Geologic Time Determining geological ages Relative dating placing




















- Slides: 61

Geologic Time

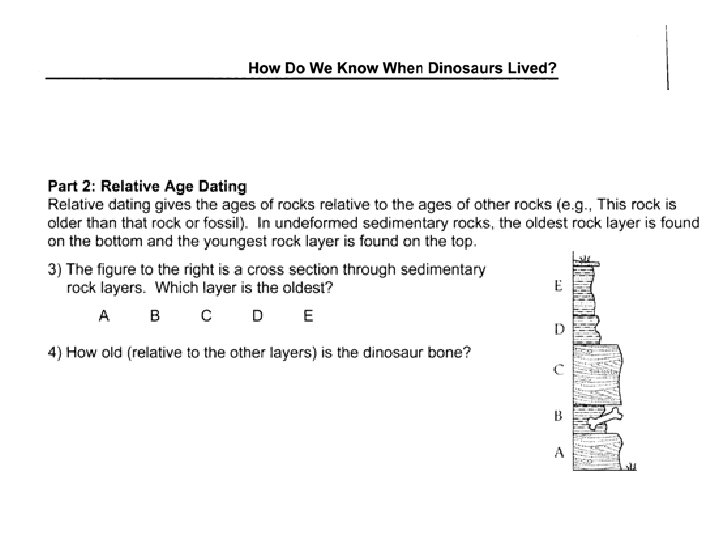
Determining geological ages • Relative dating – placing rocks and events in their proper sequence of formation, without actual dates. • Numerical dating – specifying the actual number of years that have passed since an event occurred (also known as absolute dating)

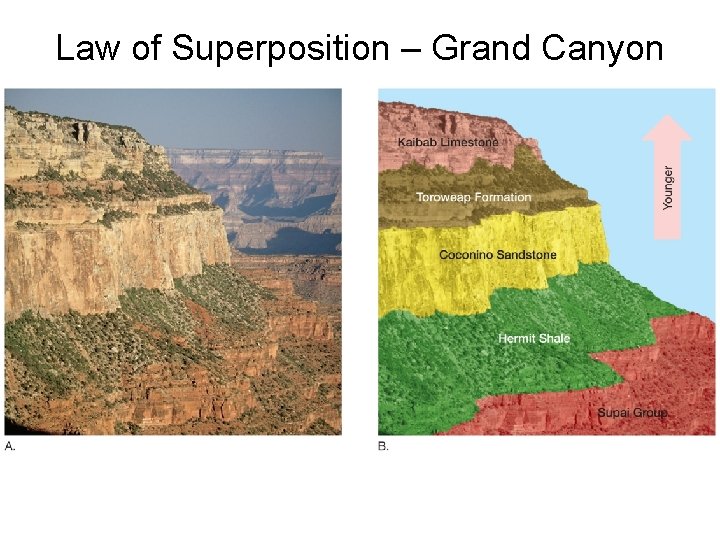
Principles of Relative Dating: Law of Superposition In an undeformed sequence of surface-deposited rocks, the oldest rocks are on the bottom. • Includes sedimentary rocks, lava flows, ash deposits and pyroclastic strata. • Does not include intrusive rocks, which intrude from below.

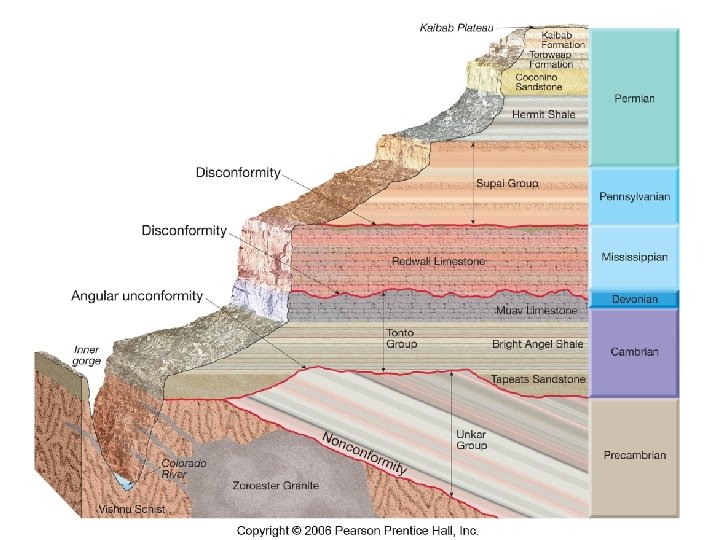
Law of Superposition – Grand Canyon

Principles of Relative Dating • Principle of original horizontality – Layers of sediment are generally deposited in a horizontal position – Rock layers that are flat have not been disturbed • Principle of cross-cutting relationships – Younger features cut across older features (faults, intrusions etc)

Figure 18. 3

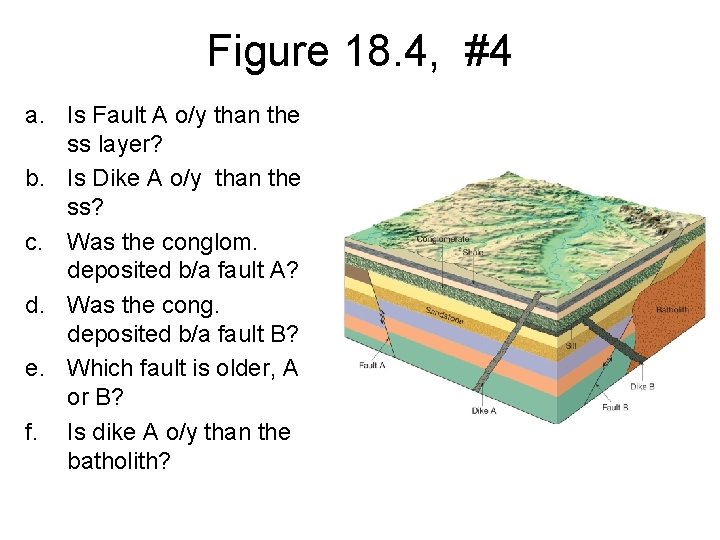
Figure 18. 4, #4 a. Is Fault A o/y than the ss layer? b. Is Dike A o/y than the ss? c. Was the conglom. deposited b/a fault A? d. Was the cong. deposited b/a fault B? e. Which fault is older, A or B? f. Is dike A o/y than the batholith?

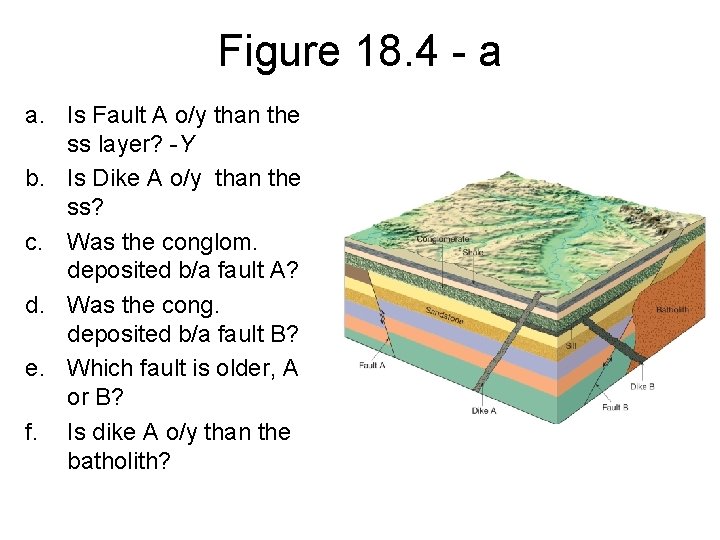
Figure 18. 4 - a a. Is Fault A o/y than the ss layer? -Y b. Is Dike A o/y than the ss? c. Was the conglom. deposited b/a fault A? d. Was the cong. deposited b/a fault B? e. Which fault is older, A or B? f. Is dike A o/y than the batholith?

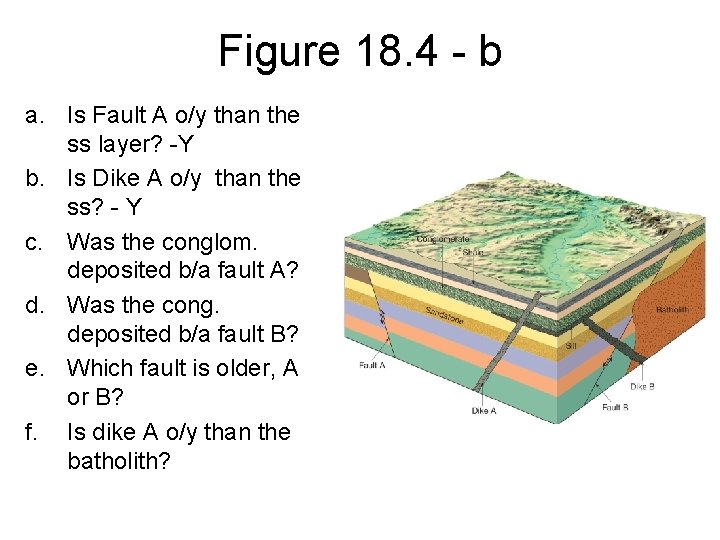
Figure 18. 4 - b a. Is Fault A o/y than the ss layer? -Y b. Is Dike A o/y than the ss? - Y c. Was the conglom. deposited b/a fault A? d. Was the cong. deposited b/a fault B? e. Which fault is older, A or B? f. Is dike A o/y than the batholith?

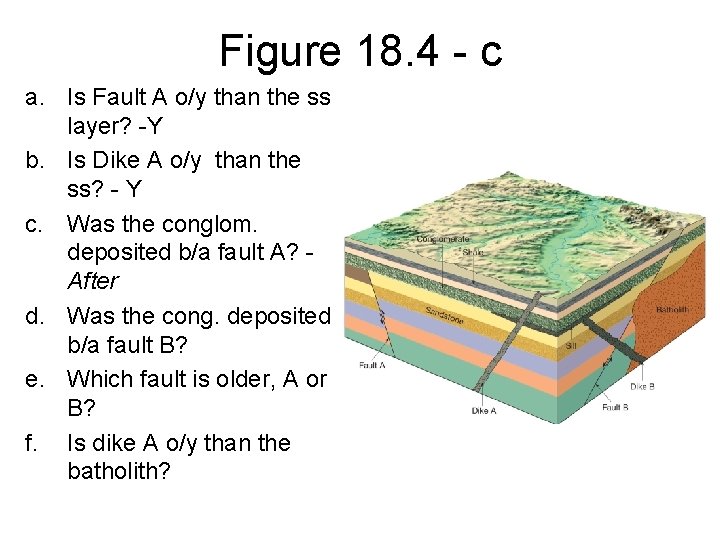
Figure 18. 4 - c a. Is Fault A o/y than the ss layer? -Y b. Is Dike A o/y than the ss? - Y c. Was the conglom. deposited b/a fault A? After d. Was the cong. deposited b/a fault B? e. Which fault is older, A or B? f. Is dike A o/y than the batholith?

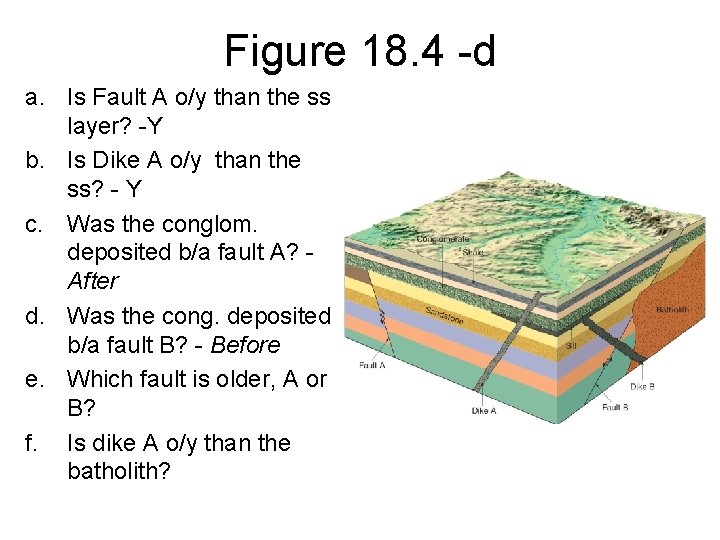
Figure 18. 4 -d a. Is Fault A o/y than the ss layer? -Y b. Is Dike A o/y than the ss? - Y c. Was the conglom. deposited b/a fault A? After d. Was the cong. deposited b/a fault B? - Before e. Which fault is older, A or B? f. Is dike A o/y than the batholith?

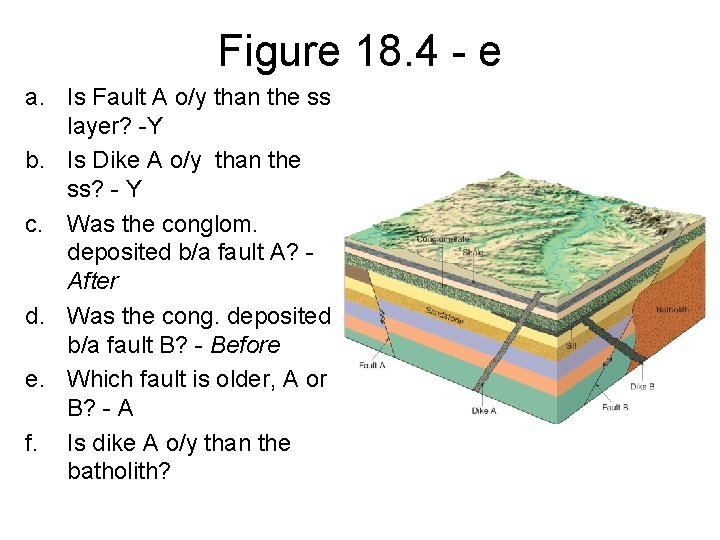
Figure 18. 4 - e a. Is Fault A o/y than the ss layer? -Y b. Is Dike A o/y than the ss? - Y c. Was the conglom. deposited b/a fault A? After d. Was the cong. deposited b/a fault B? - Before e. Which fault is older, A or B? - A f. Is dike A o/y than the batholith?

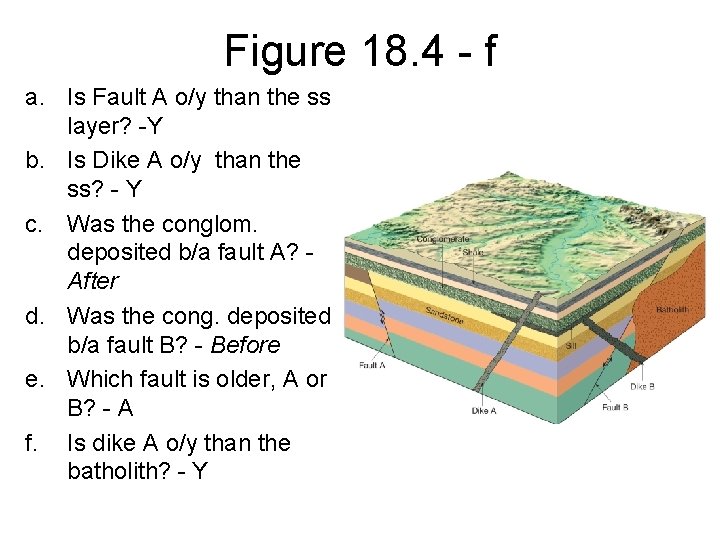
Figure 18. 4 - f a. Is Fault A o/y than the ss layer? -Y b. Is Dike A o/y than the ss? - Y c. Was the conglom. deposited b/a fault A? After d. Was the cong. deposited b/a fault B? - Before e. Which fault is older, A or B? - A f. Is dike A o/y than the batholith? - Y

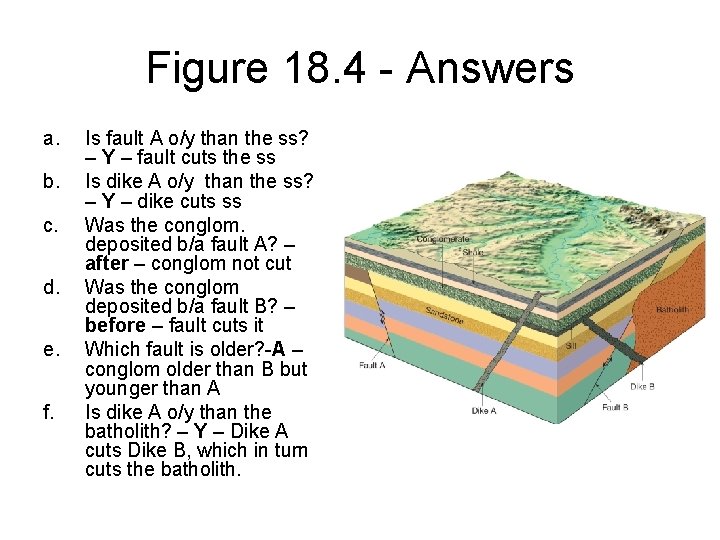
Figure 18. 4 - Answers a. b. c. d. e. f. Is fault A o/y than the ss? – Y – fault cuts the ss Is dike A o/y than the ss? – Y – dike cuts ss Was the conglom. deposited b/a fault A? – after – conglom not cut Was the conglom deposited b/a fault B? – before – fault cuts it Which fault is older? -A – conglom older than B but younger than A Is dike A o/y than the batholith? – Y – Dike A cuts Dike B, which in turn cuts the batholith.

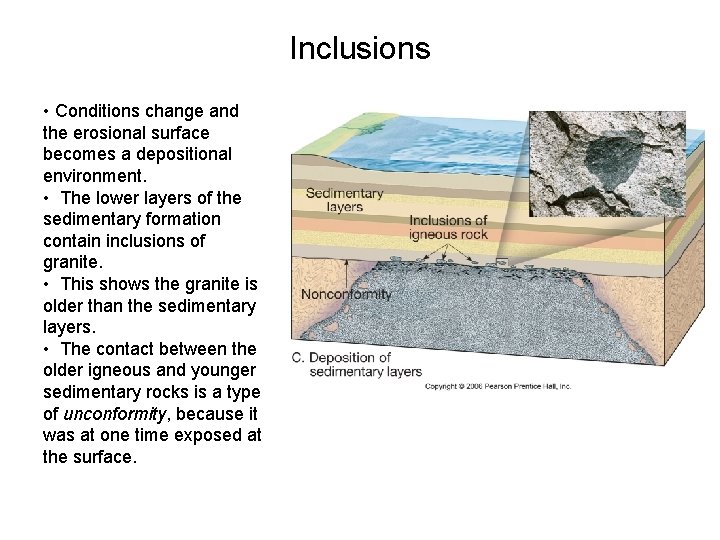
Inclusions – An inclusion is a piece of rock that is enclosed within another rock. – Principle of cross-cutting relationships tells us rock containing the inclusion is younger than the inclusion itself. – The presence of inclusions allow us to determine whether a intrusive igneous rock is older or younger than the rock above it. – Let’s see how

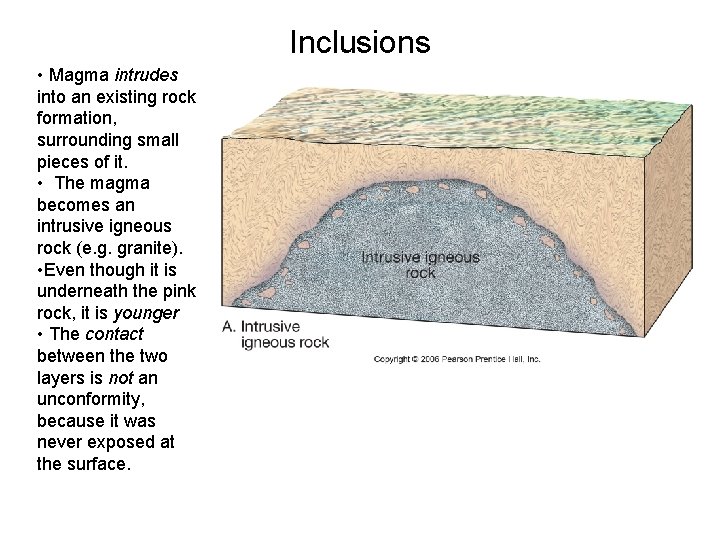
Inclusions • Magma intrudes into an existing rock formation, surrounding small pieces of it. • The magma becomes an intrusive igneous rock (e. g. granite). • Even though it is underneath the pink rock, it is younger • The contact between the two layers is not an unconformity, because it was never exposed at the surface.

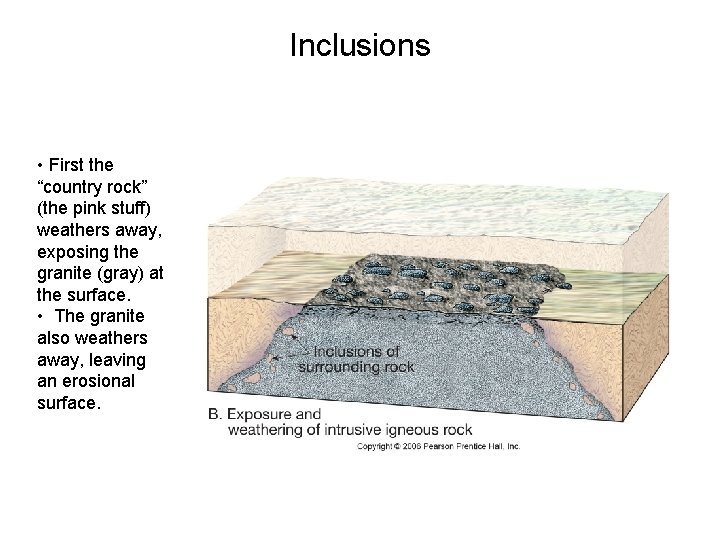
Inclusions • First the “country rock” (the pink stuff) weathers away, exposing the granite (gray) at the surface. • The granite also weathers away, leaving an erosional surface.

Inclusions • Conditions change and the erosional surface becomes a depositional environment. • The lower layers of the sedimentary formation contain inclusions of granite. • This shows the granite is older than the sedimentary layers. • The contact between the older igneous and younger sedimentary rocks is a type of unconformity, because it was at one time exposed at the surface.

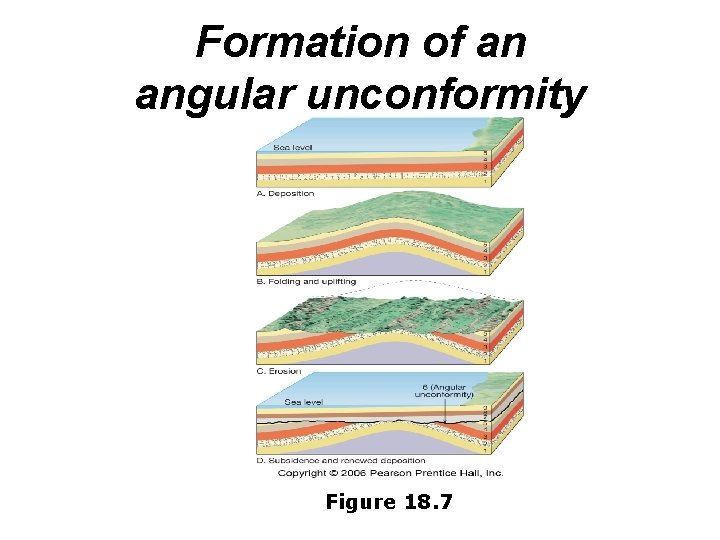
Unconformity • a break in the rock record produced by erosion of rock units and/or nondeposition of sediments – Between sedimentary rocks and crystalline (non-layered) bedrock – Between two sets of layered sedimentary rocks deposited at two different times – Angular unconformity – tilted rocks are overlain by flat-lying rocks

Formation of an angular unconformity Figure 18. 7

Unconformity Types

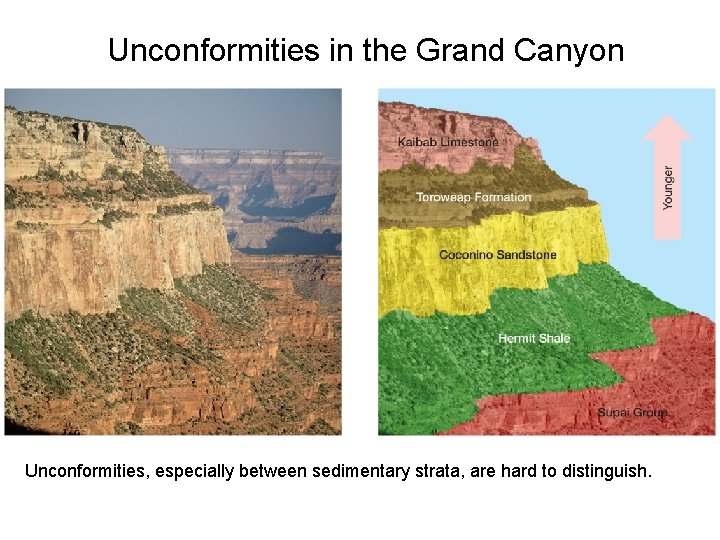
Unconformities in the Grand Canyon Unconformities, especially between sedimentary strata, are hard to distinguish.

Figure 18. 6

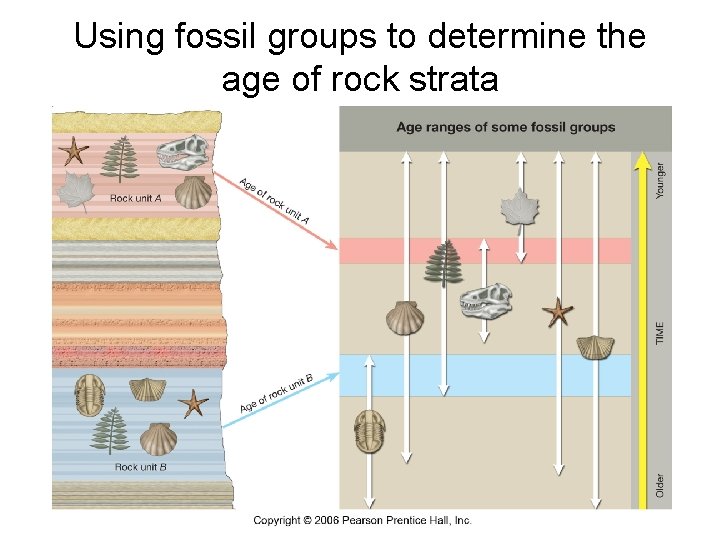
Fossils: the remains or traces of living organisms • Conditions favoring preservation • Rapid burial • Possession of hard parts (shells or bones • Correlation: Matching of rocks of similar ages in different regions • Correlation often relies upon fossils

Principle of Fossil Succession: Fossil organisms succeed one another in a definite and determinable order, so any time period can be recognized by its fossil content.

Principle of Fossil Succession: • Although developed over 50 years before Darwin’s work, it is now known that the reason this principle is valid is due to evolution. • Fossil organisms become more similar to modern organisms with geologic time • Extinct fossils organisms never reappear in the fossil record

Index Fossils – Widespread geographically – Limited to short span of geologic time – Valuable for correlation: use of index fossils can often provide numerical dates for rock units and events – Similar accuracy to radiometric dating techniques.

Using fossil groups to determine the age of rock strata

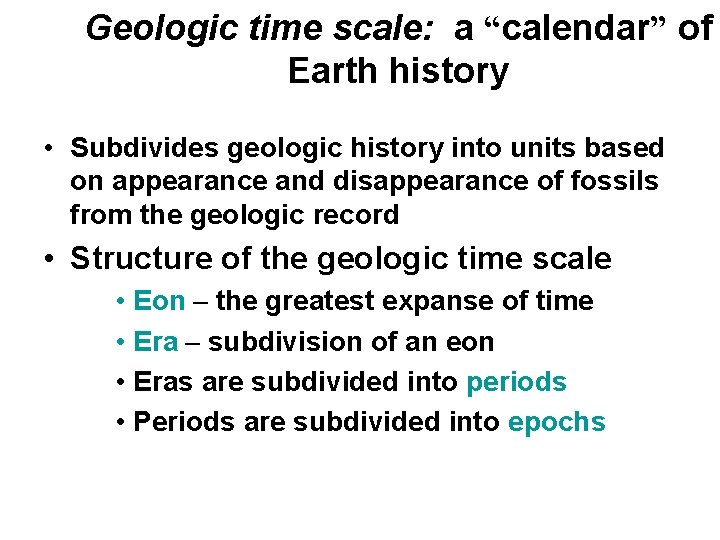
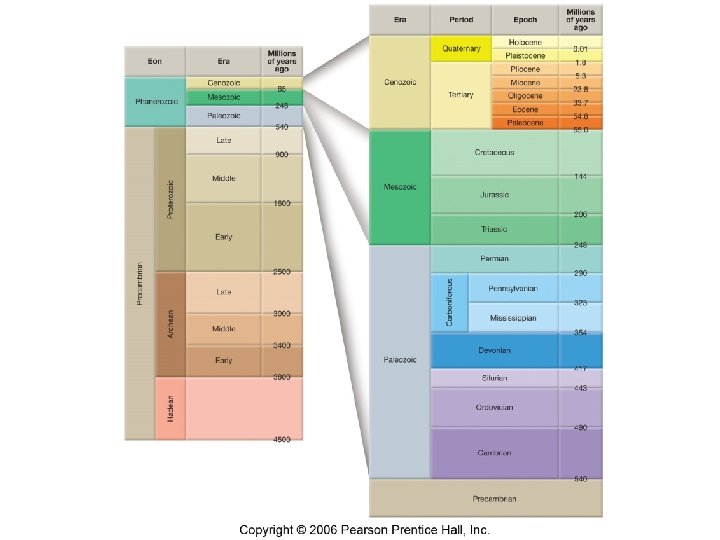
Geologic time scale: a “calendar” of Earth history • Subdivides geologic history into units based on appearance and disappearance of fossils from the geologic record • Structure of the geologic time scale • Eon – the greatest expanse of time • Era – subdivision of an eon • Eras are subdivided into periods • Periods are subdivided into epochs

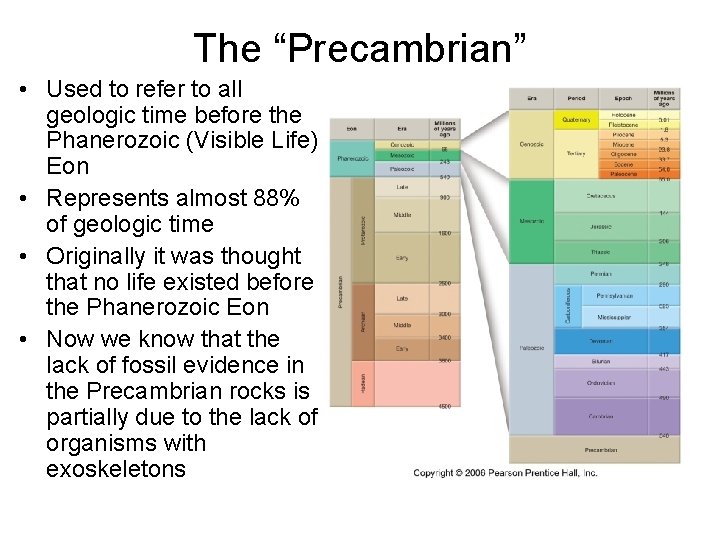
The “Precambrian” • Used to refer to all geologic time before the Phanerozoic (Visible Life) Eon • Represents almost 88% of geologic time • Originally it was thought that no life existed before the Phanerozoic Eon • Now we know that the lack of fossil evidence in the Precambrian rocks is partially due to the lack of organisms with exoskeletons

Eras of the Phanerozoic eon – Cenozoic (“recent life”) – Mesozoic (“middle life”) – Paleozoic (“ancient life”)

Notable divisions between the Eras • Paleozoic-Mesozoic – 248 mya – Mass extinction of trilobites and many other marine organisms – Possibly due to climate change that occurred with the formation of Pangaea • Mesozoic-Cenozoic – 65 mya – Mass extinction of dinosaurs and many other species – Probably caused by meteor impact – Made way for the domination of mammals • Cenozoic- ? ?

Figure 18. 16

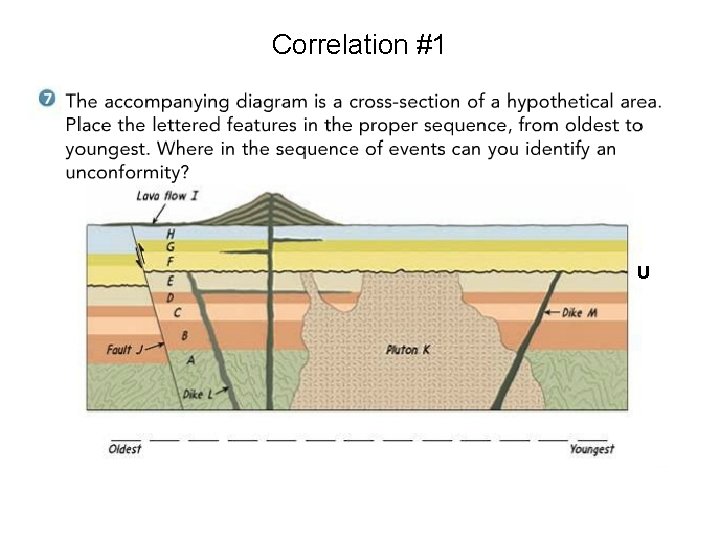
Correlation #1 U

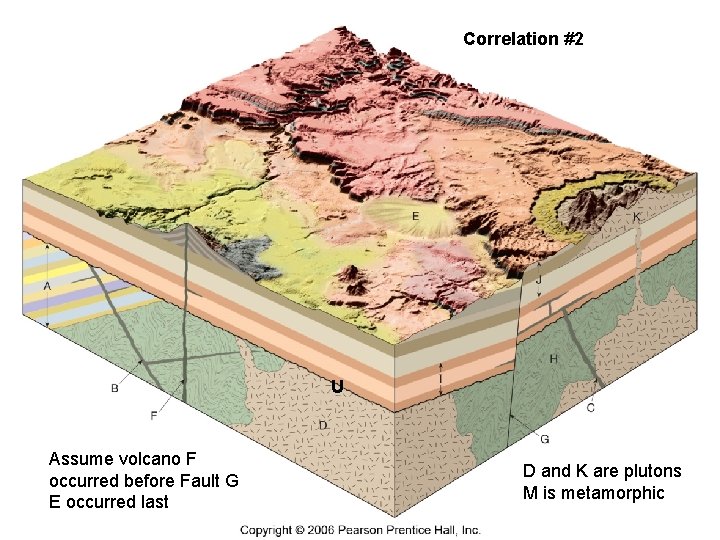
Correlation #2 Figure 18. 18 U Assume volcano F occurred before Fault G E occurred last D and K are plutons M is metamorphic


Radioactivity 2 - rhyolite

Radioactivity 3 – felsic ash




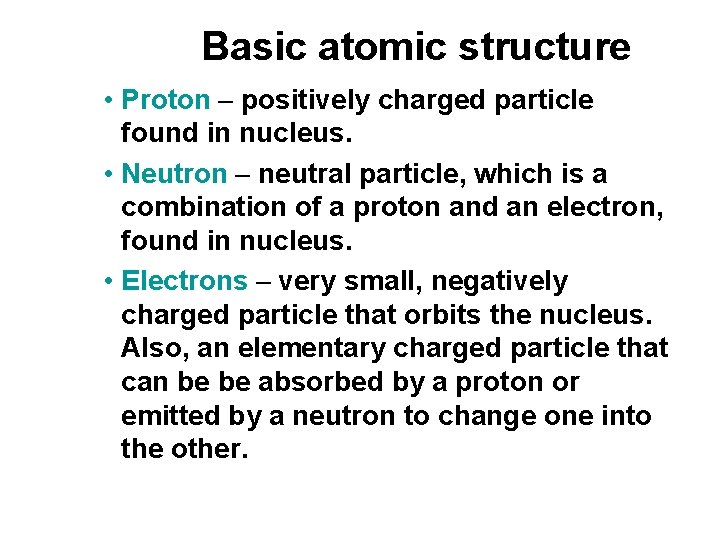
Basic atomic structure • Proton – positively charged particle found in nucleus. • Neutron – neutral particle, which is a combination of a proton and an electron, found in nucleus. • Electrons – very small, negatively charged particle that orbits the nucleus. Also, an elementary charged particle that can be be absorbed by a proton or emitted by a neutron to change one into the other.

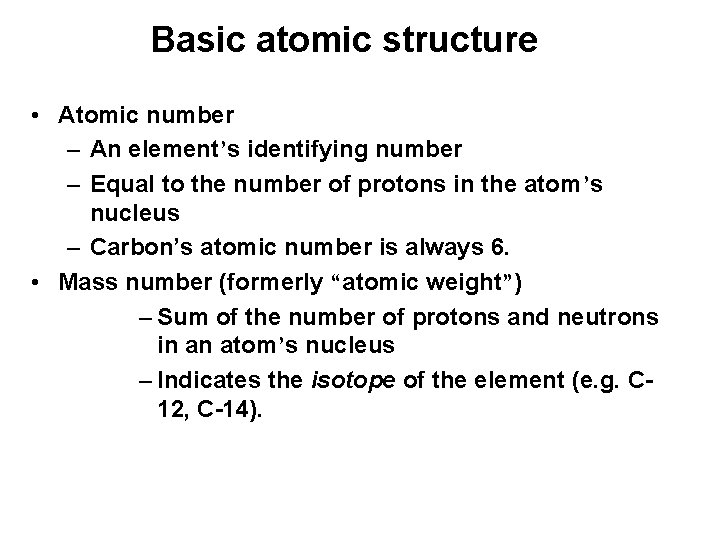
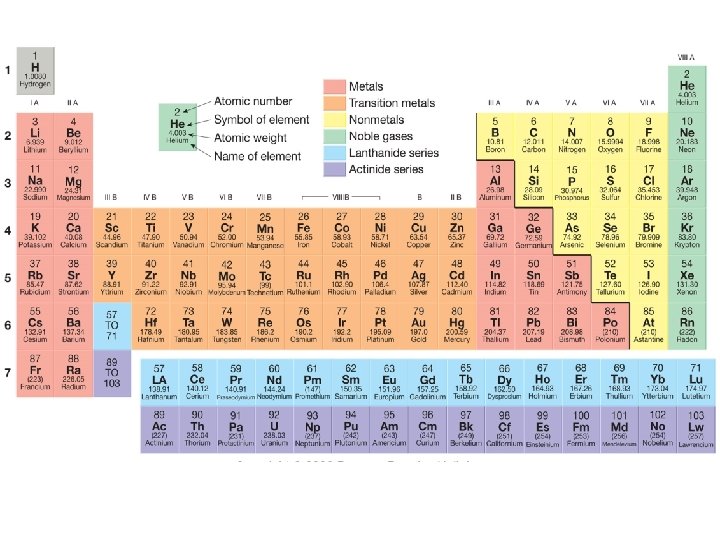
Basic atomic structure • Atomic number – An element’s identifying number – Equal to the number of protons in the atom’s nucleus – Carbon’s atomic number is always 6. • Mass number (formerly “atomic weight”) – Sum of the number of protons and neutrons in an atom’s nucleus – Indicates the isotope of the element (e. g. C 12, C-14).

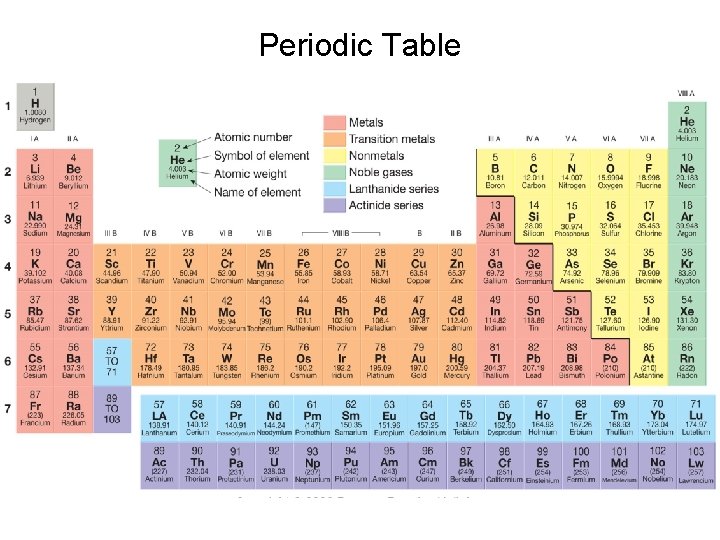
Periodic Table

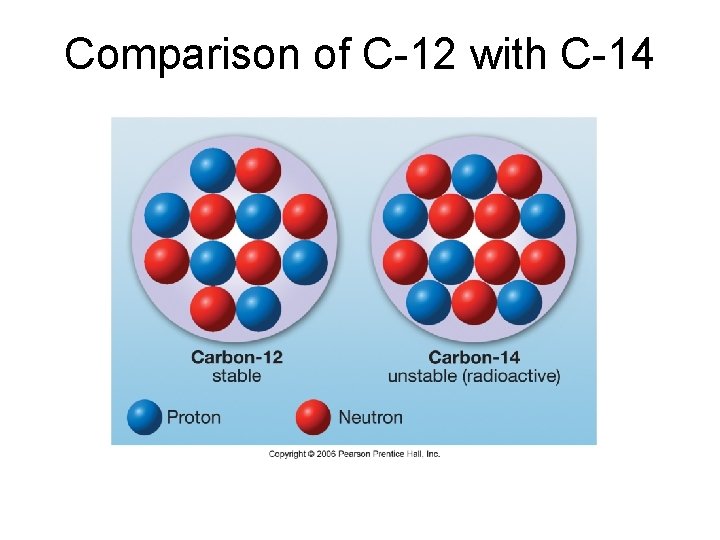
Isotopes and Radioactivity – Isotope: Variety of an atom with a different number of neutrons and mass number – Some isotopes (not all!) are inherently unstable, which means the forces binding nuclear particles together are not enough to hold the nucleus together. These are called radioactive isotopes. – Examples of isotopes include O-16, O-18, C -12, C-13, and C-14. Only the last is radioactive.

Comparison of C-12 with C-14

Radioactivity • Many common radioactive isotopes are naturally occurring. • Most radioactive processes release energy; formation of C-14 by neutron capture is an exception. It requires cosmic (solar) radiation. • They also often release energy and sometimes eject atomic particles as they “decay” or change into a more stable substance.

From Parent to Daughter • In many cases atomic particle are ejected during radioactive decay – Protons and/or neutrons ejected from nucleus – Protons become neutrons or vice verse • Atomic number changes so a new daughter element results. • How long does a radioactive parent take to turn into a stable daughter?

Figure 2. 4
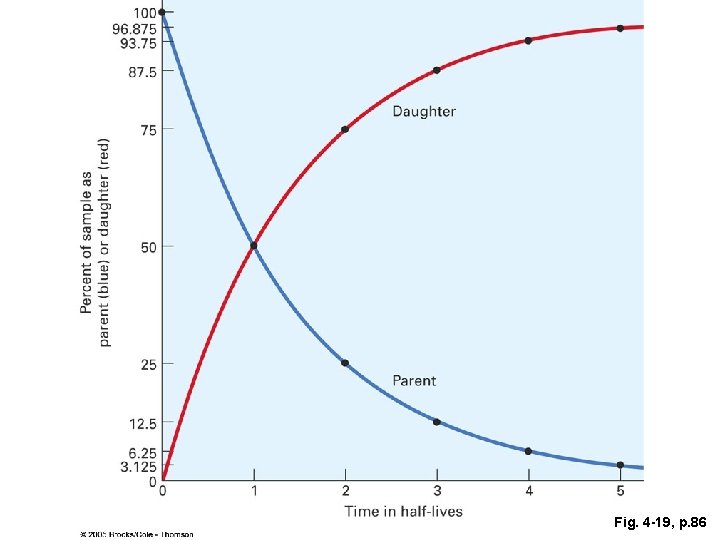
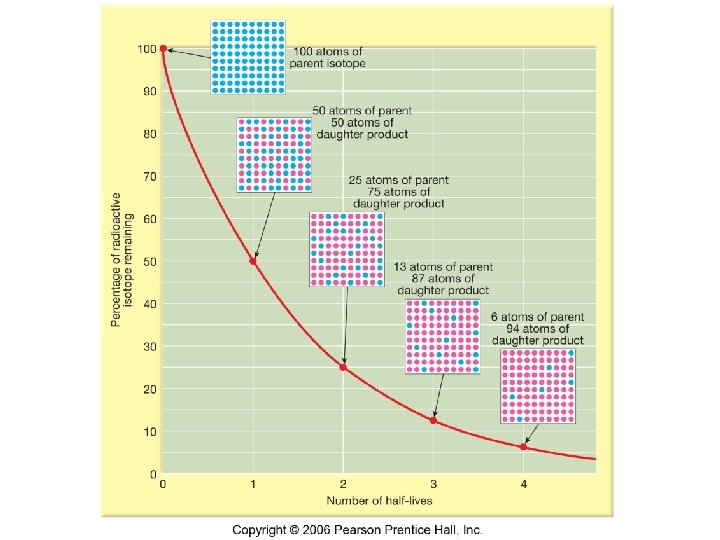
Half-life • the time required for one-half of the radioactive nuclei in a sample to change from parent isotope to daughter isotope. • Decay occurs at random. Can’t predict when an individual atom will decay. • However, decay is statistically predictable. • Comparison with coin toss

Half Life (cont’d) • After one half-life, 50% of the parent isotope will have become daughter isotope, regardless of the sample size. • After 2 half-lives, 50% of the remaining parent isotope will have become daughter isotope. This means 75% of the original parent isotope will have changed. • This is an exponential relationship.

Fig. 4 -19, p. 86

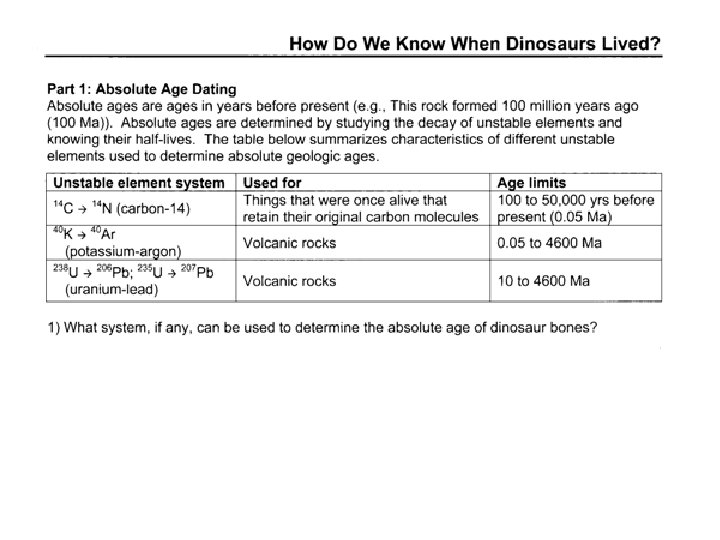
Using half-lives of radioactive isotopes in an object to determine the numerical age • Zircon (zirconium silicate) is a common accessory mineral in igneous, sedimentary and metamorphic rocks which contains traces of uranium and thorium • Potassium-40 is a radioactive isotope which occurs in K-spars and other minerals containing potassium. Zircon crystal

Using half-lives of radioactive isotopes in an object to determine the numerical age • Every radioactive isotope has a unique halflife, which can be determined by experiment • For radioactive isotopes other than C-14, the ratio of parent to daughter product in a sample determines the age of the sample • C-14 is compared to atmospheric concentration to determine age of organic material. • After approximately 10 half-lives, the method is no longer effective as the amount of parent material is too small to measure.

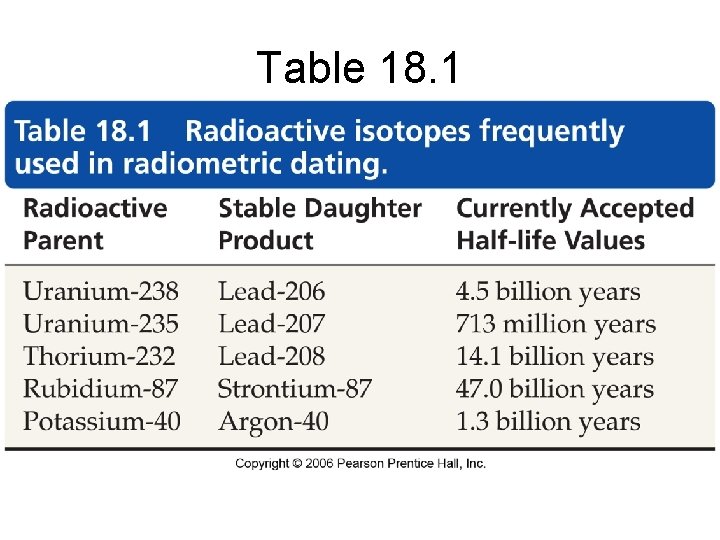
Table 18. 1

Figure 18. 14

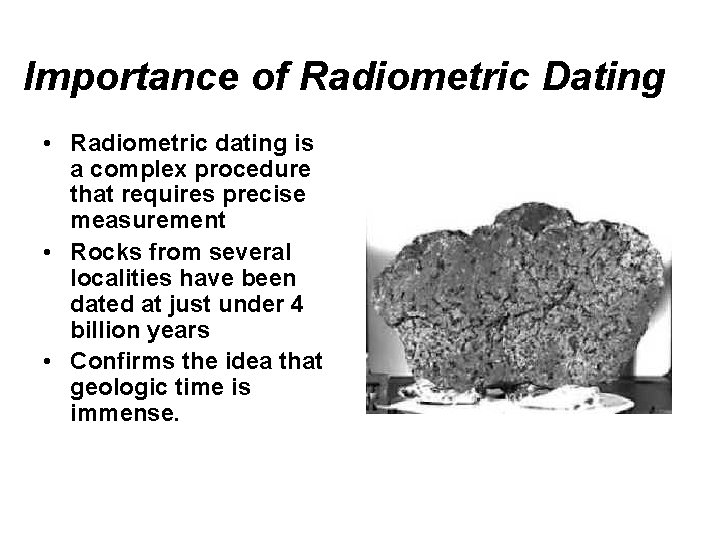
Importance of Radiometric Dating • Radiometric dating is a complex procedure that requires precise measurement • Rocks from several localities have been dated at just under 4 billion years • Confirms the idea that geologic time is immense.

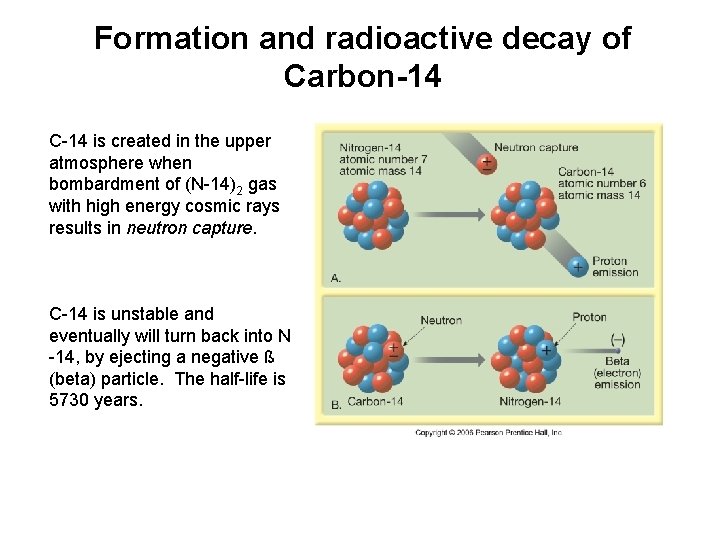
Formation and radioactive decay of Carbon-14 C-14 is created in the upper atmosphere when bombardment of (N-14)2 gas with high energy cosmic rays results in neutron capture. C-14 is unstable and eventually will turn back into N -14, by ejecting a negative ß (beta) particle. The half-life is 5730 years.

Radiometric dating with Carbon-14 • The % C-14 is equal to atmospheric C-14 in a living object, but decreases after death • To determine the age of the sample, compare % C-14 in sample with % atmospheric C-14. • Due to relatively small half-life, C-14 is used to date recent events only (10 half-lives is less than 60, 000 years) • Most useful in the fields of archeology and anthropology, also for climate change studies

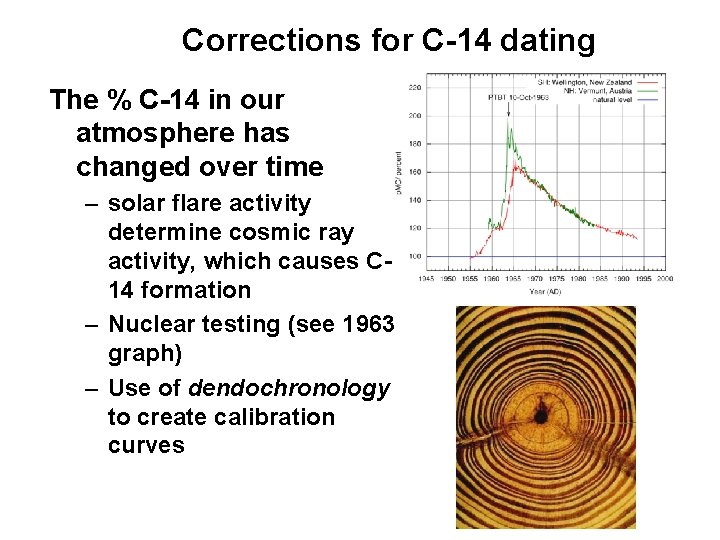
Corrections for C-14 dating The % C-14 in our atmosphere has changed over time – solar flare activity determine cosmic ray activity, which causes C 14 formation – Nuclear testing (see 1963 graph) – Use of dendochronology to create calibration curves

Conversions of Dates and Ages into years BP If the age of the object is given as a date: • AD (“year of the lord”): This is the same as a calendar date. The years BP value is how old the sample was in 1950. Ex: If an object is dated at 5 AD, the BP age is 1950 -5 or 1945 years BP • BC/BCE: This is also a date, indicating how many years before “the birth of Christ”. Ex: If an object is dated at 5 BC, it was already 5 when the AD numbering system began. In 1950, it was 1950 + 5 or 1955 years BP • If an age is given (ex: the object is 2000 years old, that’s 2000 years older than today. Assuming today is in 1999 (!) simply subtract 49 years from the age.