Giant Covalent Compounds Graphite and diamonds Allotropes of








- Slides: 24

Giant Covalent Compounds Graphite and diamonds Allotropes of carbon Silicon and Quartz

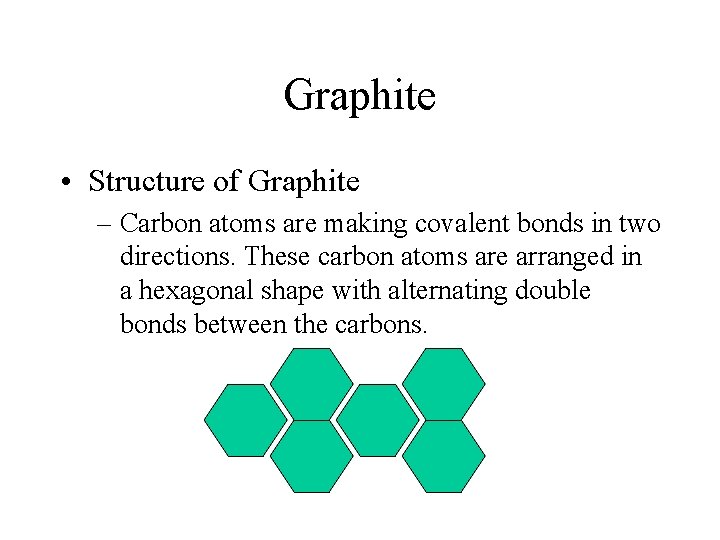
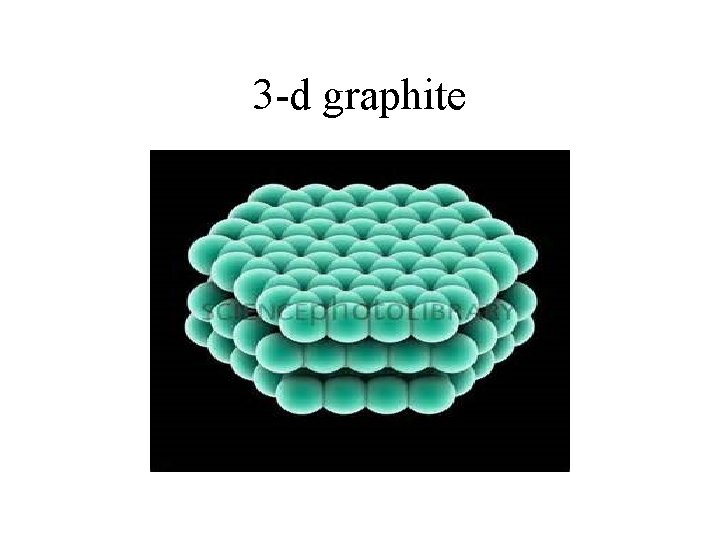
Graphite • Structure of Graphite – Carbon atoms are making covalent bonds in two directions. These carbon atoms are arranged in a hexagonal shape with alternating double bonds between the carbons.

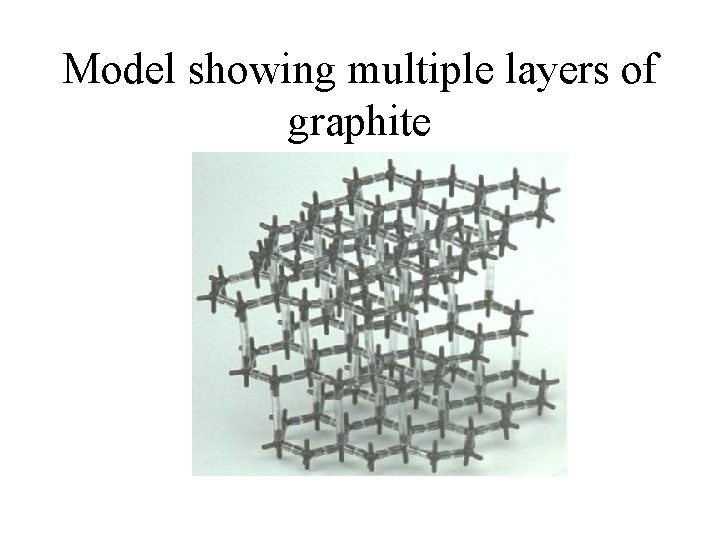
Structure of graphite cont. • The hexagonal shapes form layers one on top of the other. Between the layers are Van der Waal forces which create a weak attraction between the layers.

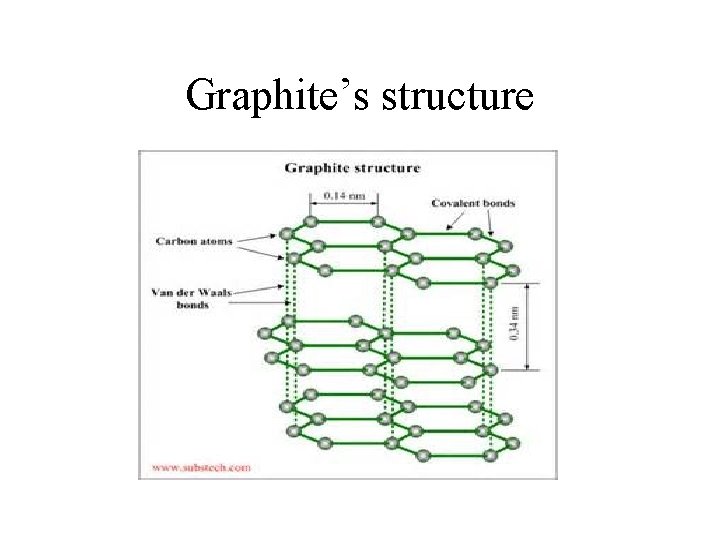
Graphite’s structure

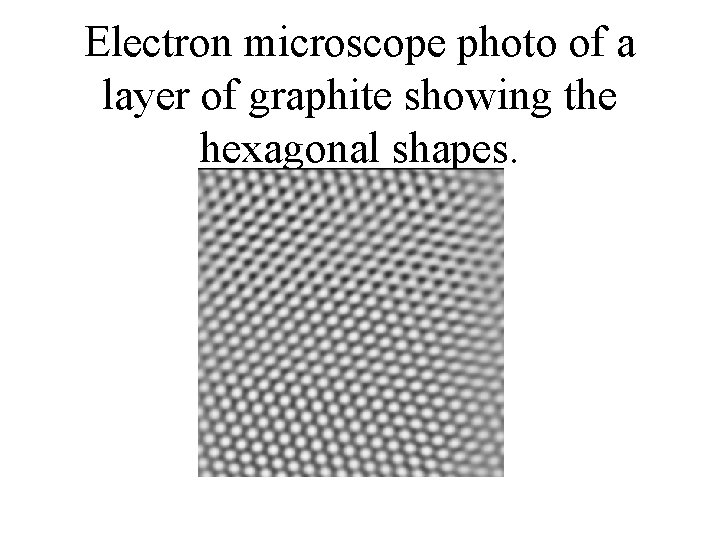
Electron microscope photo of a layer of graphite showing the hexagonal shapes.

3 -d graphite

Model showing multiple layers of graphite

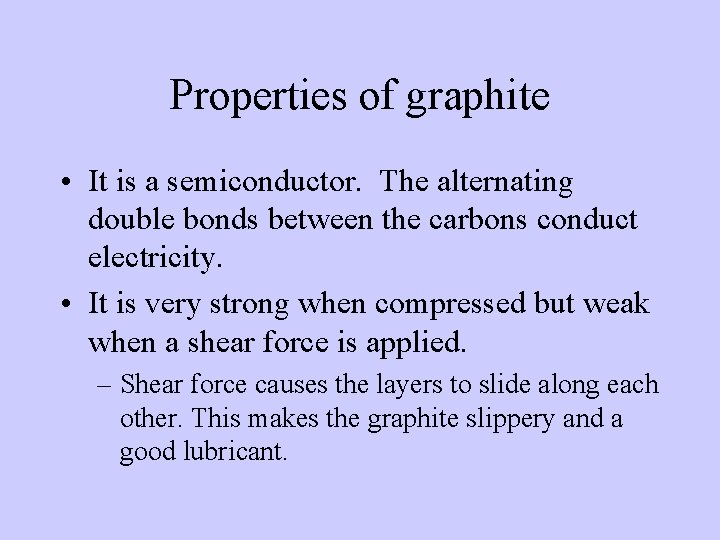
Properties of graphite • It is a semiconductor. The alternating double bonds between the carbons conduct electricity. • It is very strong when compressed but weak when a shear force is applied. – Shear force causes the layers to slide along each other. This makes the graphite slippery and a good lubricant.

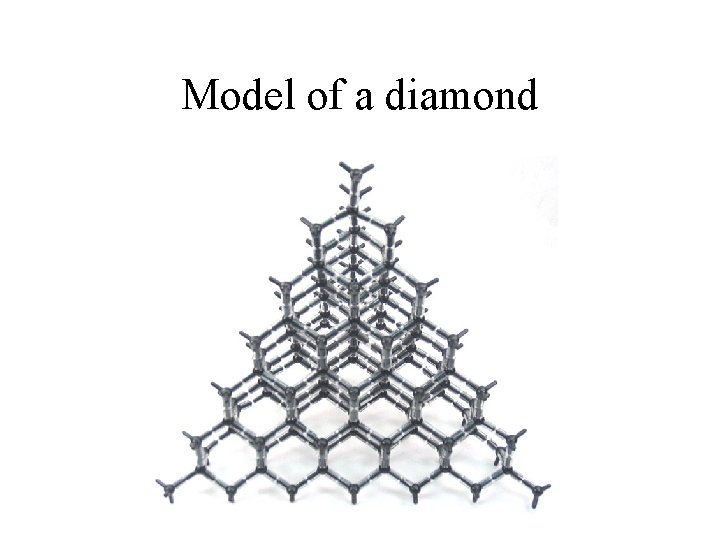
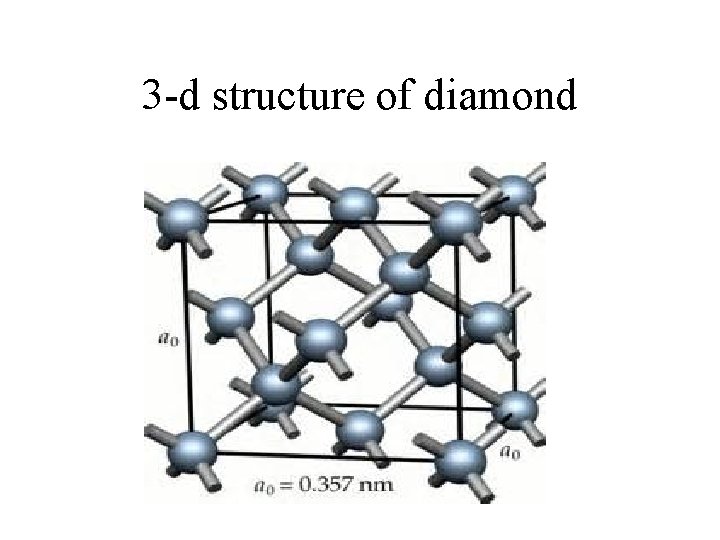
diamonds • Structure of diamonds. – All of the carbons are covalently bonded to each other in a tetrahedral arrangement. • Properties of diamonds – They are strong in all directions. The tetrahedral arrangement makes a covalent bond in all dimensions. – They are not conductive. – Extremely high melting points. – Brittle. – High index of refraction. This “traps” light and makes them “sparkle”

Model of a diamond

3 -d structure of diamond

Photos of diamonds



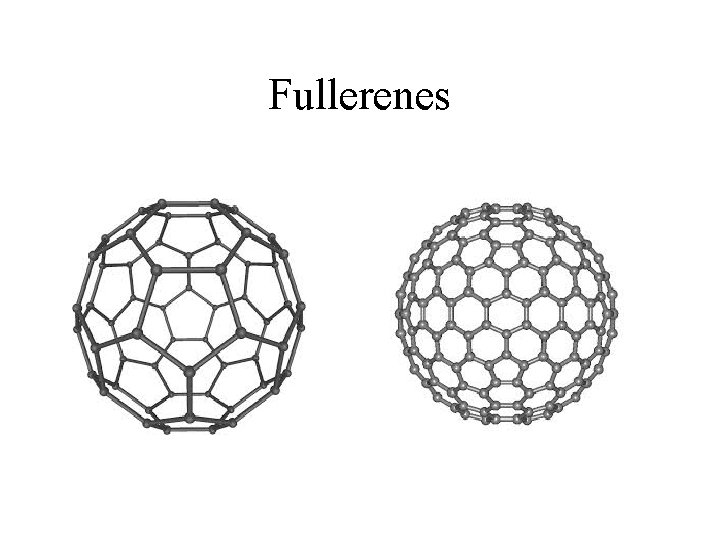
Fullerenes • Another allotrope of carbon • Most common is C 60, but other forms have been discovered. C 20 is the smallest fullerene to be discovered. • Shaped like a geodesic dome, C 60 is made of 32 pentagons bonded together to make a ball shaped molecule. • Formed when graphite is vaporized in the presence of helium. Not found in nature.

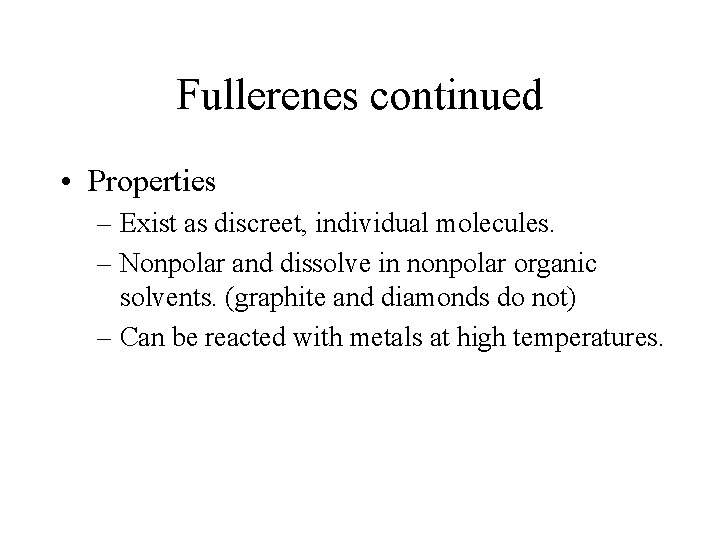
Fullerenes continued • Properties – Exist as discreet, individual molecules. – Nonpolar and dissolve in nonpolar organic solvents. (graphite and diamonds do not) – Can be reacted with metals at high temperatures.

Fullerenes

Silicon networks • Pure silicon – Has the same tetrahedral arrangement as carbon – Larger radius reduces the bond strength between the atoms; they’re not as strong as a diamond

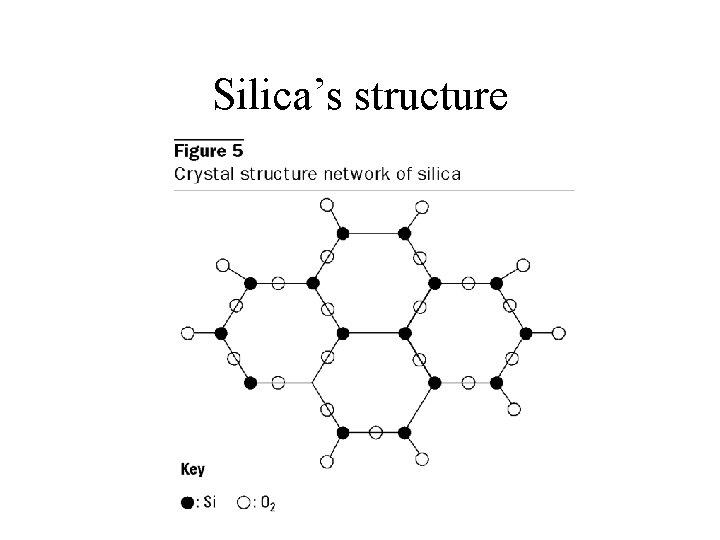
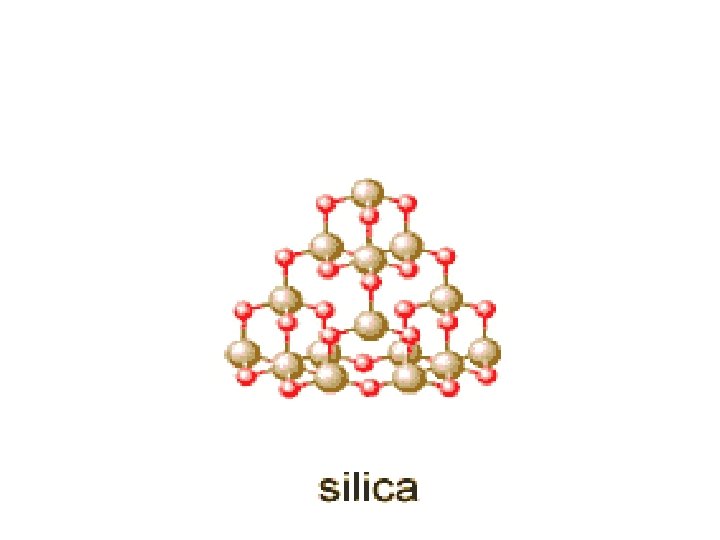
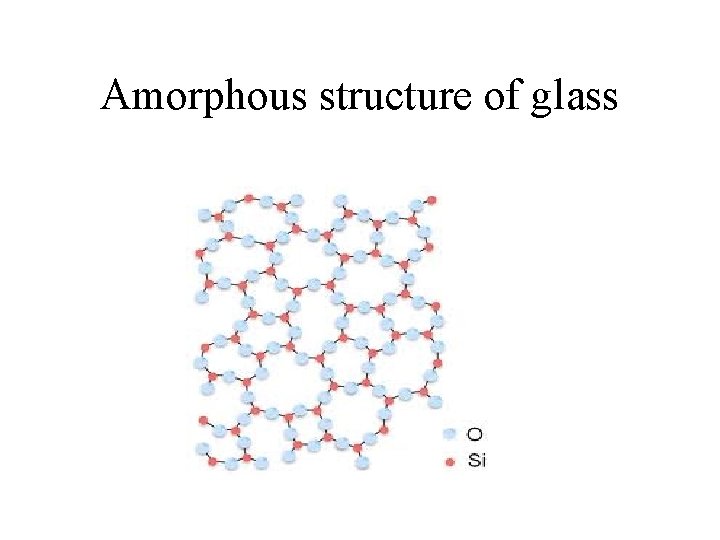
Silicon networks continued • Silicon dioxide, or silica – Empirical formula is Si. O 2 but it actually exists as Si. O 4 arranged in a tetrahedral arrangement where the Si atom is the center of a tetrahedral arrangement and the oxygen atoms are shared with other Si atoms. That is: each silicon is bonded to 4 oxygen atom, and each oxygen atom is bonded to two silicon atoms. Thus, Si. O 2 – Forms sand quartz crystal. – Melting silica (sand) above 1600 C turns it into glass, a disordered, amorphous solid.

Silica’s structure


Amorphous structure of glass

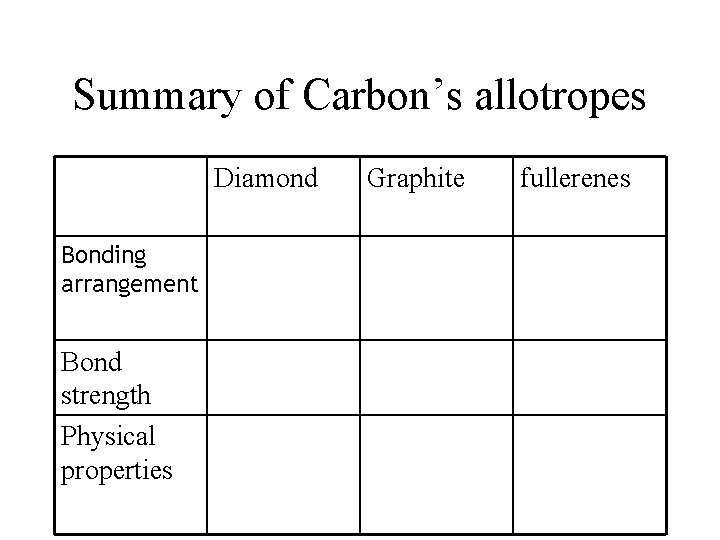
Summary of Carbon’s allotropes Diamond Bonding arrangement Bond strength Physical properties Graphite fullerenes

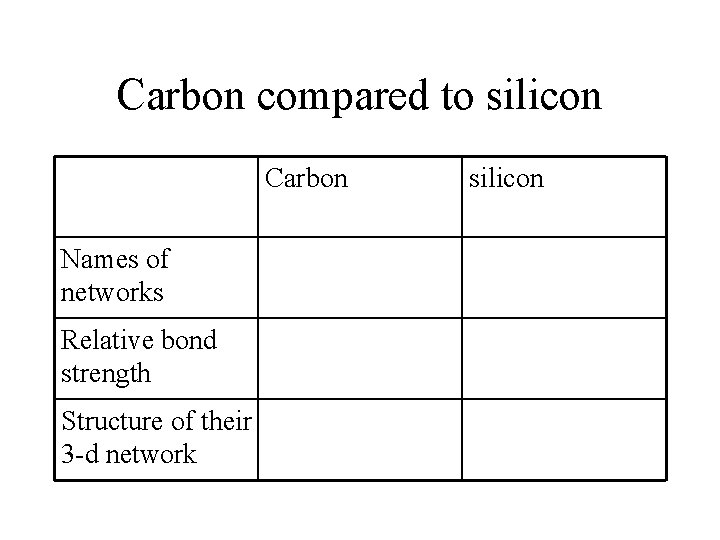
Carbon compared to silicon Carbon Names of networks Relative bond strength Structure of their 3 -d network silicon