Giant Covalent Molecules Covalent Bonds Simple molecular structure

Giant Covalent Molecules
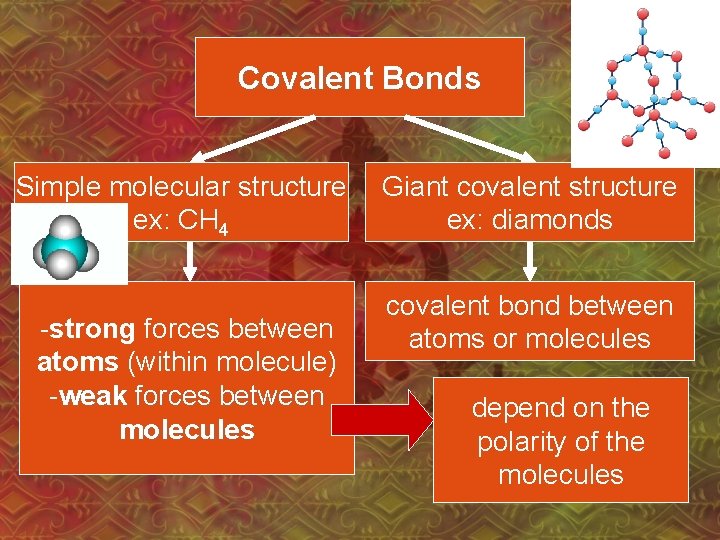
Covalent Bonds Simple molecular structure ex: CH 4 -strong forces between atoms (within molecule) -weak forces between molecules Giant covalent structure ex: diamonds covalent bond between atoms or molecules depend on the polarity of the molecules
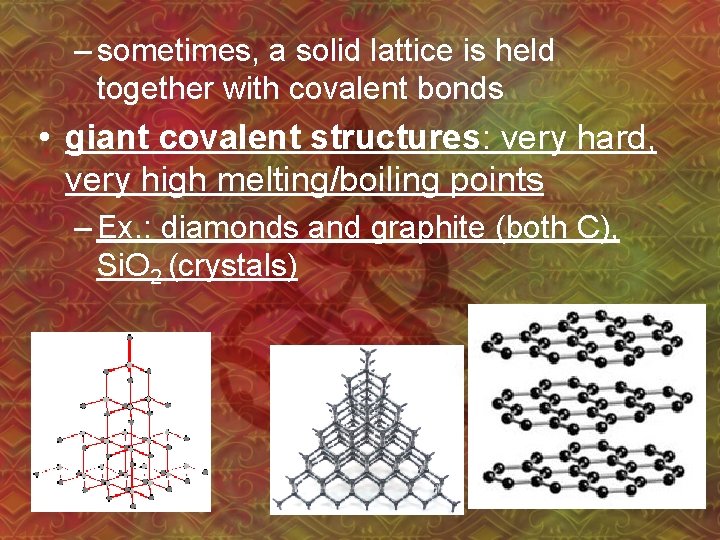
– sometimes, a solid lattice is held together with covalent bonds • giant covalent structures: very hard, very high melting/boiling points – Ex. : diamonds and graphite (both C), Si. O 2 (crystals)

Intermolecular Forces IMF

• IMF: Forces (not bonds) that hold covalent molecules together – For solids and liquids

• IMF control the physical properties of covalent compounds. – Melting and boiling point – Viscosity (how thick/sticky a liquid is) – Solubility (what will dissolve in a liquid) • IMF explains why WATER is the liquid required for life!

Three types of IMF Increasing strength • Van der Waal’s • Dipole-dipole • Hydrogen bonds

1. van der Waal’s Forces • Between non-polar molecules • Electrons move randomly, so by chance, many e- can end up at one end of a molecule – “temporary dipole” – even in non-polar molecules! • Ex: CCl 4
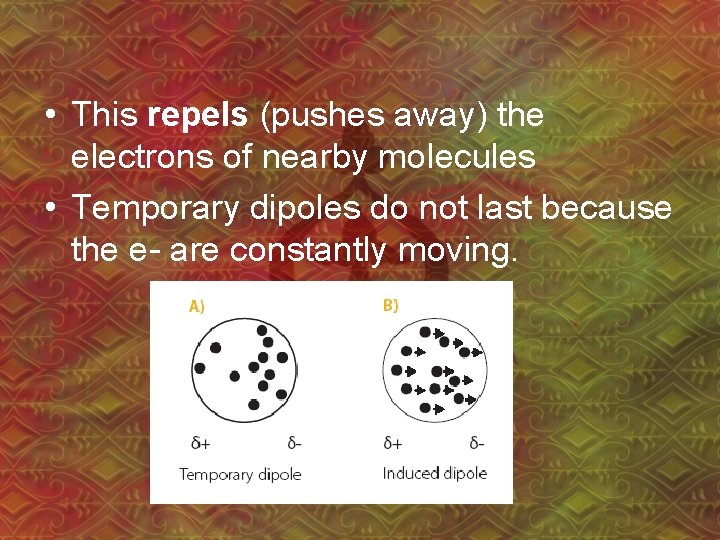
• This repels (pushes away) the electrons of nearby molecules • Temporary dipoles do not last because the e- are constantly moving.
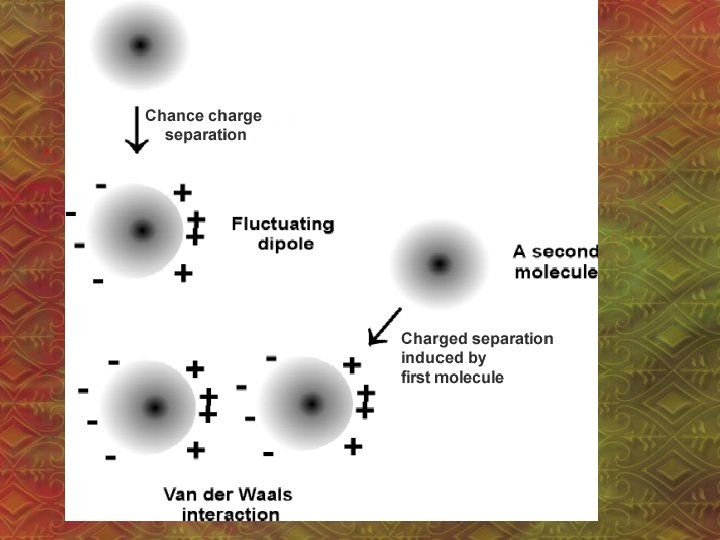
• vd. W Force depends on: – surface area of molecules – molar mass of molecules
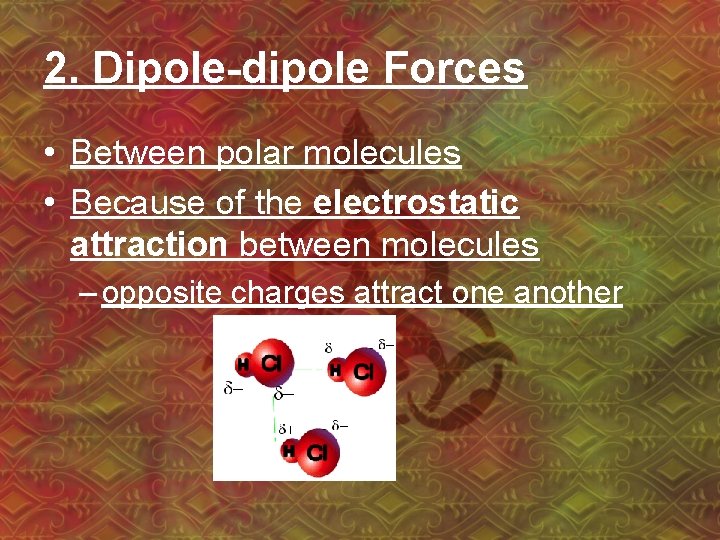
2. Dipole-dipole Forces • Between polar molecules • Because of the electrostatic attraction between molecules – opposite charges attract one another
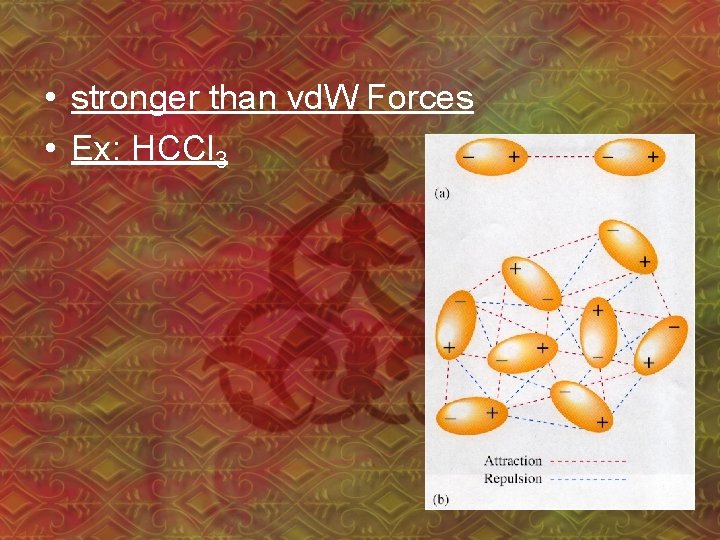
• stronger than vd. W Forces • Ex: HCCl 3
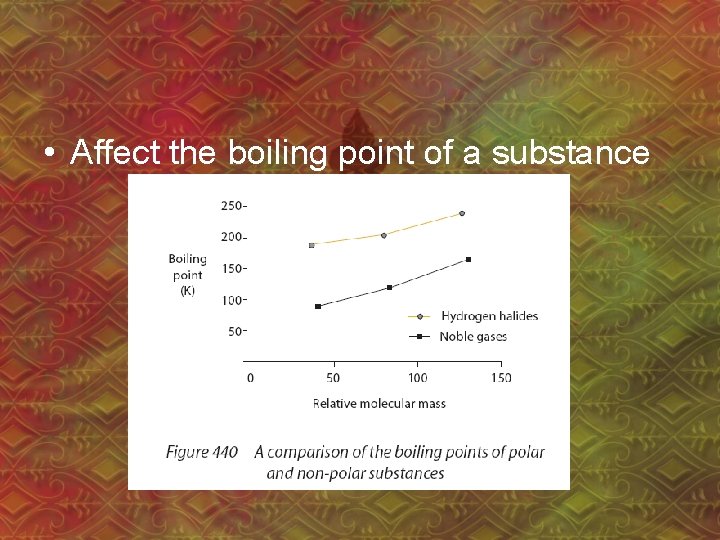
• Affect the boiling point of a substance
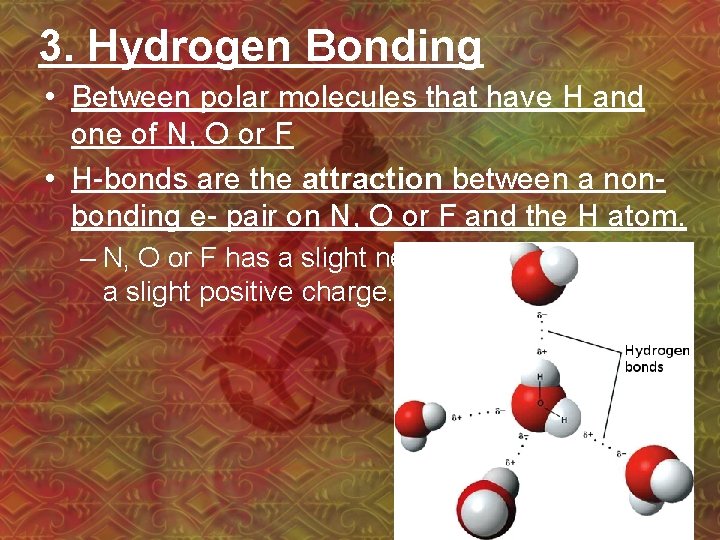
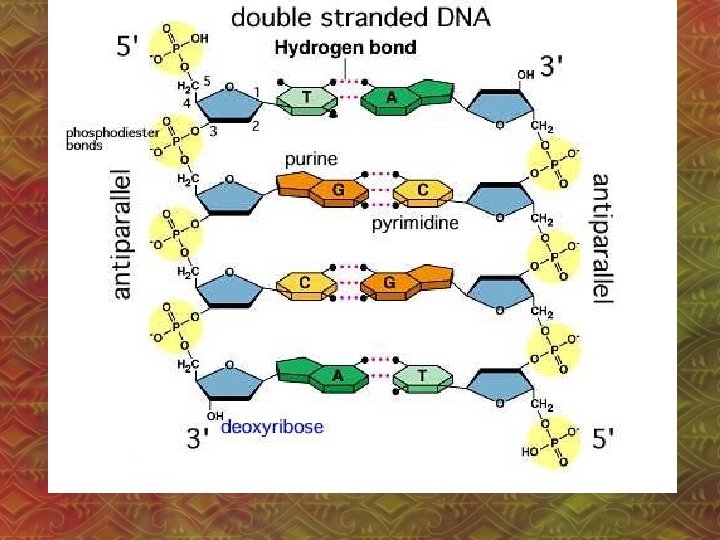
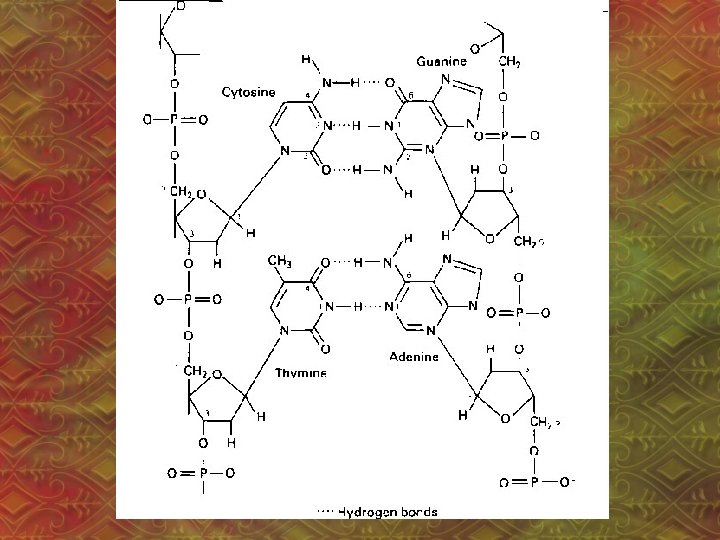
3. Hydrogen Bonding • Between polar molecules that have H and one of N, O or F • H-bonds are the attraction between a nonbonding e- pair on N, O or F and the H atom. – N, O or F has a slight negative charge, H has a slight positive charge.

• H-bond is the strongest IMF • H-bonds affect the physical properties of substances (ex: boiling point) • Ex: H 2 O
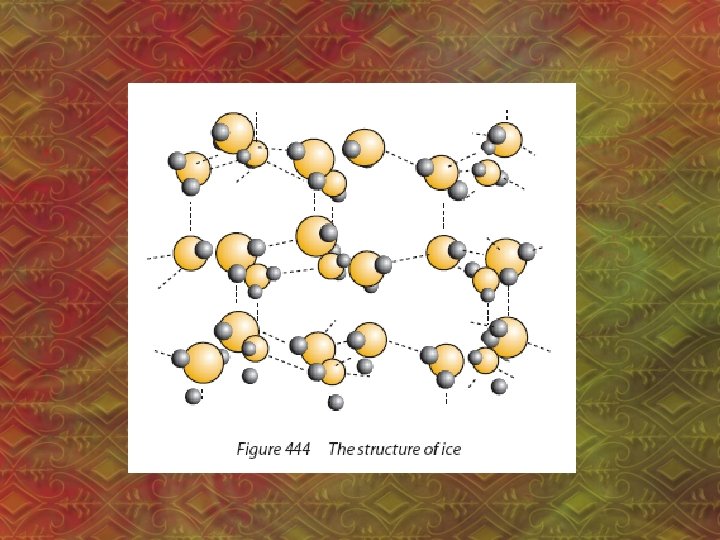

• H-bonds cause: a. higher boiling point, lower volatility • volatility: how easily something evaporates b. greater solubility in water c. higher viscosity • viscosity: resistance to flow, “thickness”

• All IMF affect the properties of a substance. • The stronger the IMF, the… – Higher the boiling point – Higher the melting point – Greater the viscosity (resistance to flow) • IMF also affect the solubility of a substance

• http: //www. youtube. com/watch? v=cgi. N k 94 Xya. I

• Practice Quiz on Thursday • I will give you the formula for a molecule. You need to provide the: – Lewis structure – Molecule shape/VSEPR Theory – Polarity – Intermolecular Force present – Also, know something about giant covalent structures

Example Quiz Question: • For the molecule HCCl 3: a) Draw the Lewis Structure b) Draw the shape including the bond angle, and label it with the name of the shape. c) Identify polarity using delta + and delta – d) Label the molecule as Polar or Non-polar. e) What kind of intermolecular forces would exist between molecules of this compound?
- Slides: 26