Germs Go Global Tuberculosis and HIVTB CoInfection Christine

Germs Go Global Tuberculosis and HIV/TB Co-Infection Christine Lubinski Vice President for Global Health Infectious Diseases Society of America April 17, 2009
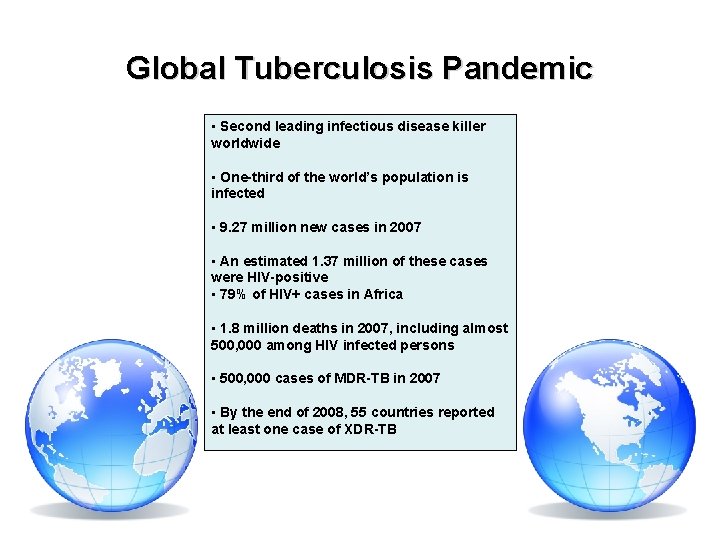
Global Tuberculosis Pandemic • Second leading infectious disease killer worldwide • One-third of the world’s population is infected • 9. 27 million new cases in 2007 • An estimated 1. 37 million of these cases were HIV-positive • 79% of HIV+ cases in Africa • 1. 8 million deaths in 2007, including almost 500, 000 among HIV infected persons • 500, 000 cases of MDR-TB in 2007 • By the end of 2008, 55 countries reported at least one case of XDR-TB
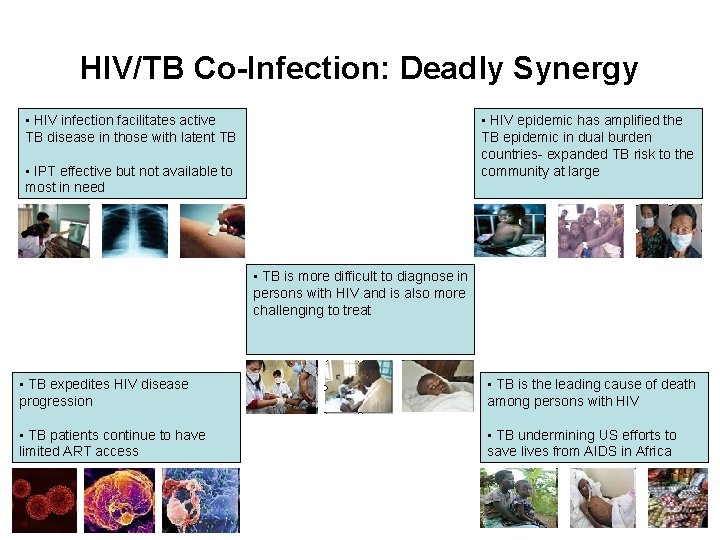
HIV/TB Co-Infection: Deadly Synergy • HIV infection facilitates active TB disease in those with latent TB • HIV epidemic has amplified the TB epidemic in dual burden countries- expanded TB risk to the community at large • IPT effective but not available to most in need • TB is more difficult to diagnose in persons with HIV and is also more challenging to treat • TB expedites HIV disease progression • TB is the leading cause of death among persons with HIV • TB patients continue to have limited ART access • TB undermining US efforts to save lives from AIDS in Africa
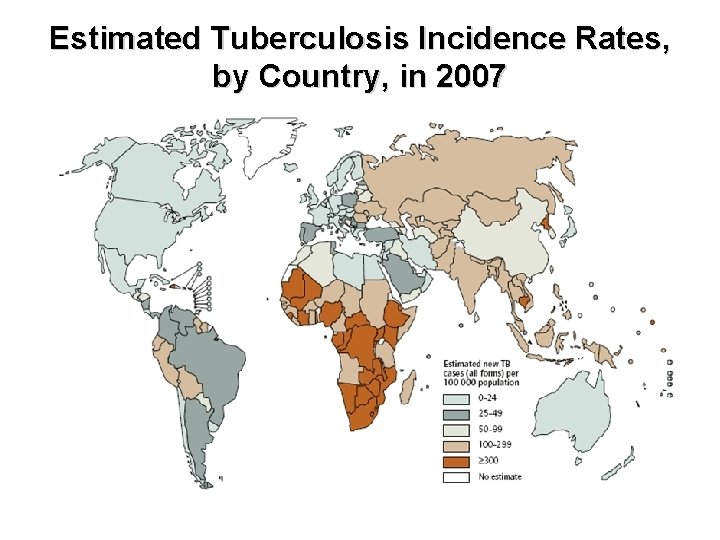
Estimated Tuberculosis Incidence Rates, by Country, in 2007
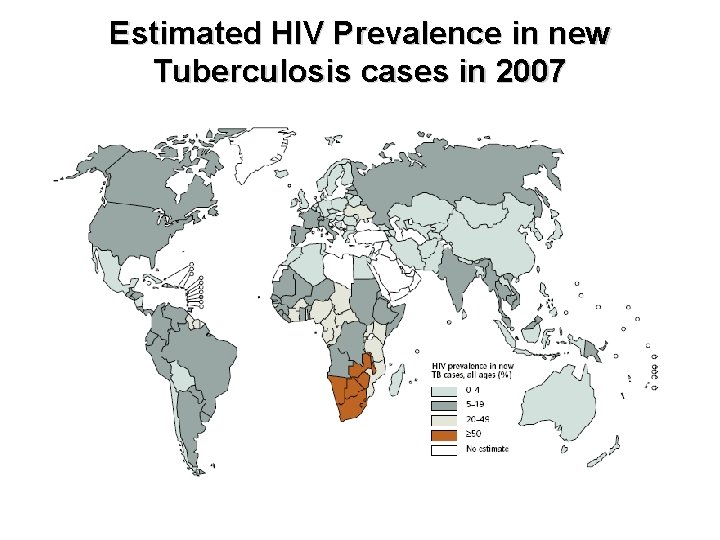
Estimated HIV Prevalence in new Tuberculosis cases in 2007
Tuberculosis: Antiquated tools for diagnosis, treatment and prevention Diagnostics • Detect only half of people tested and fewer than 20% of HIV patients with active TB • Tests for drug resistant strains not available in most of the developing world Drugs • 4 drugs must be taken for 6 -9 months– significant side effects, not compatible with important anti-HIV drugs • Drug resistant TB requires Vaccine • existing vaccine does not protect past 2 years of treatment with highly toxic drugs, which are infancy, and is not recommended in infants with HIV infection frequently not available in developing countries

Tuberculosis Research & Development • $482. 5 million spent worldwide in 2007, far short of WHO goals of $900 million per year • TB drugs received highest level of funding at $170 mil • US diagnostic research is grossly underfunded at $41. 9 million, as is operational research at $36. 8 million • Top Funder– NIAID/NIH at $160 million • No. 2 funder– Bill & Melinda Gates Foundation at $124 million in 2007. Gates Foundation funding outpaced NIH in all categories except for basic research Treatment Action Group: TB Research and Development: A Critical Analysis of Funding Trends, 2005 -2007 An Update
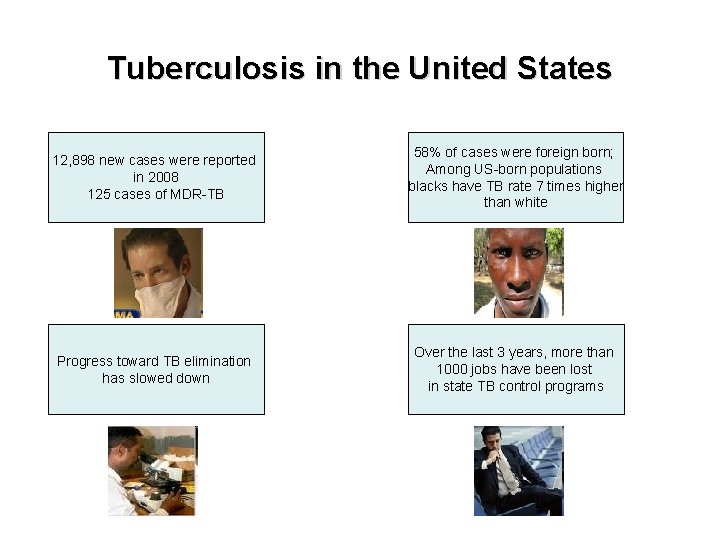
Tuberculosis in the United States 12, 898 new cases were reported in 2008 125 cases of MDR-TB 58% of cases were foreign born; Among US-born populations blacks have TB rate 7 times higher than white Progress toward TB elimination has slowed down Over the last 3 years, more than 1000 jobs have been lost in state TB control programs
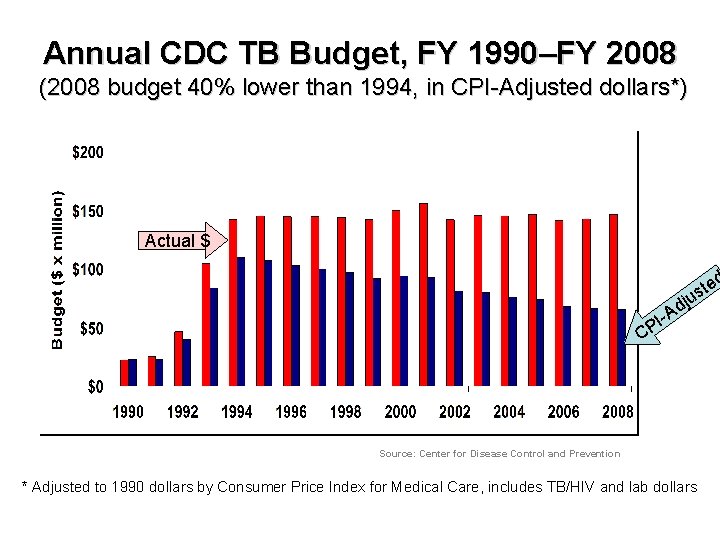
Annual CDC TB Budget, FY 1990–FY 2008 (2008 budget 40% lower than 1994, in CPI-Adjusted dollars*) Actual $ ted s ju d C -A PI Source: Center for Disease Control and Prevention * Adjusted to 1990 dollars by Consumer Price Index for Medical Care, includes TB/HIV and lab dollars

New Legislative Authorities* Comprehensive TB Elimination Act: • $200 million for TB prevention, control, and new tools FY 2009 -2013 Tom Lantos and Henry J. Hyde United States Global Leadership Against HIV/AIDS, Tuberculosis, and Malaria Reauthorization Act: • $4 billion for global TB prevention FY 2009 -2013 *Not yet appropriated
Fund the Comprehensive TB Elimination Act : Public Law 110 -392 • Authorizes $200 million for TB prevention, control and new tools FY 2009 -2013 • Shore up state TB control programs • Enhance US capacity to address drug-resistant TB • Facilitate development of new “tools”- drugs, diagnostics, vaccines • Current TB funding is inadequate for testing diagnostics, drugs, and vaccines currently in pipeline in Phase III trials

Advancing TB R&D and Global TB Control • Double TB research Spending to $320 million at NIH, providing resources for clinical trials, diagnostics and research agenda for drug-resistant TB • $100 million for CDC TB R & D • Provide $2. 7 Billion to the Global Fund– largest funding of global TB control • Enhance USAID TB Spending to $650 million to Implement Lantos/Hyde • Increase operational research through USAID and OGAC • Implement recommendations of the Federal TB Task force to respond to MDR-TB domestically and globally
HIV/TB: US Response Fund Lantos-Hyde • Continue scale-up of HIV treatment, which reduces TB morbidity/mortality in PWHIV • Fund the Global Fund to Fight HIV, TB and Malaria at $2. 7 billion- leading global funder of TB control. • Ensure that TB screening, treatment and preventive therapy are standard of care at PEPFAR-funded HIV clinics • Stop TB transmission in HIV clinics by Implementing infection control strategies
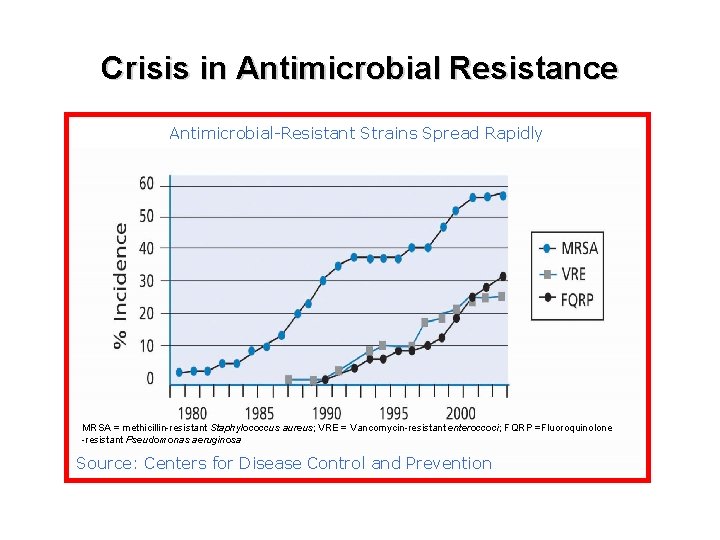
Crisis in Antimicrobial Resistance Antimicrobial-Resistant Strains Spread Rapidly MRSA = methicillin-resistant Staphylococcus aureus; VRE = Vancomycin-resistant enteroccoci; FQRP =Fluoroquinolone -resistant Pseudomonas aeruginosa Source: Centers for Disease Control and Prevention

Strategies to Address Antimicrobial Resistance (STAAR) Act To Strengthen Federal Antimicrobial Resistance Surveillance, Research and Prevention & Control Working Together We Can Enact the STAAR Act!!

IDSA’s 2004 Report: “Bad Bugs, No Drugs (BBND): As Antibiotic Discovery Stagnates, A Public Health Crisis Brews” “Only 16 new antibacterials are in late-stage clinical development at this time. ” -- Bad Bugs, No Drugs: No ESKAPE! An Update from the Infectious Diseases Society of America (Clinical Infectious Disease 2009: 48; January 1, 2009)
- Slides: 16