Gibbs Free Energy How can we use H

Gibbs Free Energy

How can we use ΔH and ΔS to predict whether a given reaction occuring at constant temperature and pressure will be spontaneous?
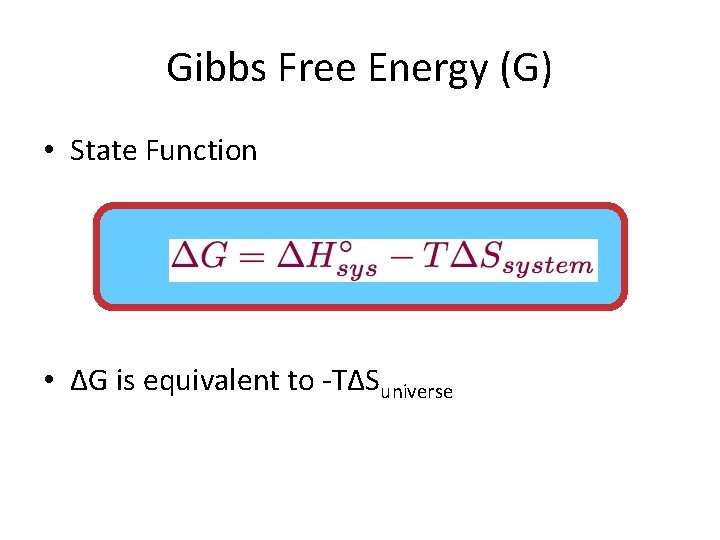
Gibbs Free Energy (G) • State Function • ΔG is equivalent to -TΔSuniverse

Gibbs Free Energy • If Suniv > 0 , G < 0 ( spontaneous rxn) • If G > 0 (nonspontaneous rxn) • If G = 0 (rxn at equilibrium )
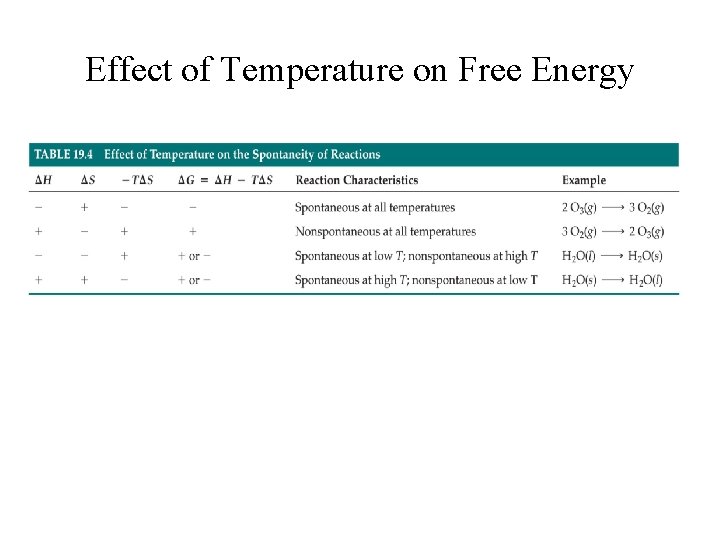
Effect of Temperature on Free Energy

Free energy always decreases for any spontaneous process carried out at constant temperature and pressure
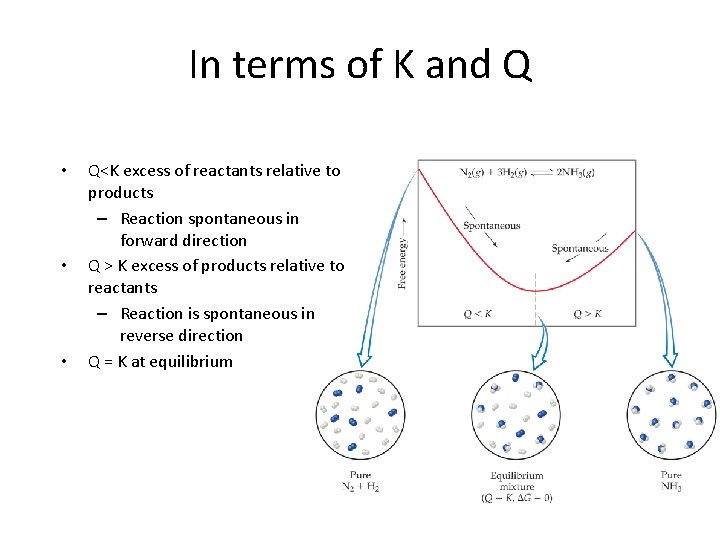
In terms of K and Q • • • Q<K excess of reactants relative to products – Reaction spontaneous in forward direction Q > K excess of products relative to reactants – Reaction is spontaneous in reverse direction Q = K at equilibrium

Calculating Gibbs Free Energy 3 equations to use:

Gibb’s Free Energy Practice Problem 1
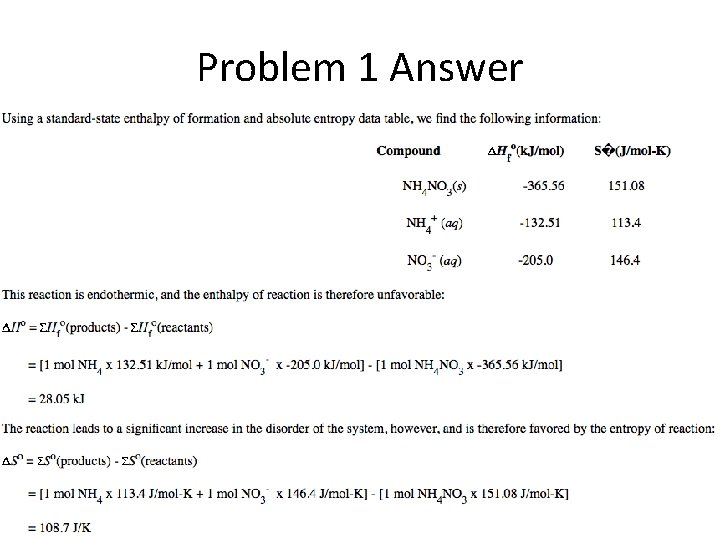
Problem 1 Answer

Problem 1 Answer Cont.
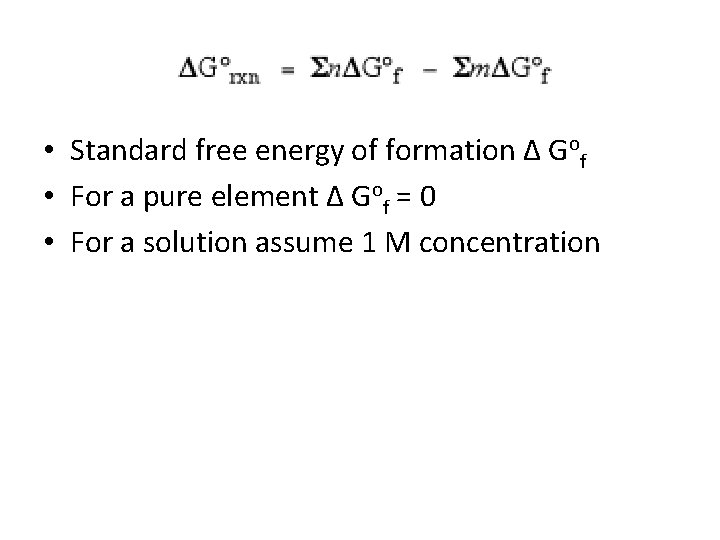
• Standard free energy of formation Δ Gof • For a pure element Δ Gof = 0 • For a solution assume 1 M concentration
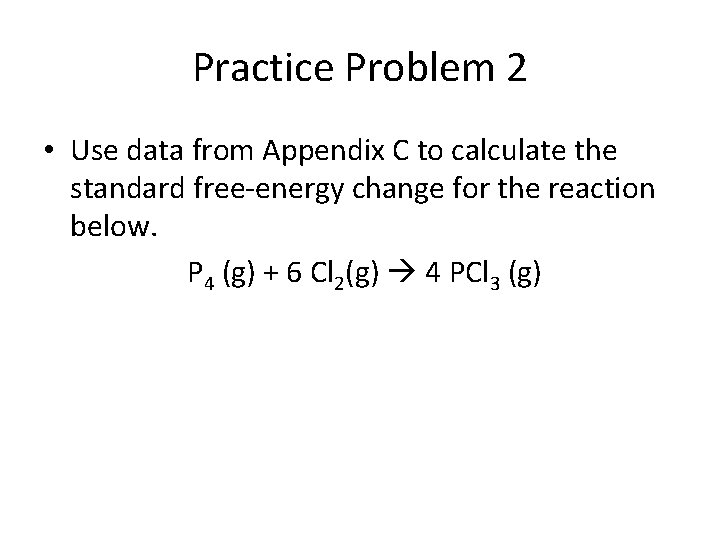
Practice Problem 2 • Use data from Appendix C to calculate the standard free-energy change for the reaction below. P 4 (g) + 6 Cl 2(g) 4 PCl 3 (g)
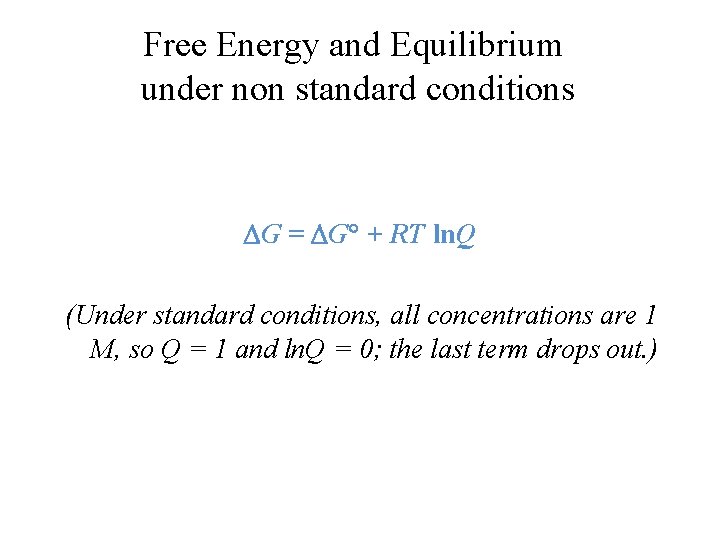
Free Energy and Equilibrium under non standard conditions G = G + RT ln. Q (Under standard conditions, all concentrations are 1 M, so Q = 1 and ln. Q = 0; the last term drops out. )

Practice Problem 3 • Calculate Δ G at 298 K for a mixture of 1. 0 atm N 2, 3. 0 atm H 2, and 0. 50 atm NH 3 being used in the equation below: N 2(g) + 3 H 2(g) 2 NH 3
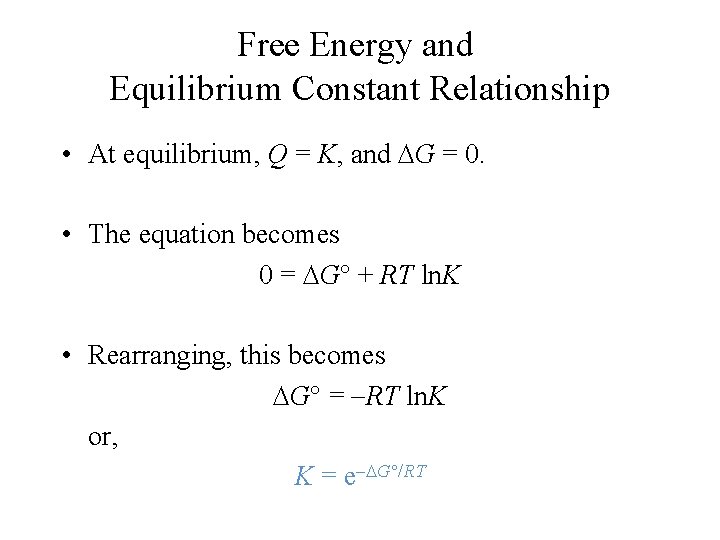
Free Energy and Equilibrium Constant Relationship • At equilibrium, Q = K, and G = 0. • The equation becomes 0 = G + RT ln. K • Rearranging, this becomes G = RT ln. K or, K = e G /RT
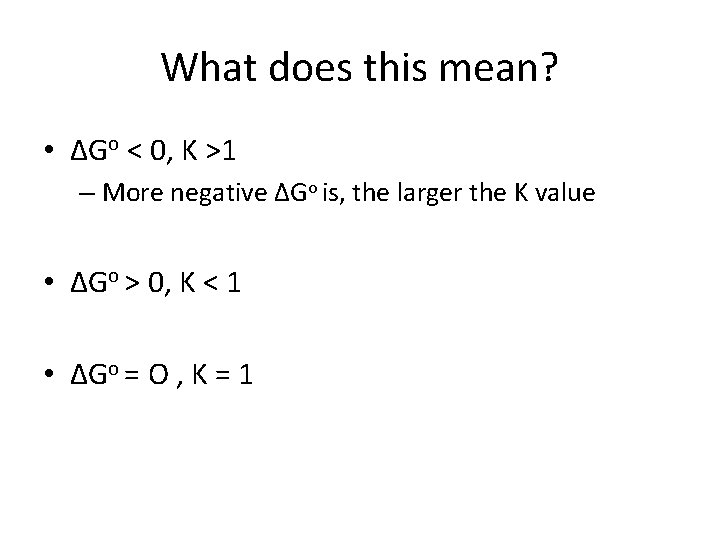
What does this mean? • ΔGo < 0, K >1 – More negative ΔGo is, the larger the K value • ΔGo > 0, K < 1 • ΔGo = O , K = 1

Online Practice Problems Visit the websites listed. Complete the practice problems on a separate sheet of paper. Check your answers
Practice Problem Websites • Entropy • http: //chemed. chem. purdue. edu/genchem/to picreview/bp/ch 21/entropy. php • Gibb’s Free Energy • http: //chemed. chem. purdue. edu/genchem/to picreview/bp/ch 21/gibbs. php • Free Energy and Equilibrium • http: //www. mhhe. com/physsci/chemistry/ch ang 7/ssg/chap 18_5 sg. html

Closure Practice Problems • Chapter 19 in textbook (2015 Edition) • Spontaneous Processes 19. 11, 19. 17 – (8 th Edition – 19. 1, 19. 2) • Entropy 19. 25, 19. 27, 19. 41, 19. 43, 19. 47, 19. 53, 19. 54 – (8 th Edition – 19. 25, 19. 27, 19. 29, 19. 31, 19. 33)

Closure Practice Problems Cont • Gibb’s Free Energy – 19. 57, 19. 59, 19. 60, 19. 61, 19. 69 – 8 th edition – 19. 49, 19. 51, 19. 53, 19. 54, 19. 56, 19. 58, 19. 63, 19. 66) • Free energy and equilibrium – 19. 79, 19. 81, 19. 83 – 8 th edition – 19, 71, 19. 76, 19. 79 • Additional Exercises – 8 th edition – 19. 82, 19. 87, 19. 88, 19. 89, 19. 90, 19. 92
- Slides: 21