Gibbs Free Energy most chemical reactions are exothermic
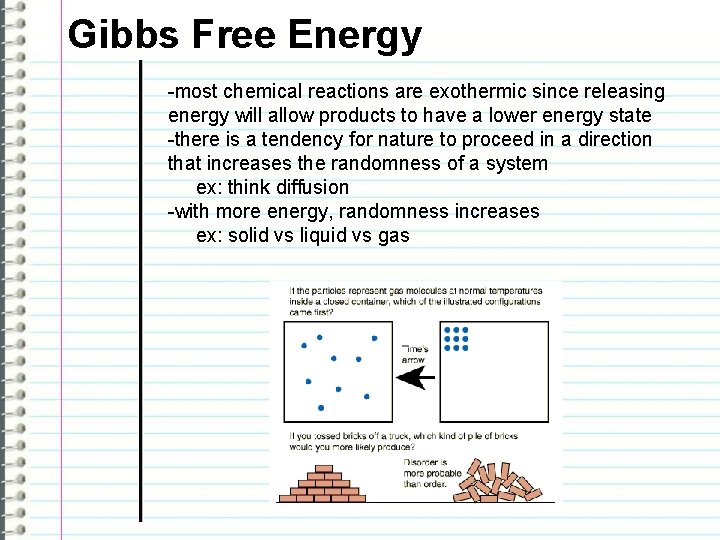
Gibbs Free Energy -most chemical reactions are exothermic since releasing energy will allow products to have a lower energy state -there is a tendency for nature to proceed in a direction that increases the randomness of a system ex: think diffusion -with more energy, randomness increases ex: solid vs liquid vs gas
Gibbs Free Energy -entropy is the degree of randomness of particles within a system -processes in nature are driven in two directions: toward the least enthalpy (heat energy) and toward the largest entropy (randomness); this happens spontaneously or naturally -often, these two are aligned, but what happens if exothermic reactions produce more order or if endothermic reactions produce more randomness? -there is an equation we can use to determine whether a reaction will occur spontaneously given the heat transferred (enthalpy), its tendency toward randomness (entropy), and its temperature
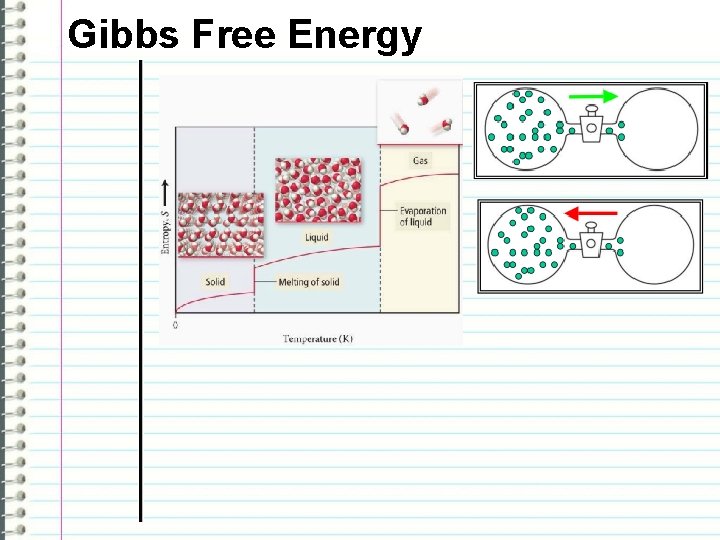
Gibbs Free Energy
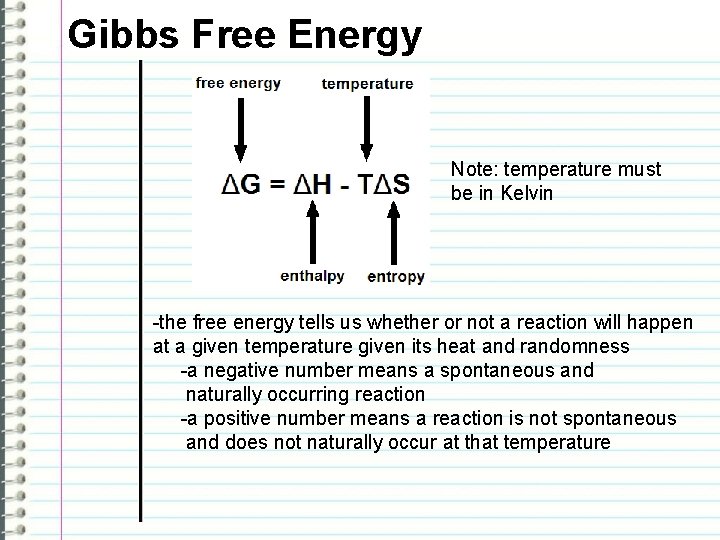
Gibbs Free Energy Note: temperature must be in Kelvin -the free energy tells us whether or not a reaction will happen at a given temperature given its heat and randomness -a negative number means a spontaneous and naturally occurring reaction -a positive number means a reaction is not spontaneous and does not naturally occur at that temperature
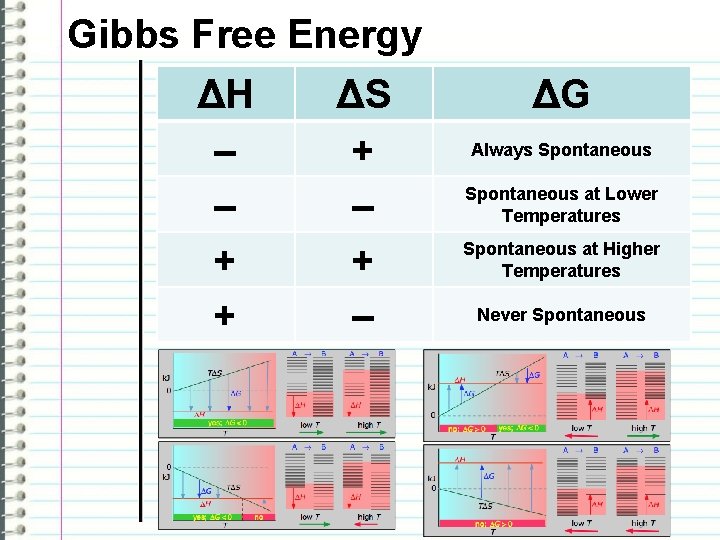
Gibbs Free Energy ΔH – – + + ΔS + – ΔG Always Spontaneous at Lower Temperatures Spontaneous at Higher Temperatures Never Spontaneous

Gibbs Free Energy Practice 1. For the reaction below, NH 4 Cl (s) NH 2 (g) + HCl (g) At 298. 15 K, ΔH = 176 k. J and ΔS = 0. 285 k. J/K. Calculate ΔG, and tell whether this reaction is spontaneous at 298. 15 K. 2. For the vaporization reaction, Br 2 (l) Br 2 (g) ΔH = 31. k. J and ΔS = 93 J/K at a temperature of 70°C. Will this reaction be spontaneous at that temperature?
- Slides: 6