Getting started with electrochemistry in polymer electrolyte membrane
Getting started with electrochemistry in polymer electrolyte membrane fuel cells (PEMFC): Francois Lapicque Laboratoire des Sciences du Génie Chimique, CNRS –ENSIC, Nancy • Background of electrochemical phenomena in FC • Features of electrochemical reactions • Transport and transfer • Available electrochemical methods for their investigation Presented by: Dr Bradley Ladewig brad. ladewig@gmail. com Electrochemistry in membrane fuel cells 1
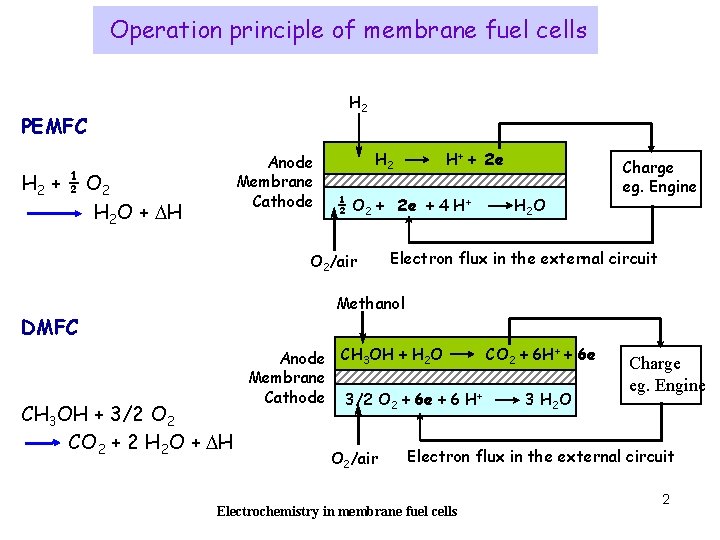
Operation principle of membrane fuel cells H 2 PEMFC Anode Membrane Cathode H 2 + ½ O 2 H 2 O + DH H 2 H+ + 2 e ½ O 2 + 2 e + 4 H+ O 2/air H 2 O Charge eg. Engine Electron flux in the external circuit Methanol DMFC CH 3 OH + 3/2 O 2 CO 2 + 2 H 2 O + DH CO 2 + 6 H+ + 6 e Anode CH 3 OH + H 2 O Membrane Cathode 3/2 O 2 + 6 e + 6 H+ 3 H 2 O O 2/air Charge eg. Engine Electron flux in the external circuit Electrochemistry in membrane fuel cells 2
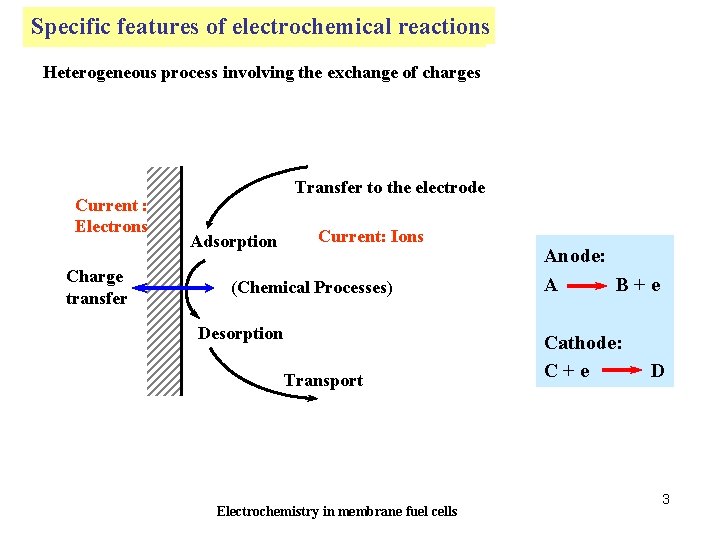
Specific features electrochemical reactions Particularités de of la réaction électrochimique Heterogeneous process involving the exchange of charges Current : Electrons Charge transfer Transfer to the electrode Adsorption Current: Ions (Chemical Processes) Desorption Transport Electrochemistry in membrane fuel cells Anode: A B+e Cathode: C+e D 3
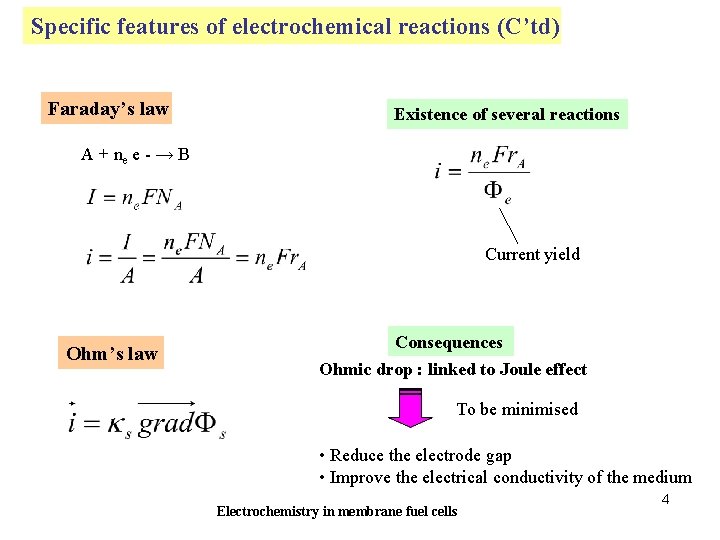
Specific features of electrochemical reactions (C’td) Faraday’s law Existence of several reactions A + ne e - → B Current yield Ohm’s law Consequences Ohmic drop : linked to Joule effect To be minimised • Reduce the electrode gap • Improve the electrical conductivity of the medium Electrochemistry in membrane fuel cells 4

Split view of a polymer electrolyte membrane fuel cell Feed H 2 O 2 External plate O 2 Outlet Bipolar plate H 2 Membrane-electrode assembly Backing PEMFC: Electrolyte = Conducting polymer • Reduce the membrane thickness • Improve the electrical connections Electrochemistry in membrane fuel cells 5

Electrochemistry in membrane fuel cells 6
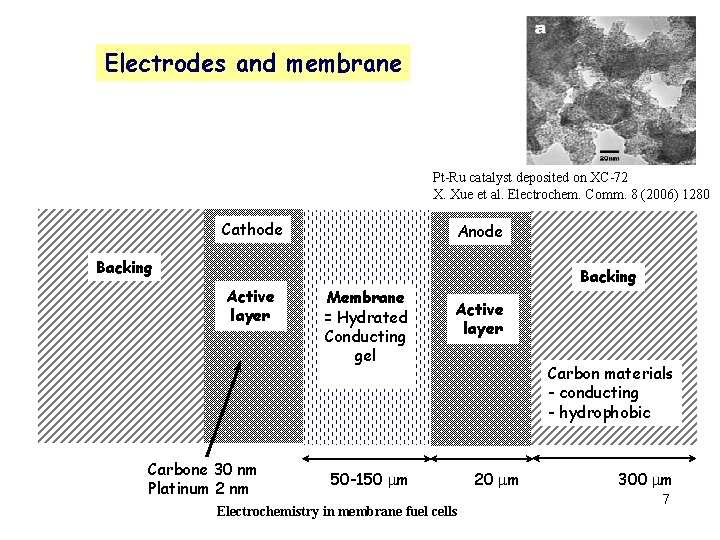
Electrodes and membrane Pt-Ru catalyst deposited on XC-72 X. Xue et al. Electrochem. Comm. 8 (2006) 1280 Cathode Anode Backing Active layer Carbone 30 nm Platinum 2 nm Membrane = Hydrated Conducting gel Backing Active layer 50 -150 mm Electrochemistry in membrane fuel cells Carbon materials - conducting - hydrophobic 20 mm 300 mm 7
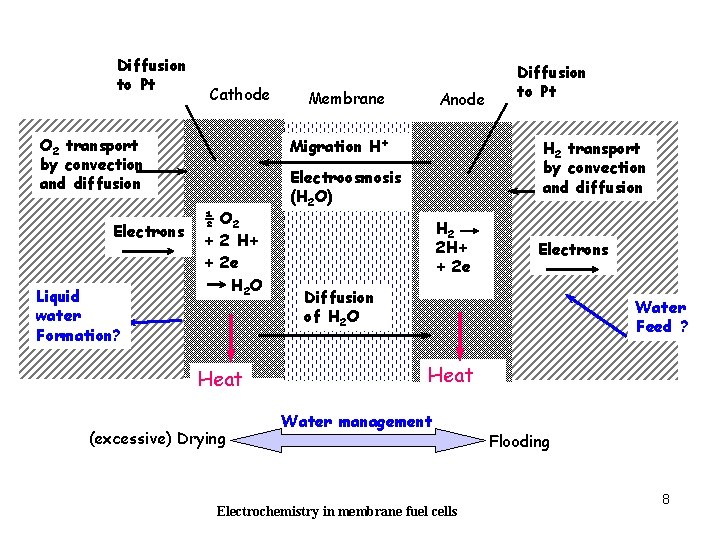
Diffusion to Pt Cathode O 2 transport by convection and diffusion Electrons Liquid water Formation? Membrane Anode Migration H+ ½ O 2 + 2 H+ + 2 e H 2 O Heat (excessive) Drying Diffusion to Pt H 2 transport by convection and diffusion Electroosmosis (H 2 O) H 2 2 H+ + 2 e Electrons Diffusion of H 2 O Water Feed ? Heat Water management Electrochemistry in membrane fuel cells Flooding 8
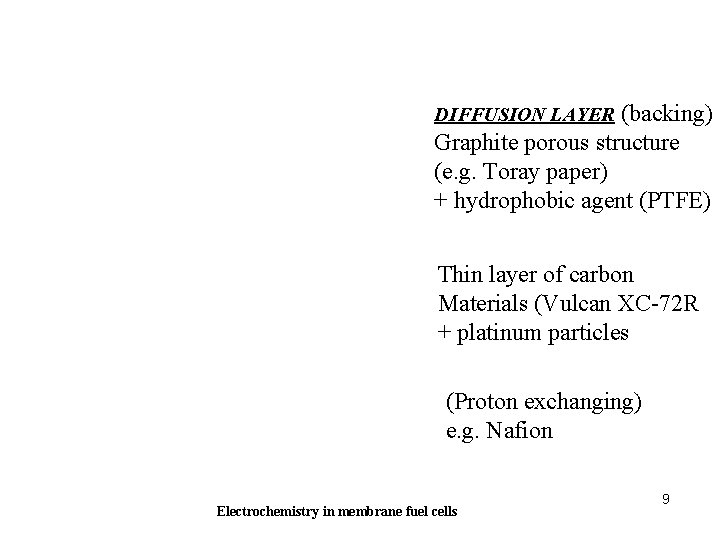
DIFFUSION LAYER (backing) Graphite porous structure (e. g. Toray paper) + hydrophobic agent (PTFE) Thin layer of carbon Materials (Vulcan XC-72 R + platinum particles (Proton exchanging) e. g. Nafion Electrochemistry in membrane fuel cells 9
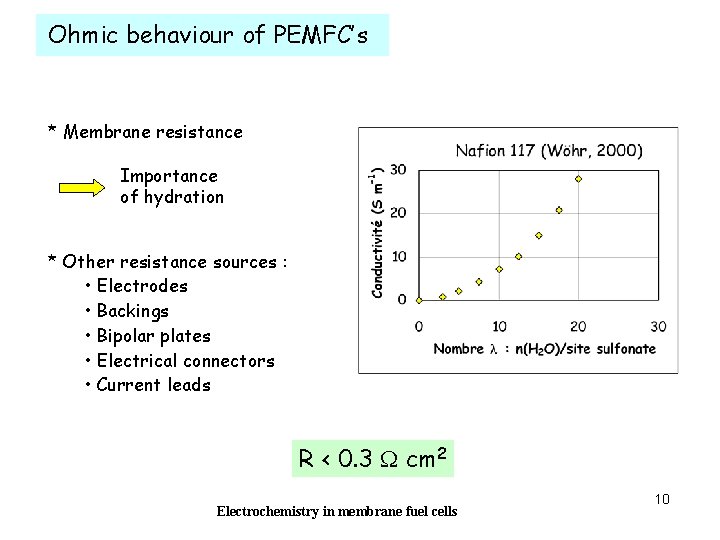
Ohmic behaviour of PEMFC’s * Membrane resistance Importance of hydration * Other resistance sources : • Electrodes • Backings • Bipolar plates • Electrical connectors • Current leads R < 0. 3 W cm 2 Electrochemistry in membrane fuel cells 10
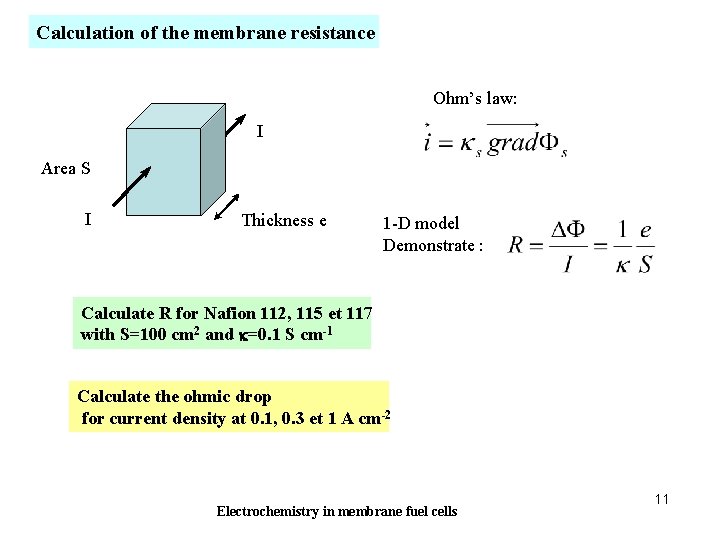
Calculation of the membrane resistance Ohm’s law: I Area S I Thickness e 1 -D model Demonstrate : Calculate R for Nafion 112, 115 et 117 with S=100 cm 2 and k=0. 1 S cm-1 Calculate the ohmic drop for current density at 0. 1, 0. 3 et 1 A cm-2 Electrochemistry in membrane fuel cells 11
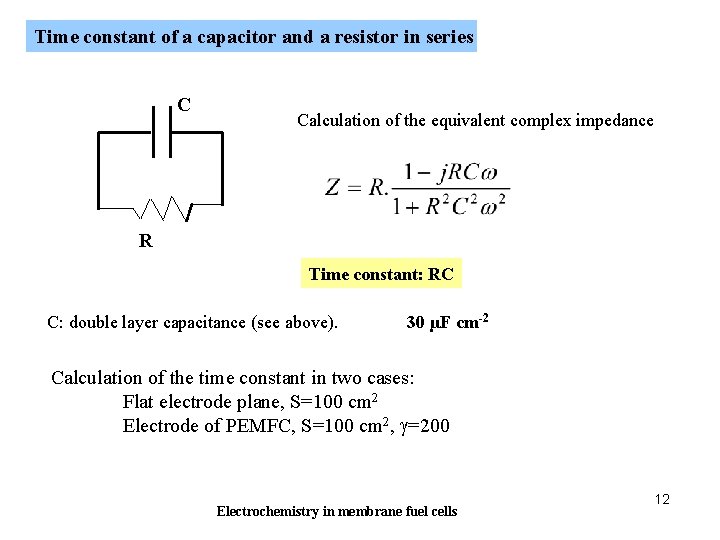
Time constant of a capacitor and a resistor in series C Calculation of the equivalent complex impedance R Time constant: RC C: double layer capacitance (see above). 30 µF cm-2 Calculation of the time constant in two cases: Flat electrode plane, S=100 cm 2 Electrode of PEMFC, S=100 cm 2, g=200 Electrochemistry in membrane fuel cells 12
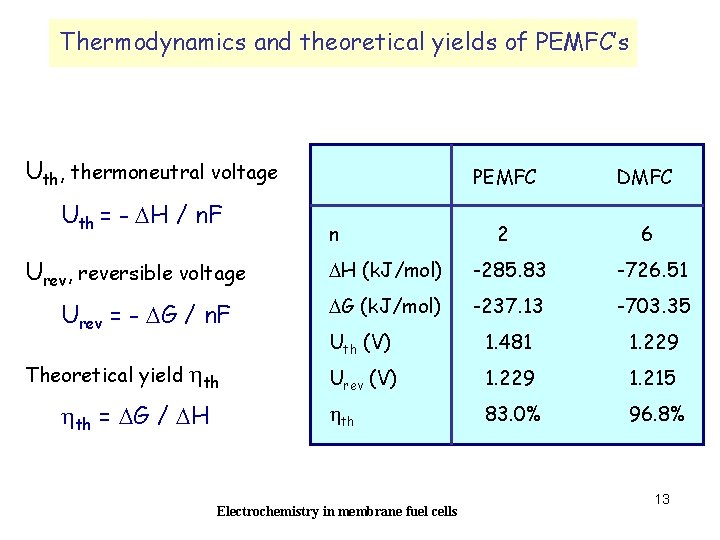
Thermodynamics and theoretical yields of PEMFC’s Uth, thermoneutral voltage Uth = - DH / n. F Urev, reversible voltage Urev = - DG / n. F Theoretical yield hth = DG / DH n PEMFC DMFC 2 6 DH (k. J/mol) -285. 83 -726. 51 DG (k. J/mol) -237. 13 -703. 35 Uth (V) 1. 481 1. 229 Urev (V) 1. 229 1. 215 hth 83. 0% 96. 8% Electrochemistry in membrane fuel cells 13
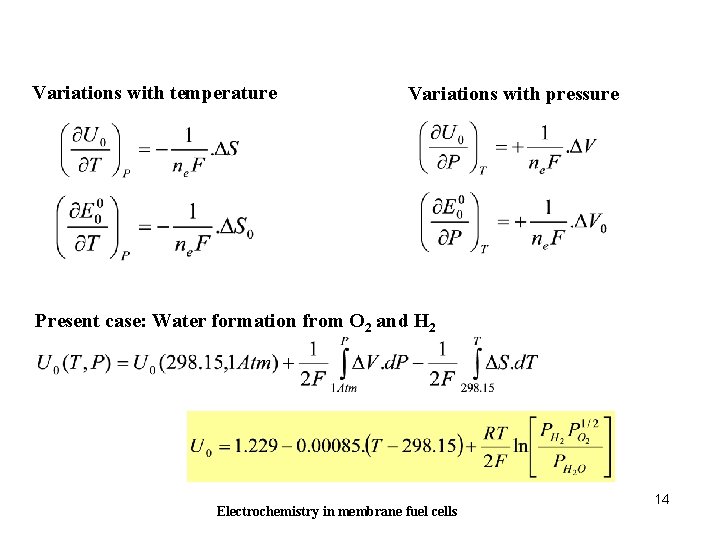
Variations with temperature Variations with pressure Present case: Water formation from O 2 and H 2 Electrochemistry in membrane fuel cells 14
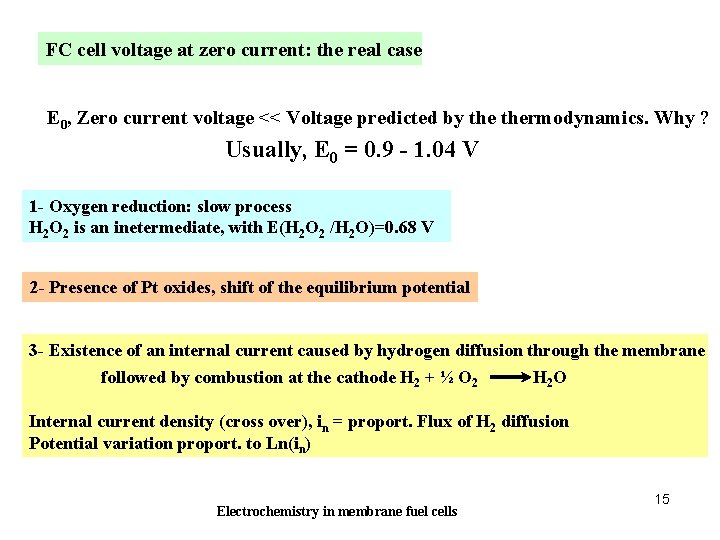
FC cell voltage at zero current: the real case E 0, Zero current voltage << Voltage predicted by thermodynamics. Why ? Usually, E 0 = 0. 9 - 1. 04 V 1 - Oxygen reduction: slow process H 2 O 2 is an inetermediate, with E(H 2 O 2 /H 2 O)=0. 68 V 2 - Presence of Pt oxides, shift of the equilibrium potential 3 - Existence of an internal current caused by hydrogen diffusion through the membrane followed by combustion at the cathode H 2 + ½ O 2 H 2 O Internal current density (cross over), in = proport. Flux of H 2 diffusion Potential variation proport. to Ln(in) Electrochemistry in membrane fuel cells 15
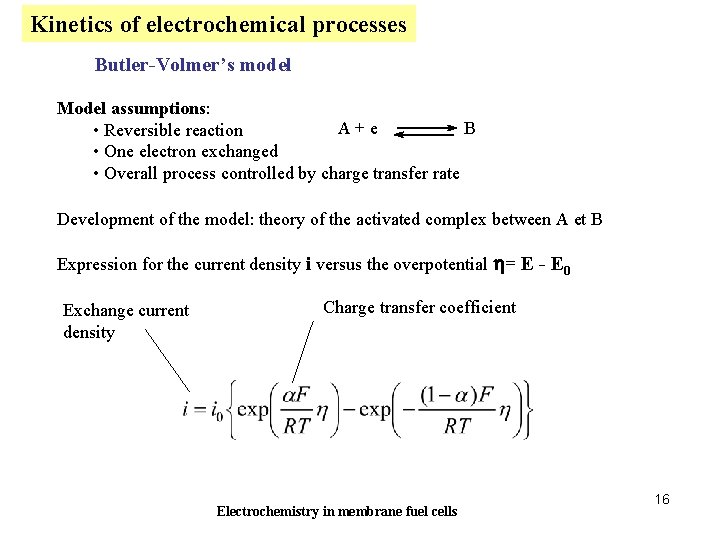
Kinetics of electrochemical processes Butler-Volmer’s model Model assumptions: A + e B • Reversible reaction • One electron exchanged • Overall process controlled by charge transfer rate Development of the model: theory of the activated complex between A et B Expression for the current density i versus the overpotential h= E - E 0 Exchange current density Charge transfer coefficient Electrochemistry in membrane fuel cells 16
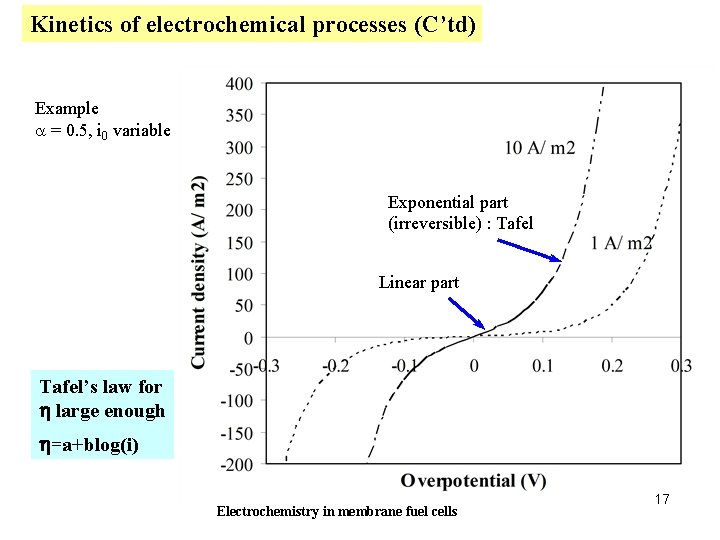
Kinetics of electrochemical processes (C’td) Example a = 0. 5, i 0 variable Exponential part (irreversible) : Tafel Linear part Tafel’s law for h large enough h=a+blog(i) Electrochemistry in membrane fuel cells 17
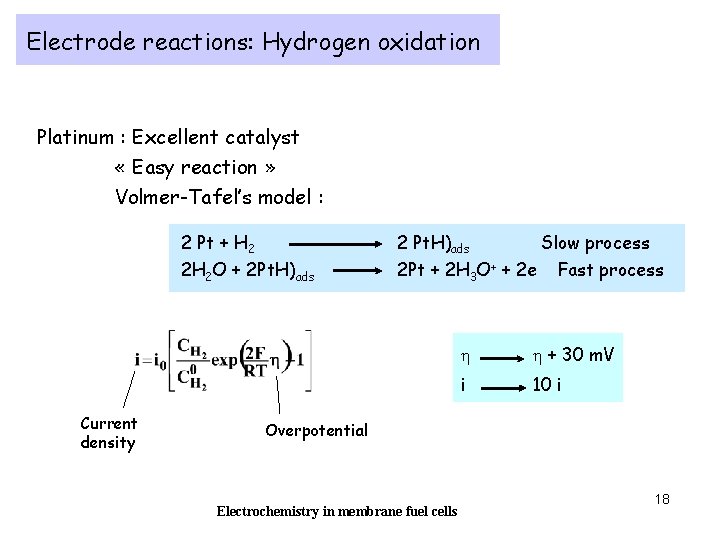
Electrode reactions: Hydrogen oxidation Platinum : Excellent catalyst « Easy reaction » Volmer-Tafel’s model : 2 Pt + H 2 2 H 2 O + 2 Pt. H)ads Current density 2 Pt. H)ads Slow process 2 Pt + 2 H 3 O+ + 2 e Fast process h h + 30 m. V i 10 i Overpotential Electrochemistry in membrane fuel cells 18
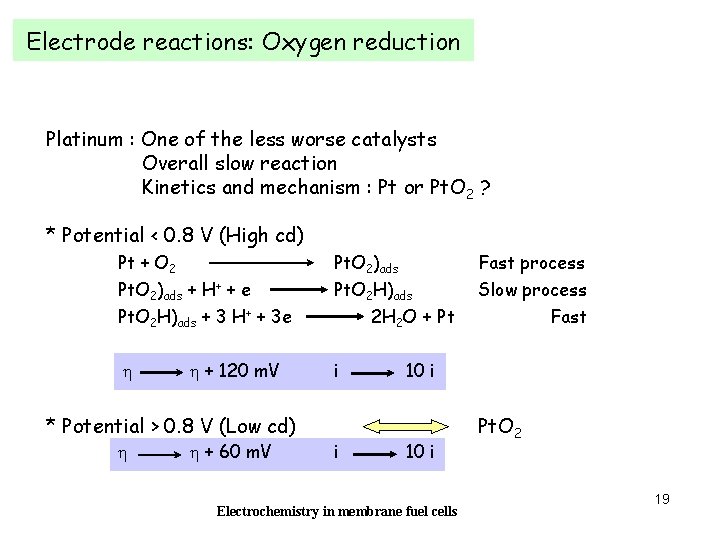
Electrode reactions: Oxygen reduction Platinum : One of the less worse catalysts Overall slow reaction Kinetics and mechanism : Pt or Pt. O 2 ? * Potential < 0. 8 V (High cd) Pt + O 2 Pt. O 2)ads + H+ + e Pt. O 2 H)ads + 3 H+ + 3 e h h + 120 m. V * Potential > 0. 8 V (Low cd) h h + 60 m. V Pt. O 2)ads Pt. O 2 H)ads 2 H 2 O + Pt i i Fast process Slow process Fast 10 i Electrochemistry in membrane fuel cells Pt. O 2 19

Charge transfer resistance , Ract T=60°C S=100 cm 2 i=0. 5 A/cm 2 b=17. 4 V-1 (56 m. V/decade) and Ract = 1. 13 m. W C Calculation of the time constant Ract. C Ract Electrochemistry in membrane fuel cells 20
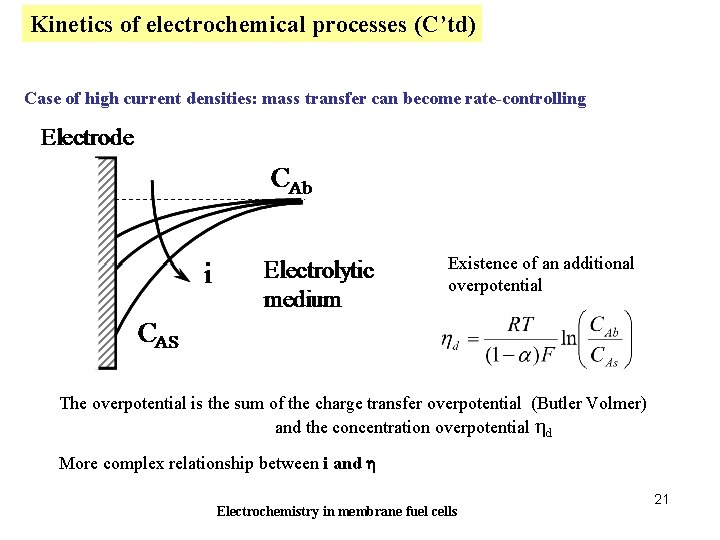
Kinetics of electrochemical processes (C’td) Case of high current densities: mass transfer can become rate-controlling Existence of an additional overpotential The overpotential is the sum of the charge transfer overpotential (Butler Volmer) and the concentration overpotential hd More complex relationship between i and h Electrochemistry in membrane fuel cells 21
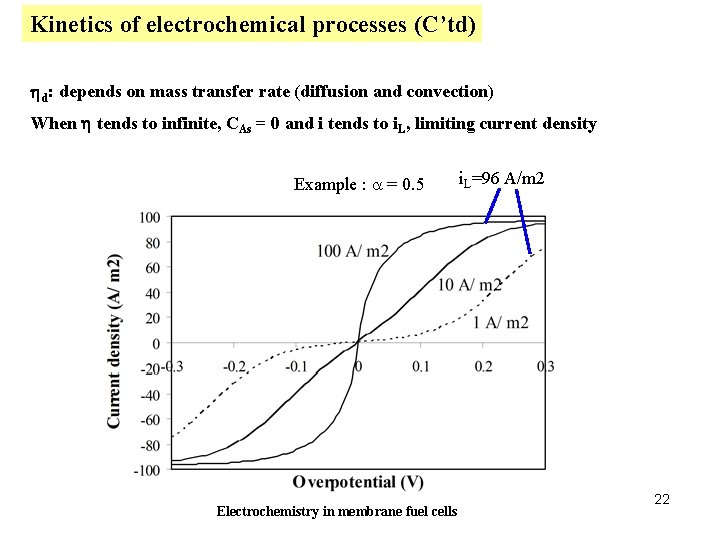
Kinetics of electrochemical processes (C’td) hd: depends on mass transfer rate (diffusion and convection) When h tends to infinite, CAs = 0 and i tends to i. L, limiting current density Example : a = 0. 5 Electrochemistry in membrane fuel cells i. L=96 A/m 2 22
Control by mass transfer phenomena in FC’s The involved phenomena Gas Convection (bipolar plates, backing) Diffusion (backing, active layers) Knudsen diffusion (active layers) Sharper problems For dilute reacting gases (air, reforming hydrogen) Water Transport through the membrane Problems raised by liquid water: Flow hindrance in the various parts: lower transfer rates i(lim) = 0. 5 – 2 A cm-2 Electrochemistry in membrane fuel cells 23
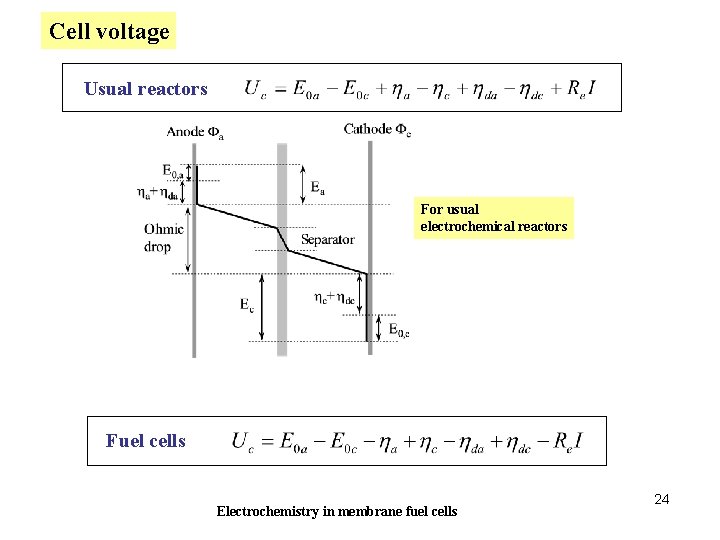
Cell voltage Usual reactors For usual electrochemical reactors Fuel cells Electrochemistry in membrane fuel cells 24
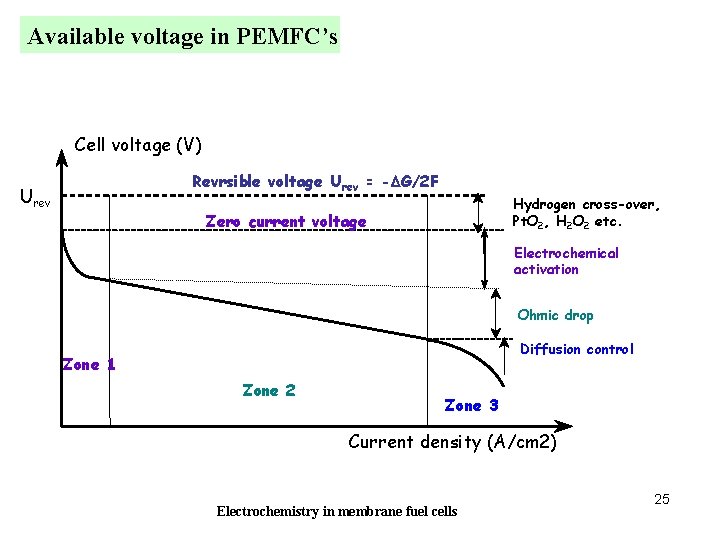
Available voltage in PEMFC’s Cell voltage (V) Revrsible voltage Urev = -DG/2 F Urev Hydrogen cross-over, Pt. O 2, H 2 O 2 etc. Zero current voltage Electrochemical activation Ohmic drop Diffusion control Zone 1 Zone 2 Zone 3 Current density (A/cm 2) Electrochemistry in membrane fuel cells 25
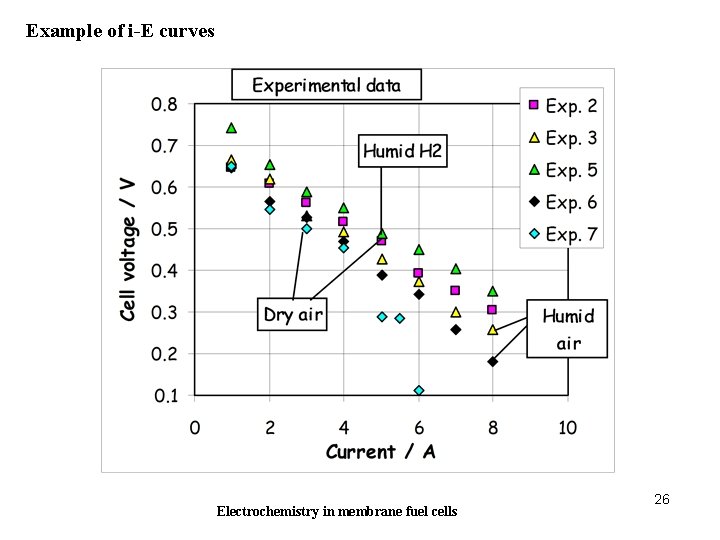
Example of i-E curves Electrochemistry in membrane fuel cells 26
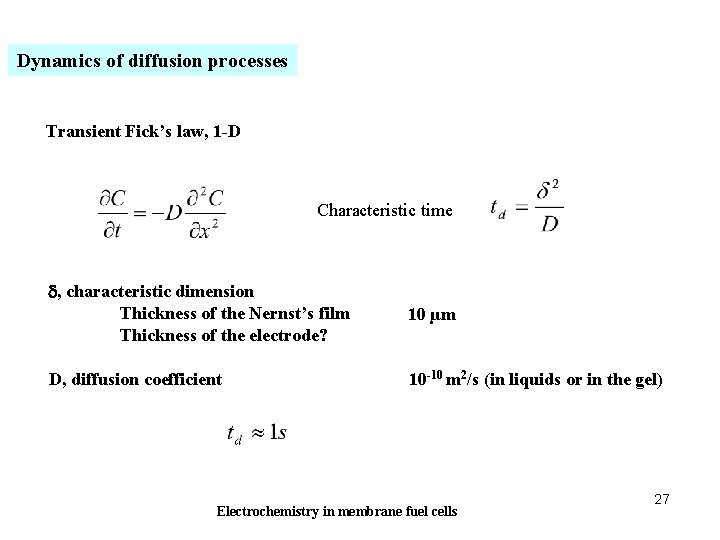
Dynamics of diffusion processes Transient Fick’s law, 1 -D Characteristic time d, characteristic dimension Thickness of the Nernst’s film Thickness of the electrode? 10 µm D, diffusion coefficient 10 -10 m 2/s (in liquids or in the gel) Electrochemistry in membrane fuel cells 27
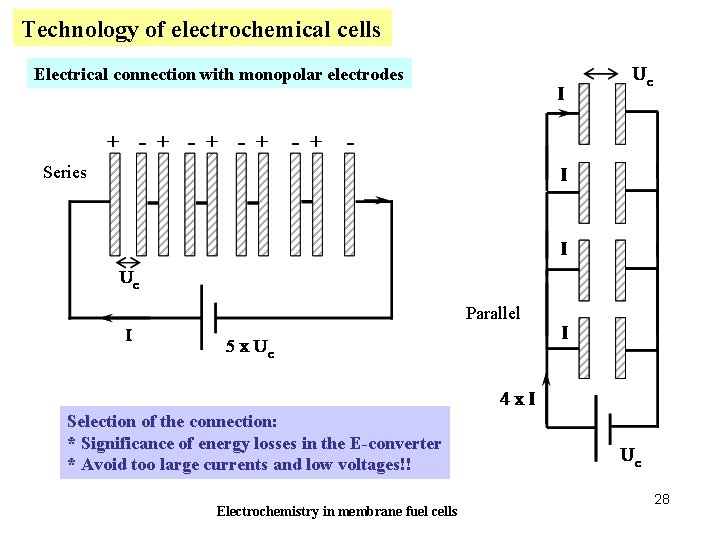
Technology of electrochemical cells Electrical connection with monopolar electrodes Series Parallel Selection of the connection: * Significance of energy losses in the E-converter * Avoid too large currents and low voltages!! Electrochemistry in membrane fuel cells 28
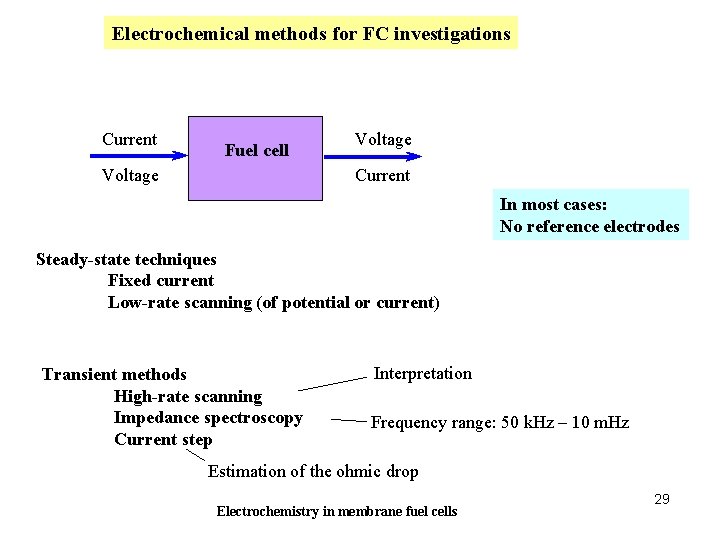
Electrochemical methods for FC investigations Current Fuel cell Voltage Current Voltage In most cases: No reference electrodes Steady-state techniques Fixed current Low-rate scanning (of potential or current) Transient methods High-rate scanning Impedance spectroscopy Current step Interpretation Frequency range: 50 k. Hz – 10 m. Hz Estimation of the ohmic drop Electrochemistry in membrane fuel cells 29
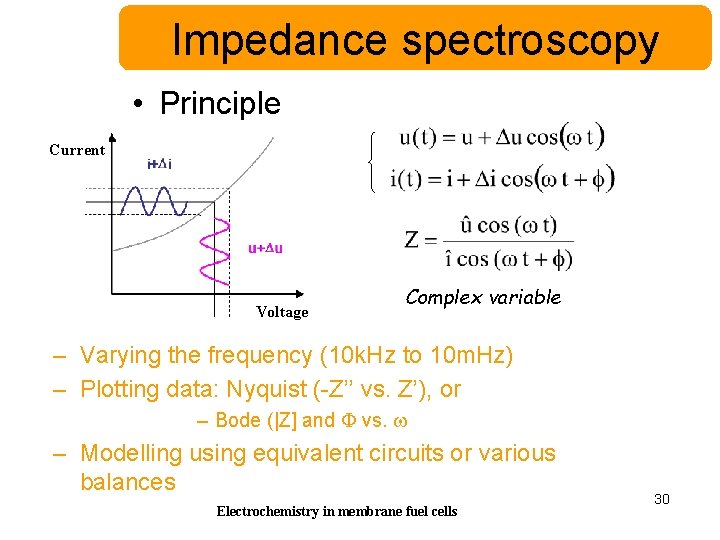
Impedance spectroscopy • Principle Current Voltage Complex variable – Varying the frequency (10 k. Hz to 10 m. Hz) – Plotting data: Nyquist (-Z’’ vs. Z’), or – Bode (|Z] and F vs. w – Modelling using equivalent circuits or various balances Electrochemistry in membrane fuel cells 30
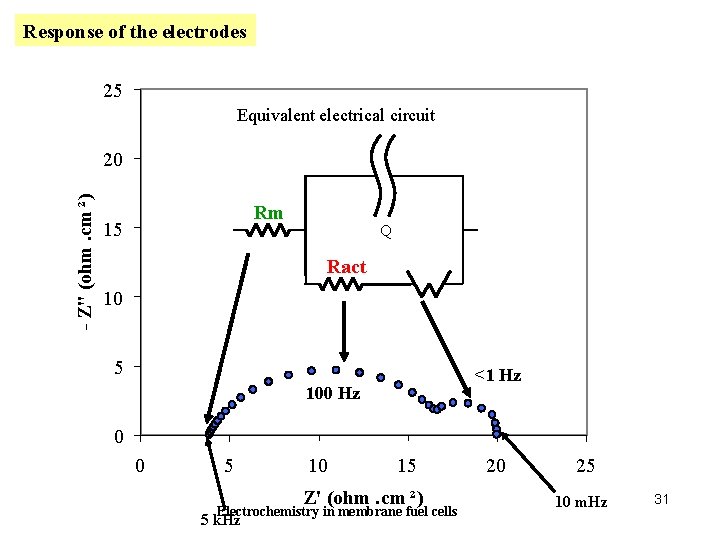
Response of the electrodes 25 Equivalent electrical circuit - Z" (ohm. cm ²) 20 Rm 15 Q Ract 10 Tension 5 <1 Hz 100 Hz 0 0 5 10 15 Z' (ohm. cm ²) Electrochemistry in membrane fuel cells 5 k. Hz 20 25 10 m. Hz 31
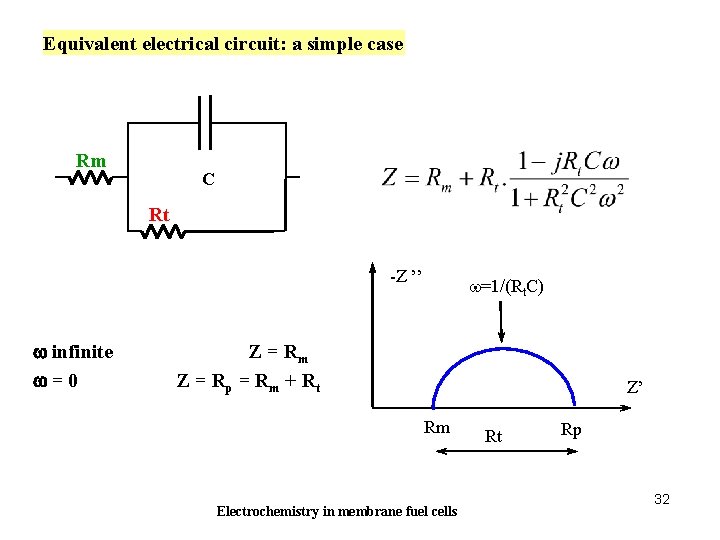
Equivalent electrical circuit: a simple case Rm C Rt Tension -Z ’’ w infinite w=0 w=1/(Rt. C) Z = Rm Z = Rp = Rm + Rt Z’ Rm Electrochemistry in membrane fuel cells Rt Rp 32
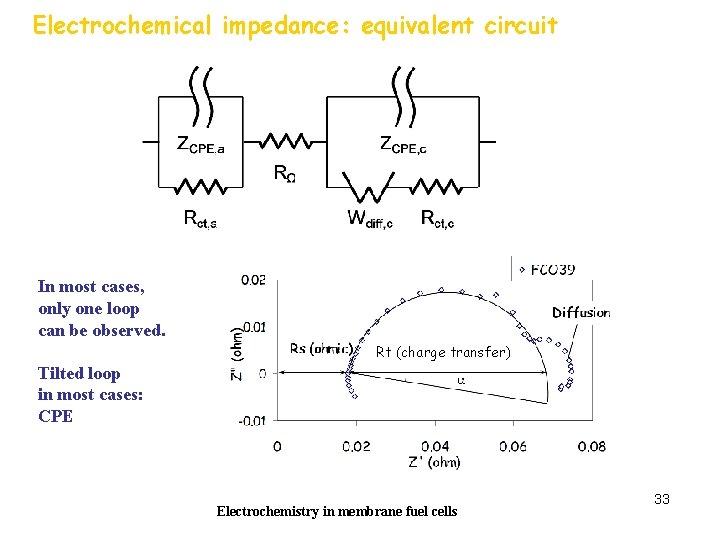
Electrochemical impedance: equivalent circuit In most cases, only one loop can be observed. Rt (charge transfer) Tilted loop in most cases: CPE Electrochemistry in membrane fuel cells 33
Some fuel cell references • Larminie, J. and Dicks, A. (2000) Fuel Cell Systems Explained, Wiley, England. • Vielstich W (2003) Handbook of Fuel Cells (4 volumes), Wiley, England. • Grove, W. (1839) On voltaic series and the combination of gases by platinum, Philosophical Magazine Series 3 14: 127 – 130. • Fuel Cell Today www. fuelcelltoday. com [funded by Johnson Matthey, worlds largest producer of Platinum, including that used by Mr Grove, producer of catalyst and MEAs] Electrochemistry in membrane fuel cells 34
- Slides: 34