

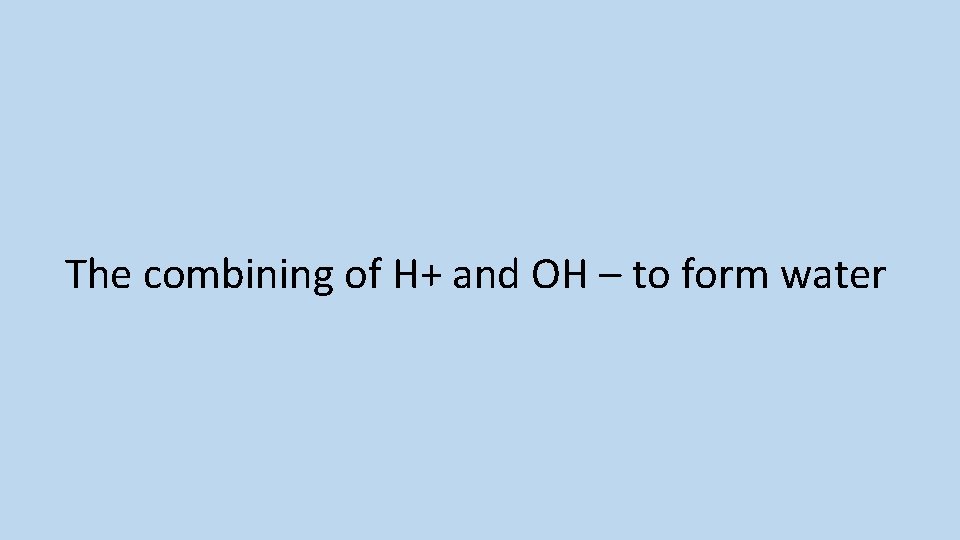
What is neutralisation?

The combining of H+ and OH – to form water

List the 3 types of bases and state which base does not form water when reacted with an acid

• Metal oxides • Hydroxides • Ammonia does not form water when reated with an acid

State 5 substances that acids react with to produce salts

• • • Metal Hydroxide Metal oxide Carbonates Ammonium compounds

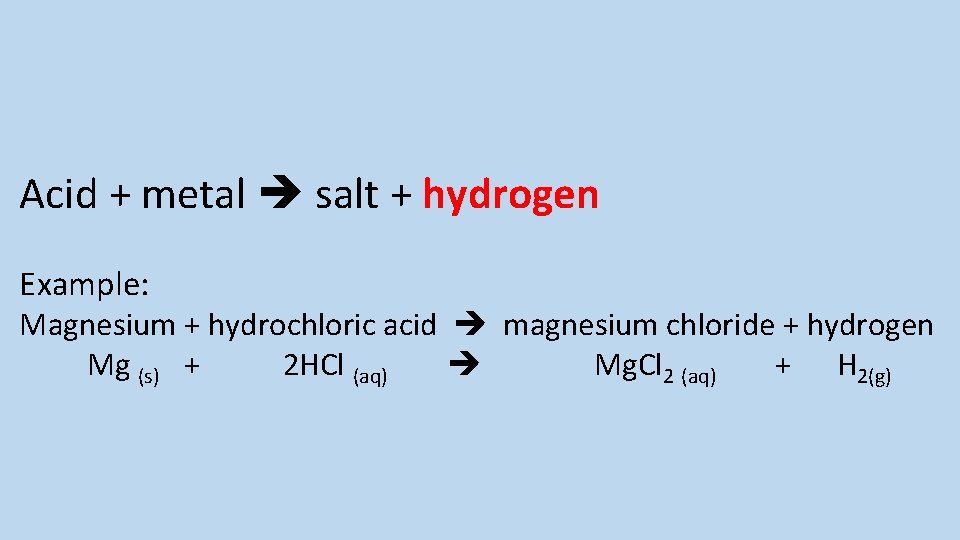
Describe the reaction between an acid and a metal and give an example of this type of reaction

Acid + metal salt + hydrogen Example: Magnesium + hydrochloric acid magnesium chloride + hydrogen Mg (s) + 2 HCl (aq) Mg. Cl 2 (aq) + H 2(g)

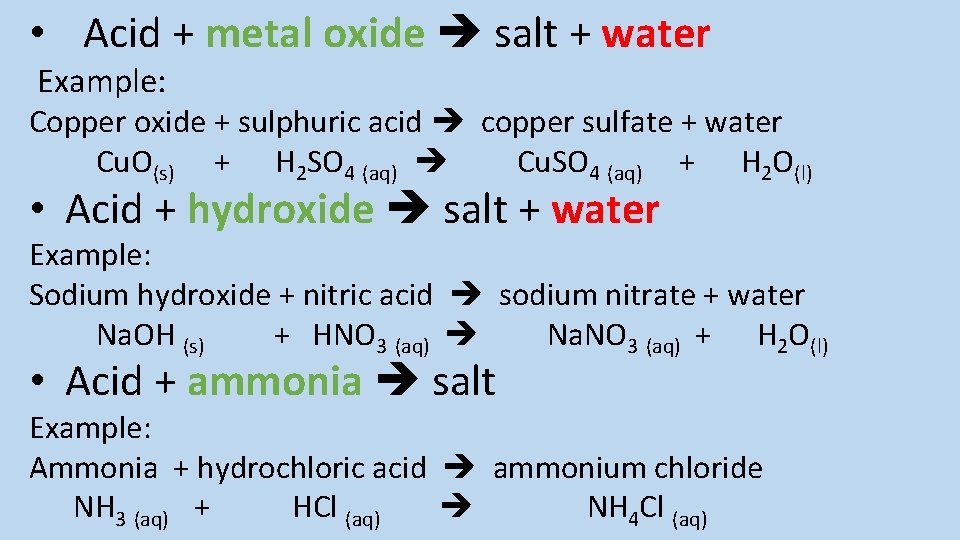
Describe the reaction between an acid and these bases : - Metal oxides - Hydroxides - Ammonia and give examples of each reaction

• Acid + metal oxide salt + water Example: Copper oxide + sulphuric acid copper sulfate + water Cu. O(s) + H 2 SO 4 (aq) Cu. SO 4 (aq) + H 2 O(l) • Acid + hydroxide salt + water Example: Sodium hydroxide + nitric acid sodium nitrate + water Na. OH (s) + HNO 3 (aq) Na. NO 3 (aq) + H 2 O(l) • Acid + ammonia salt Example: Ammonia + hydrochloric acid ammonium chloride NH 3 (aq) + HCl (aq) NH 4 Cl (aq)

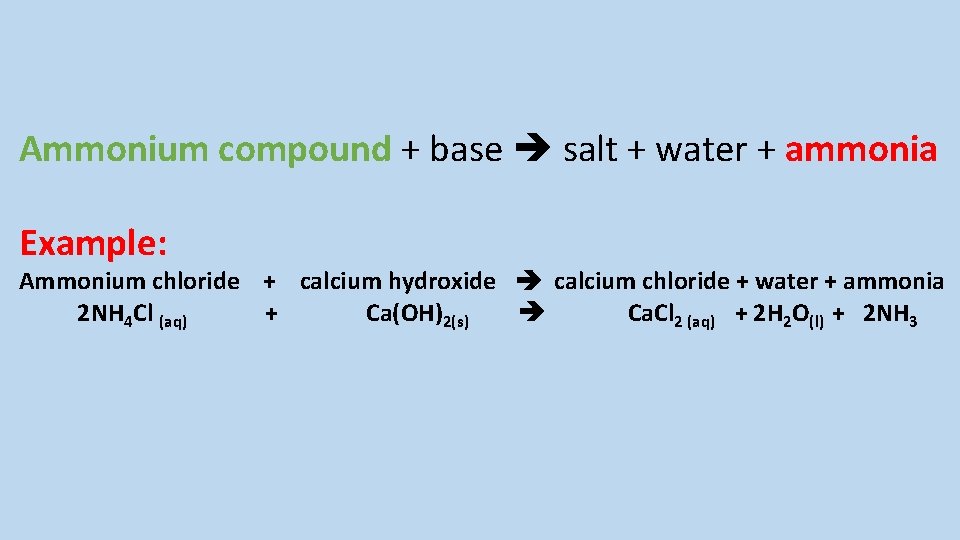
Describe the displacement reaction between an ammonium compound a base and give an example

Ammonium compound + base salt + water + ammonia Example: Ammonium chloride + calcium hydroxide calcium chloride + water + ammonia 2 NH 4 Cl (aq) + Ca(OH)2(s) Ca. Cl 2 (aq) + 2 H 2 O(l) + 2 NH 3

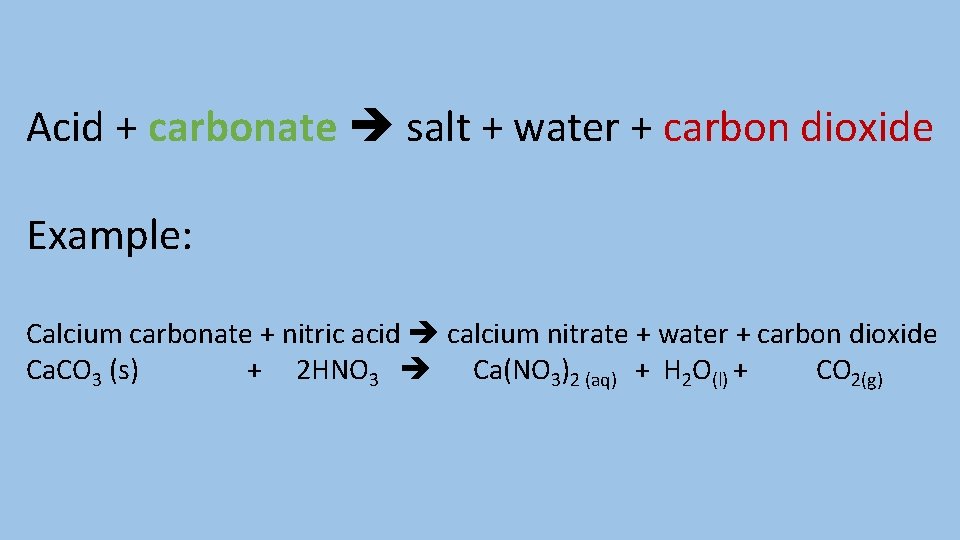
Describe the reaction between an acid and a carbonate and give an example of this type of reaction

Acid + carbonate salt + water + carbon dioxide Example: Calcium carbonate + nitric acid calcium nitrate + water + carbon dioxide Ca. CO 3 (s) + 2 HNO 3 Ca(NO 3)2 (aq) + H 2 O(l) + CO 2(g)
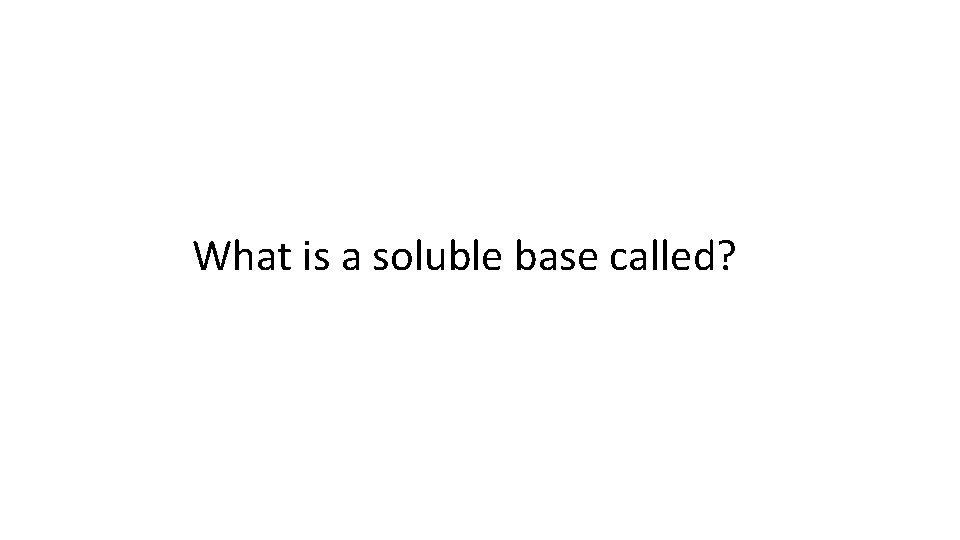
What is a soluble base called?

An alkali

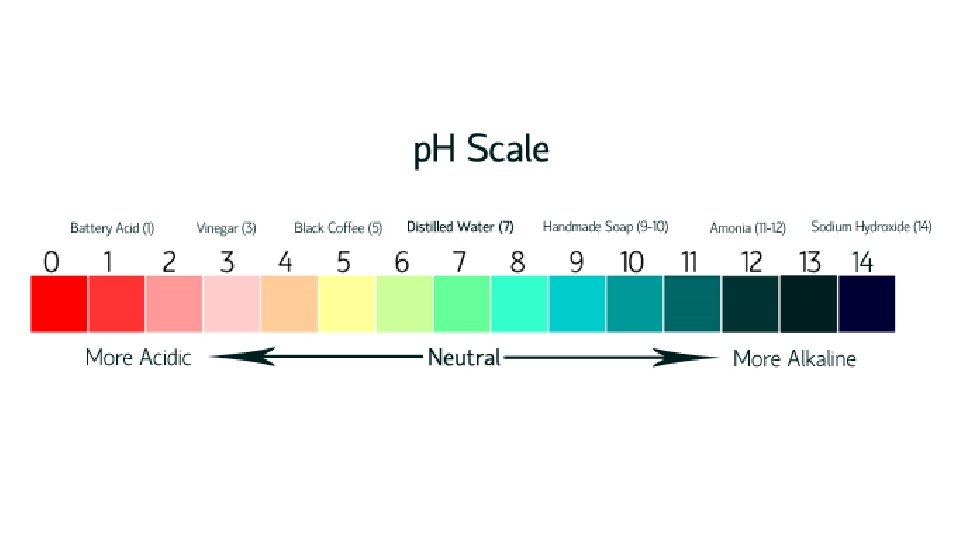
Describe the relative acidity and alkalinity with reference to the p. H scale


Describe how universal indicator is used to measure p. H

The colour of universal indicator changes depending on the p. H 1 -2 red p. H 3 -4 orange p. H 5 -6 yellow p. H 7 -9 green p. H 10 -11 blue p. H 12 -14 purple-violet

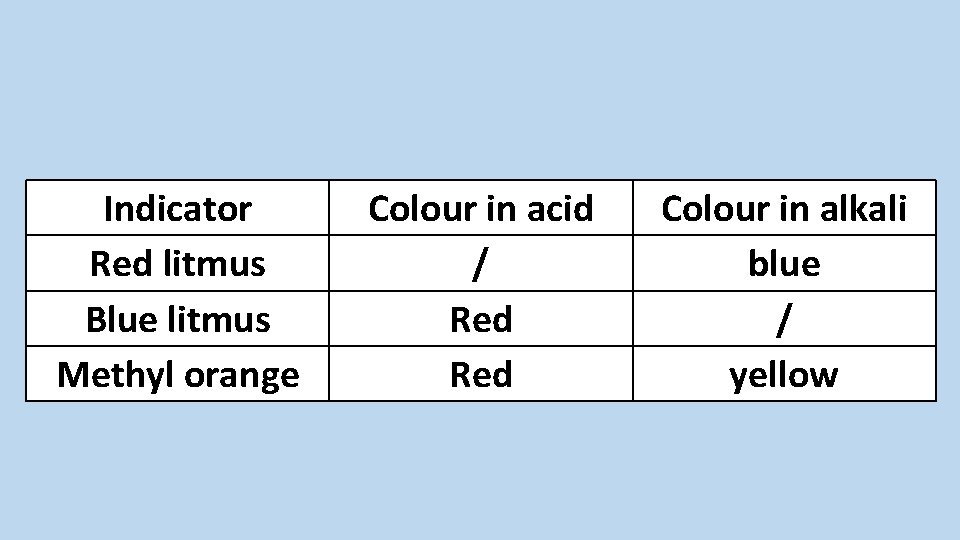
Describe the use of litmus and methyl orange indicators

Indicator Red litmus Blue litmus Methyl orange Colour in acid / Red Colour in alkali blue / yellow

Describe and explain the importance of controlling acidity in soil

If soil is too acidic plants are unable to absorb nutrients Calcium oxide, calcium hydroxide and calcium carbonate are spread on the soil to neutralise it This allows plants to absorb nutrients better and so they grow faster and larger and the farmer gets a larger yield

Explain why calcium oxide, calcium hydroxide and calcium carbonate are chosen to neutralise soil

• They are cheap • Only slightly soluble in water so rain will not wash them away from the soil

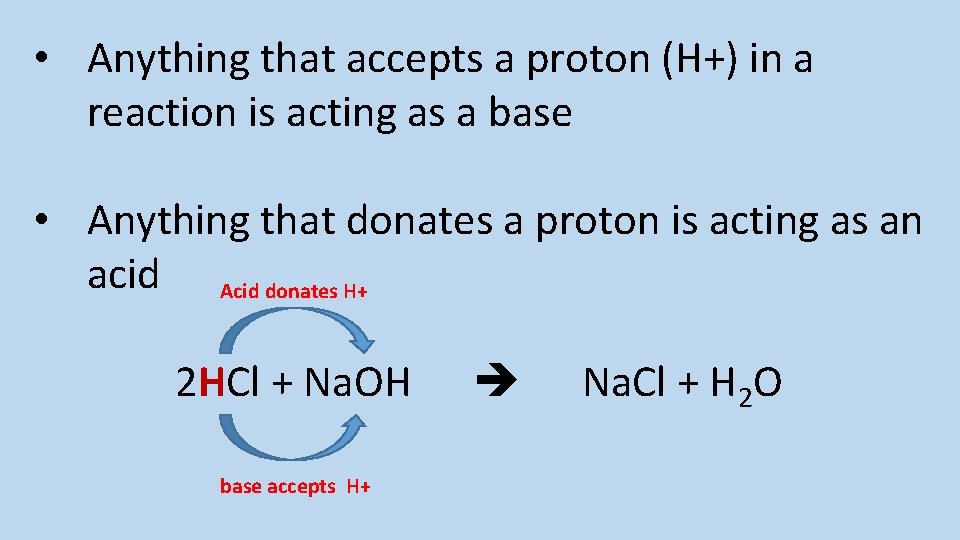
Define acids and bases in terms of proton transfer

• Anything that accepts a proton (H+) in a reaction is acting as a base • Anything that donates a proton is acting as an acid Acid donates H+ 2 HCl + Na. OH base accepts H+ Na. Cl + H 2 O

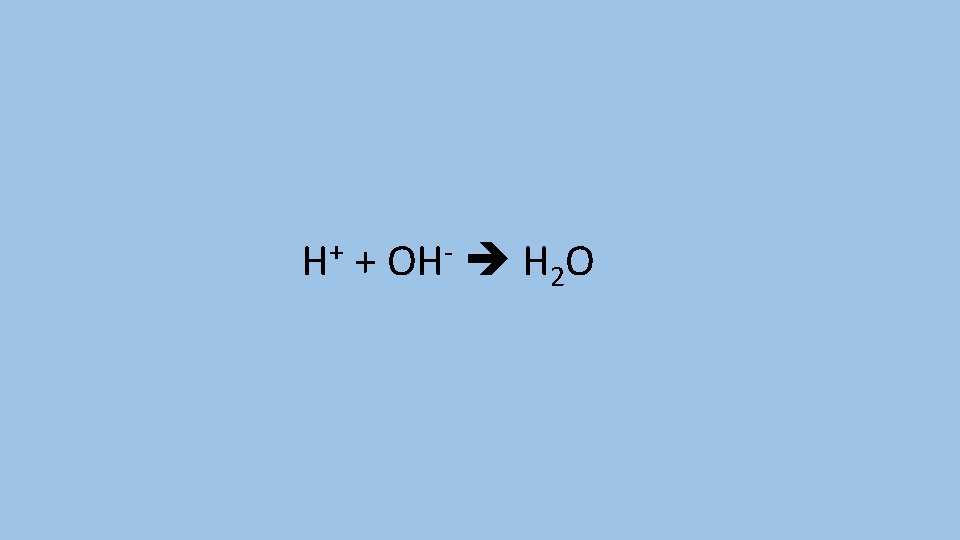
Write an ionic equation for the reaction of an acid and a base

H+ + OH- H 2 O

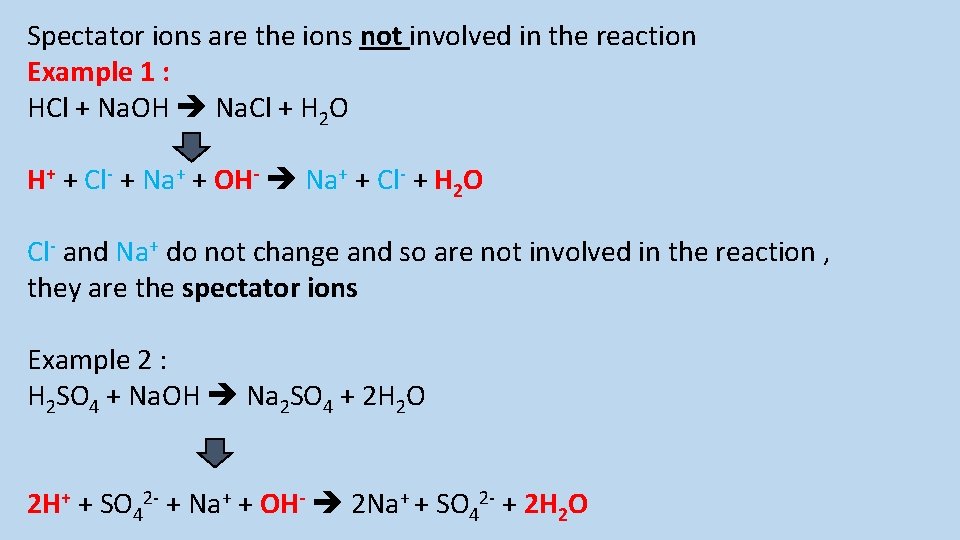
What are spectator ions?

Spectator ions are the ions not involved in the reaction Example 1 : HCl + Na. OH Na. Cl + H 2 O H+ + Cl- + Na+ + OH- Na+ + Cl- + H 2 O Cl- and Na+ do not change and so are not involved in the reaction , they are the spectator ions Example 2 : H 2 SO 4 + Na. OH Na 2 SO 4 + 2 H 2 O 2 H+ + SO 42 - + Na+ + OH- 2 Na+ + SO 42 - + 2 H 2 O

Explain the meaning of weak and strong acids and bases

• The strength of an acid or base depends on the extent to which H+ and OH- form • Strong acids completely dissociate into H+ ions • Weak acids only partially dissociate into H+ ions • Strong alkali completely dissociate into OH- ions • Weak alkali only partially dissociate into OH- ions

Give examples of strong and weak acids and strong and weak alkali

Strong acids: • Hydrochloric acid • Sulfuric acid • Nitric acid Weak acids: • Ethanoic acid • Citric acid Strong alkali: • Sodium hydroxide • Potassium hydroxide Weak alkali: • Ammonia solution

Explain why a the same concentration of a weak acid and a strong acid will completely neutralise a particular alkali

A weak acids will have a lower concentration of H+ ions than the strong acid but as the H+ ions are used up in neutralisation more will dissociate from the weak acid to continue neutralising the alkali

Describe how oxides can be classified as either acidic or basic

In general metal oxides form basic oxides which will neutralise acids In general, non metal oxides form acidic oxides which will neutralise bases

Give a examples of basic oxides
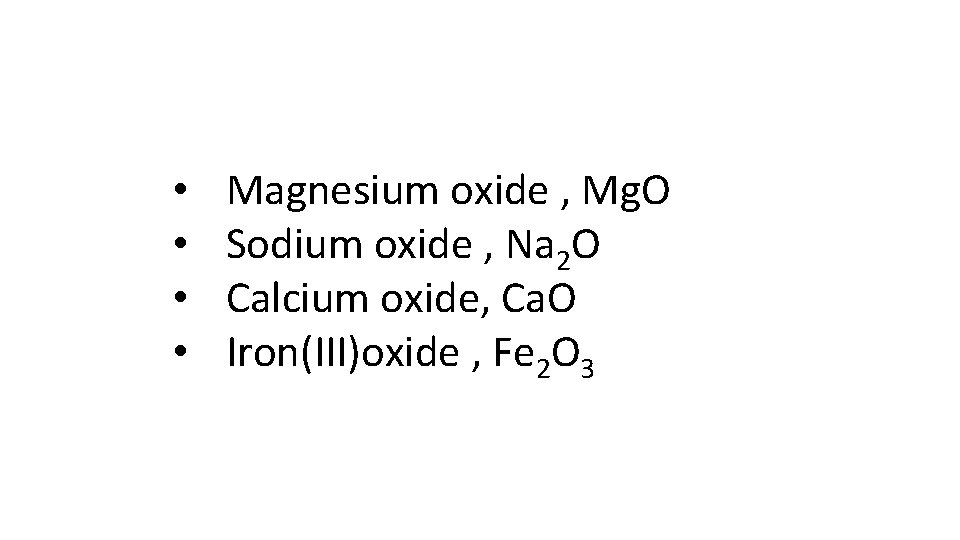
• • Magnesium oxide , Mg. O Sodium oxide , Na 2 O Calcium oxide, Ca. O Iron(III)oxide , Fe 2 O 3
Give a examples of acidic oxides

Sulfur dioxide , SO 2 Carbon dioxide , CO 2 Nitrogen dioxide , NO 2 Phosphorus pentoxide , P 4 O 10

Describe how oxides can be classified as neutral or amphoteric

A neutral oxide is neither acidic nor basic and has no effect on litmus An amphoteric oxide is both acidic and basic so they will react with either acids or bases to produce a salt and water
Give examples of amphoteric oxides

• Aluminium oxide • Zinc oxide , Zn. O • Lead(II)oxide , Pb. O

Give examples of neutral oxides

• Carbon monoxide, CO • Water , H 2 O • Dinitrogen oxide , N 2 O