GEOLOGY 101 Today Chapter 6 Weathering and Erosion










![Biological activity Weathering of rock from activities of ] organisms ] plants ] burrowing Biological activity Weathering of rock from activities of ] organisms ] plants ] burrowing](https://slidetodoc.com/presentation_image_h2/fa4b9e9b1b4639fa2f7c2ccdb40c35ca/image-19.jpg)







- Slides: 35

GEOLOGY 101 Today: Chapter 6 “Weathering and Erosion” Instructor: TA: Professor Matt Fouch Email: fouch 101@asu. edu Office: PSF-540 965 -9292 Ayelet Blattstein Email: ayelet@asu. edu Office: PSH-452 Course Website: http: //fouch 101. asu. edu

How do we move material to lower elevations? • Weathering – Physical – smaller pieces, but composition isn’t altered – Chemical – composition altered • Erosion – Transfer of material by water, wind, ice • Mass Wasting – Transfer of rock & soil downhill via gravity

Weathering • Physical and chemical changes that occur in sediments and rocks when they are exposed to the atmosphere and biosphere • Not the same as erosion!

Why Care About Weathering and Erosion? • Among other reasons, they produce soil • Important natural resource – Supports plant life which support us – Acts as a storage site for CO 2 • • • Causes of landslides Septic system design Building foundation design Landfill design Climate history

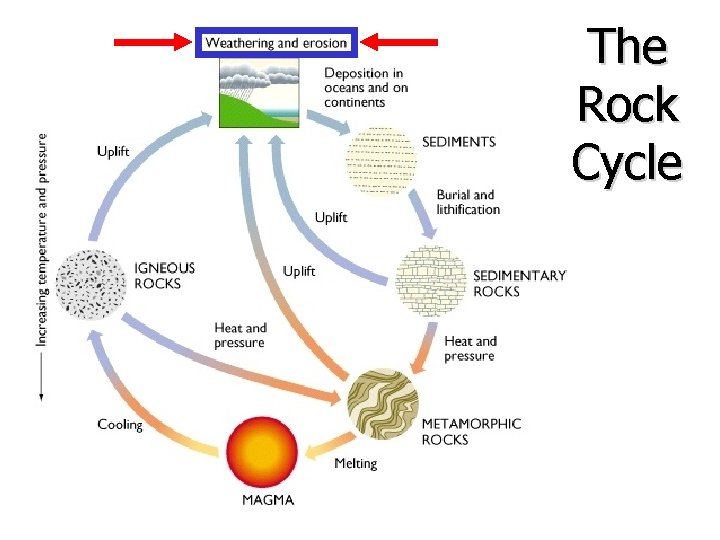
The Rock Cycle

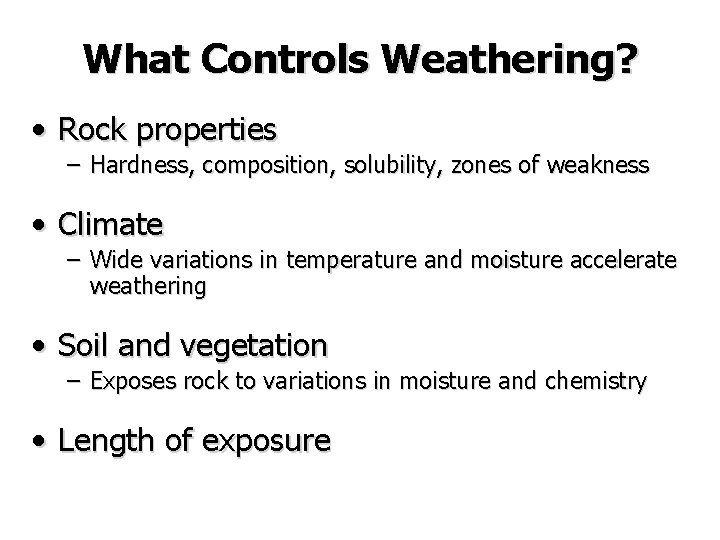
What Controls Weathering? • Rock properties – Hardness, composition, solubility, zones of weakness • Climate – Wide variations in temperature and moisture accelerate weathering • Soil and vegetation – Exposes rock to variations in moisture and chemistry • Length of exposure

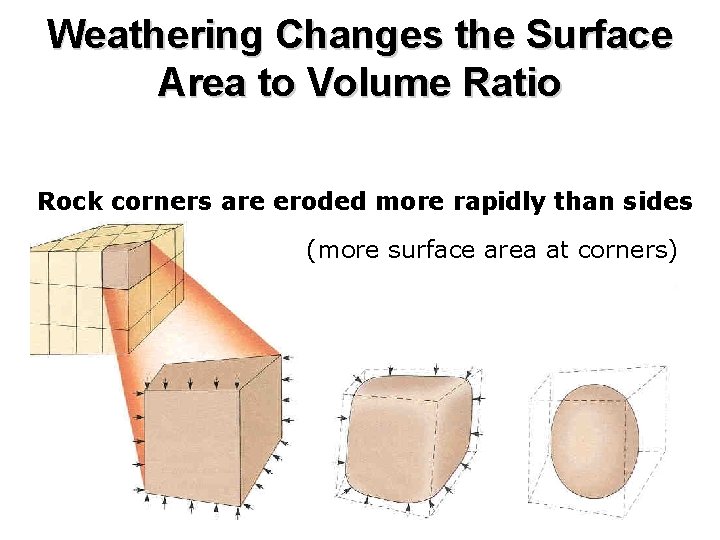
Weathering Changes the Surface Area to Volume Ratio Rock corners are eroded more rapidly than sides (more surface area at corners)

Physical Weathering • Physical forces break rock into smaller pieces w/out changing mineral composition Types of physical weathering • Frost wedging • Unloading • Expansion and contraction • Biological activity

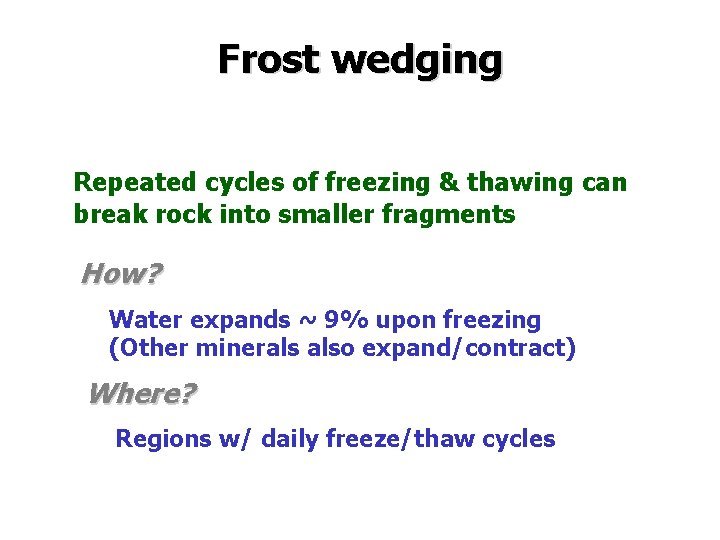
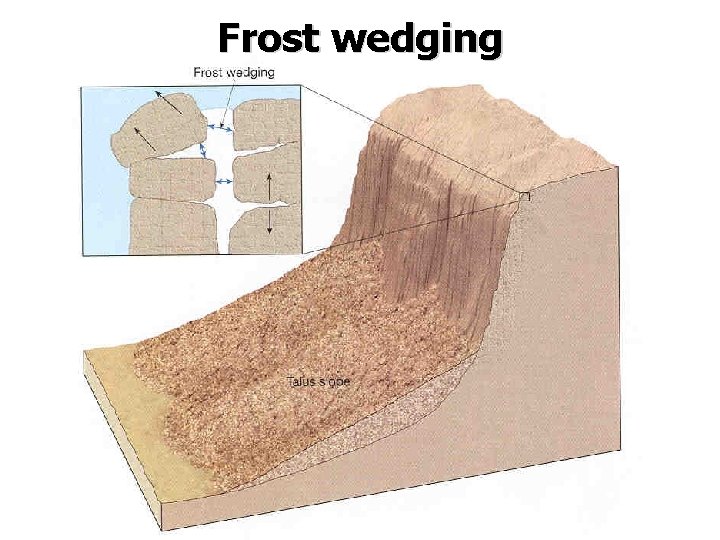
Frost wedging Repeated cycles of freezing & thawing can break rock into smaller fragments How? Water expands ~ 9% upon freezing (Other minerals also expand/contract) Where? Regions w/ daily freeze/thaw cycles

Frost wedging

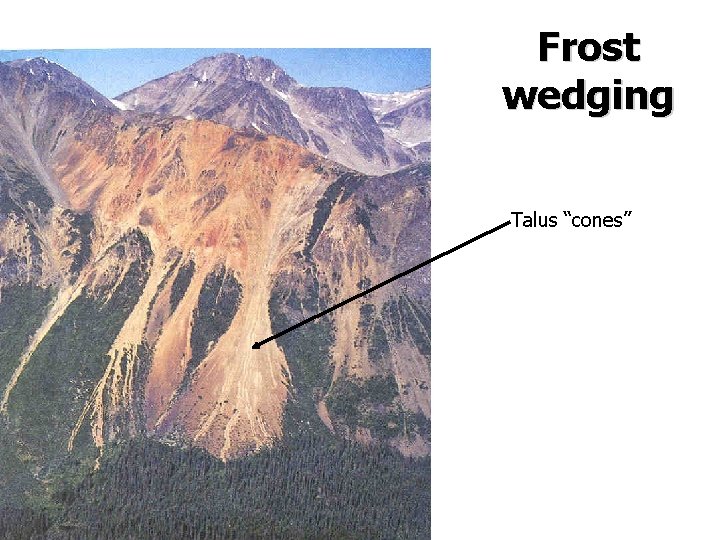
Frost wedging Talus slope

Frost wedging Talus “cones”

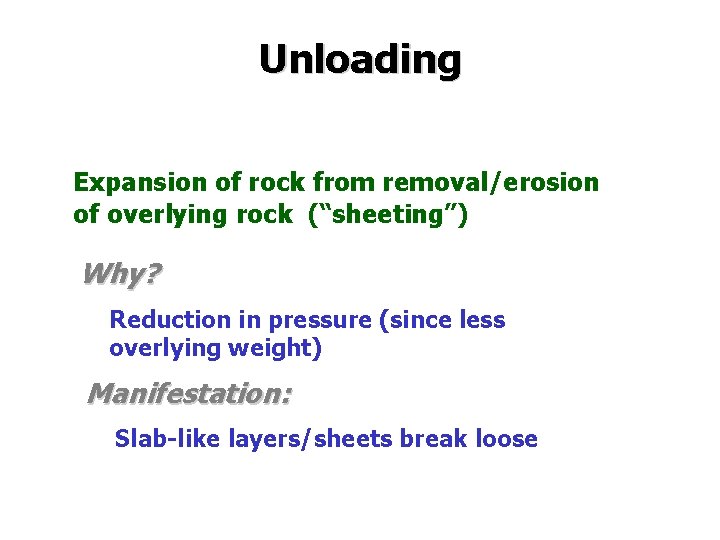
Unloading Expansion of rock from removal/erosion of overlying rock (“sheeting”) Why? Reduction in pressure (since less overlying weight) Manifestation: Slab-like layers/sheets break loose

Unloading Continued weathering causes rock slabs to separate & fall HALF DOME YOSEMITE NATIONAL PARK (California) “exfoliation domes”

Another “sheeting” example

“Devil’s Marbles” Australia

Spheroidal weathering

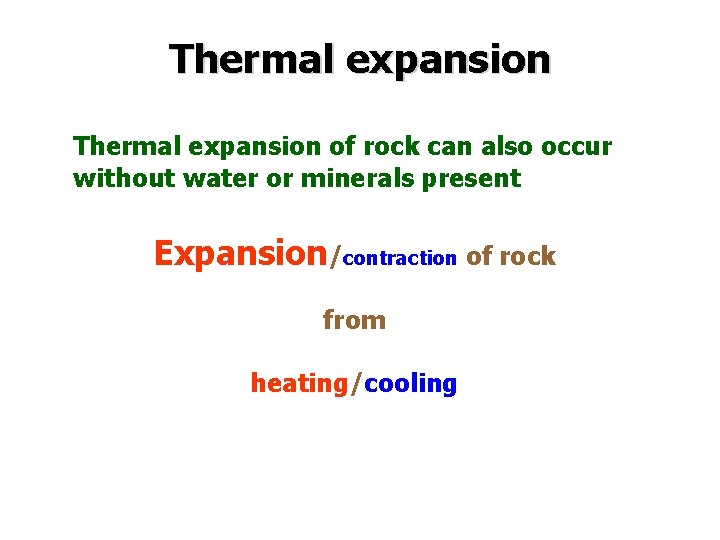
Thermal expansion of rock can also occur without water or minerals present Expansion/contraction of rock from heating/cooling
![Biological activity Weathering of rock from activities of organisms plants burrowing Biological activity Weathering of rock from activities of ] organisms ] plants ] burrowing](https://slidetodoc.com/presentation_image_h2/fa4b9e9b1b4639fa2f7c2ccdb40c35ca/image-19.jpg)
Biological activity Weathering of rock from activities of ] organisms ] plants ] burrowing animals ] humans

Chemical Weathering • Processes that break rock components and internal structures of minerals, making new minerals Ways to chemically weather rock • Oxidation (add oxygen) • Dissolution (dissolve) • Hydration (add water)

Chemical Weathering • All minerals are chemically unstable once removed from where they were formed • Water is the primary agent of chemical weathering

Products of Chemical Weathering • • • Clays Oxides Salts – Halite • Silica and quartz sand

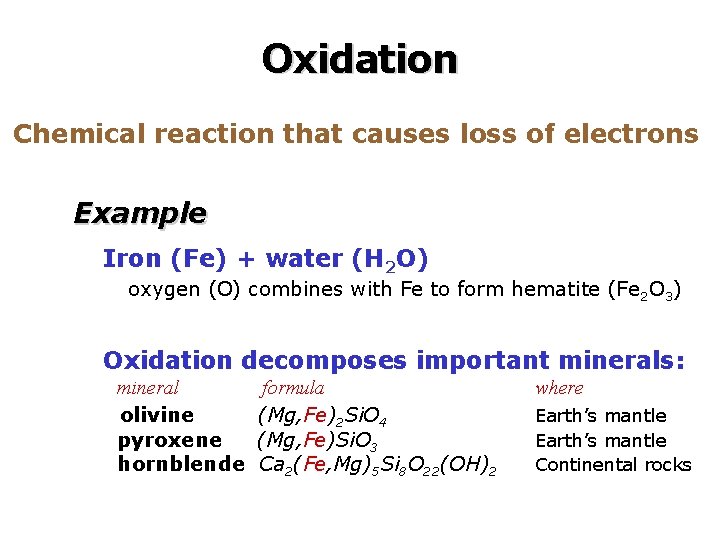
Oxidation Chemical reaction that causes loss of electrons Example Iron (Fe) + water (H 2 O) oxygen (O) combines with Fe to form hematite (Fe 2 O 3) Oxidation decomposes important minerals: mineral olivine pyroxene hornblende formula (Mg, Fe)2 Si. O 4 (Mg, Fe)Si. O 3 Ca 2(Fe, Mg)5 Si 8 O 22(OH)2 where Earth’s mantle Continental rocks

Dissolution Dissolving minerals by a liquid agent (such as water) How? Many minerals are water-soluble example: Halite (salt) Adding acid (H+) increases corrosiveness example: carbon dioxide and rain

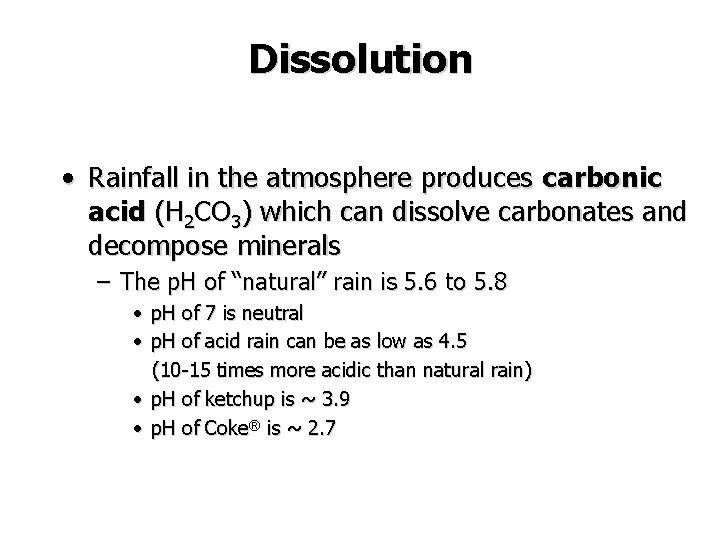
Dissolution • Rainfall in the atmosphere produces carbonic acid (H 2 CO 3) which can dissolve carbonates and decompose minerals – The p. H of “natural” rain is 5. 6 to 5. 8 • p. H of 7 is neutral • p. H of acid rain can be as low as 4. 5 (10 -15 times more acidic than natural rain) • p. H of ketchup is ~ 3. 9 • p. H of Coke® is ~ 2. 7

Hydration The reaction of any substance w/ water. Example Silicates primarily decomposed by hydration Water molecules break down from H 2 O into H+ and (OH-)

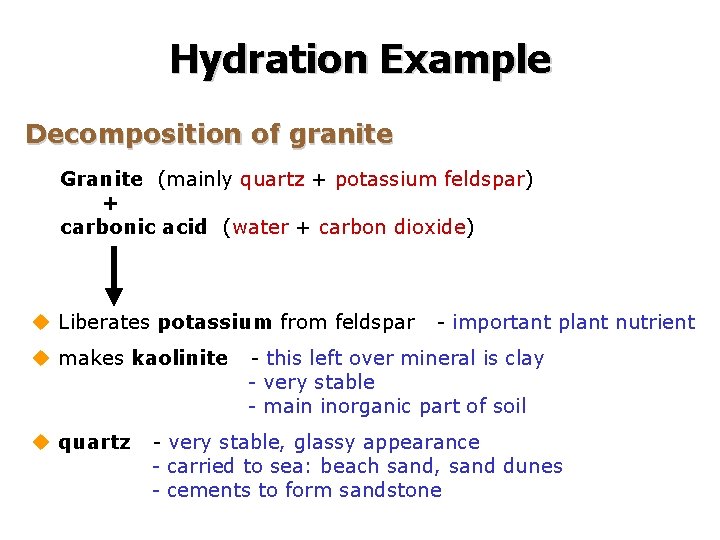
Hydration Example Decomposition of granite Granite (mainly quartz + potassium feldspar) + carbonic acid (water + carbon dioxide) u Liberates potassium from feldspar u makes kaolinite u quartz - important plant nutrient - this left over mineral is clay - very stable - main inorganic part of soil - very stable, glassy appearance - carried to sea: beach sand, sand dunes - cements to form sandstone

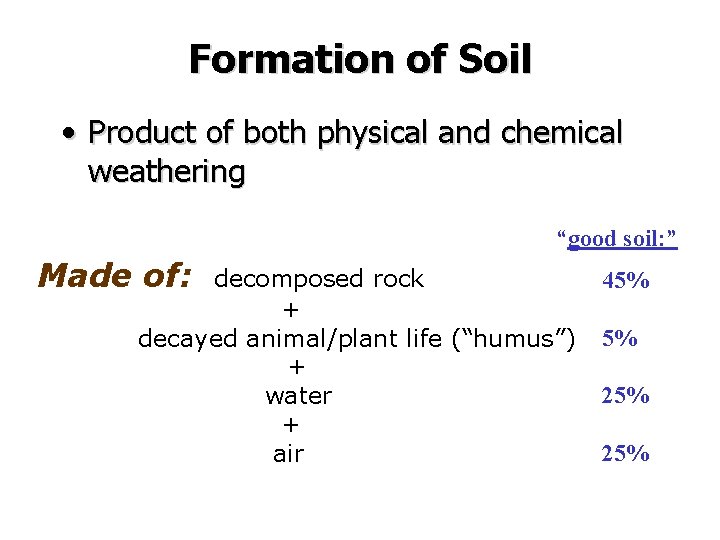
Formation of Soil • Product of both physical and chemical weathering “good soil: ” Made of: decomposed rock + decayed animal/plant life (“humus”) + water + air 45% 5% 25%

Important Soil-Forming Factors • Climate – Temperature & precipitation • Time – Longer time = thicker soil • Plants/Animals – Organic matter • Slope – If too steep, little/no soil

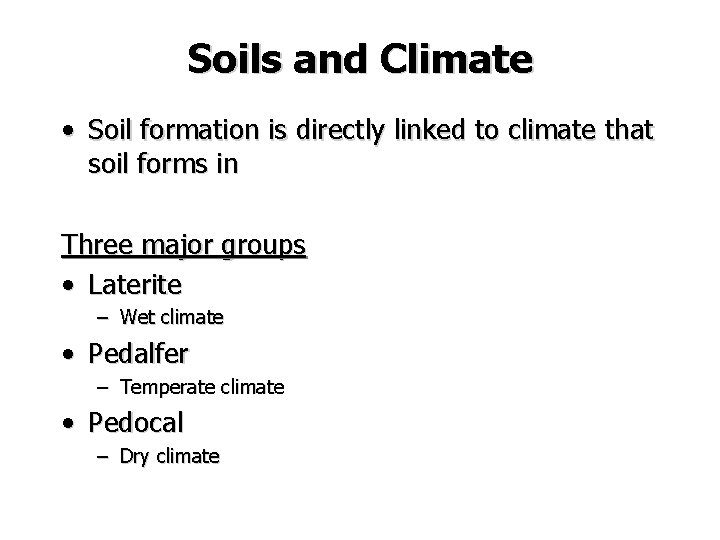
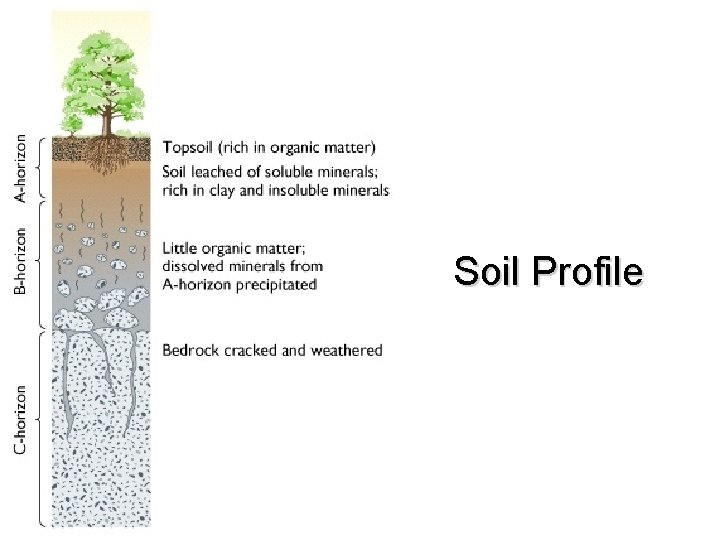
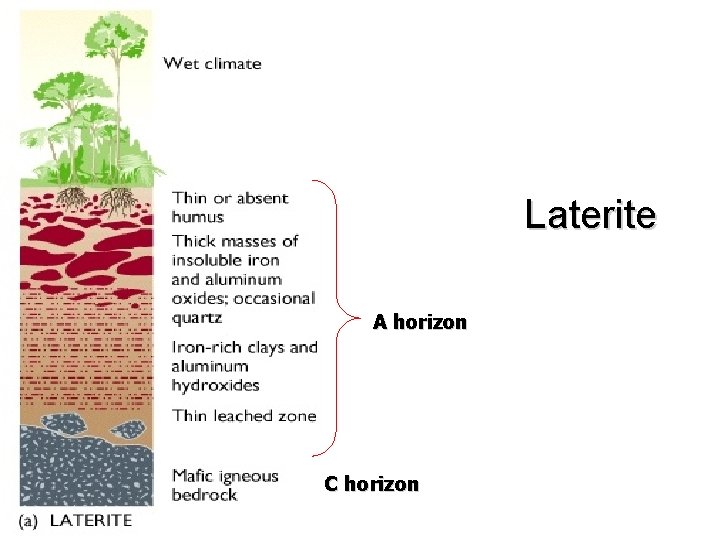
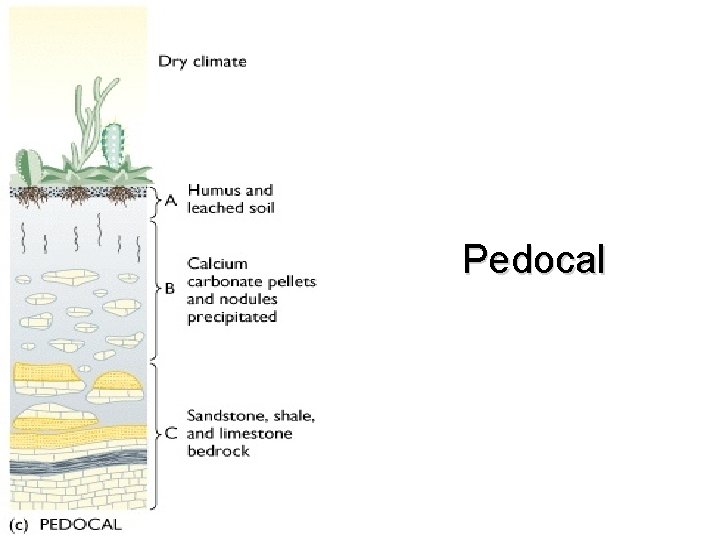
Soils and Climate • Soil formation is directly linked to climate that soil forms in Three major groups • Laterite – Wet climate • Pedalfer – Temperate climate • Pedocal – Dry climate

Soil Profile

Laterite A horizon C horizon

Pedalfer

Pedocal

Soil Erosion • The biggest problem facing the developing world because of deforestation – Much of Madagascar’s soils has been lost due to deforestation • Read Box 6. 1 (pp. 132 -133)