Geologic Time and Earth History Part 2 Absolute

Geologic Time and Earth History Part 2 – Absolute Age

Uniformitarianism • A principle that states that geologic processes that have been occurring today have been occurring since Earth formed. • Key to the science of geology and studying Earth’s past!

Essential Questions • How can the amount of parent material in a rock sample be determined by using halflives? • How does the amount of parent material in a sample change as time increases?

Absolute Dating • Radioactive isotope content can be used to identify the amount of time passed since the rock formed. • Rocks that can be dated like this are mostly igneous, but sometimes metamorphic. • Sedimentary rocks cannot be dated this way. • The process is also known as radiometric analysis.
Radioactive Decay • Method that is used to determine the absolute age of a rock or fossil. • When an atom breaks down or decays over time until it is stable.

Half-Life • Half-life: The time required for half of a sample of a radioactive isotope to break down by radioactive decay to form a daughter isotope. • Ranges from seconds to billions of years depending on the material.
Half-Life • For example, carbon-14 takes 5730 years to break down. So, if I have 100 g of carbon-14, after 5730 years I will only have 50 g. How much will be left after another 5730 years? • How much time would have passed? Answer: • You would have 25 g of carbon-14 • 11, 460 years of total time would have passed.

Using Half-Life • Parent: unstable radioactive isotope • Daughter: isotope resulting from the decay of the parent. # of Half Lives Parent Isotopes Daughter Isotopes 0 100% 0% 1 50% 2 25% 75% 3 12. 5% 87. 5% 4 6. 25% 93. 75%
Parent and Daughter material in terms of half-life • By comparing the percentage of an original element (parent) to the percentage of the decay element (daughter), the age of rock can be calculated. • The ratio of the two atom types is a direct function of its age because when a rock was formed, it had all parent atoms and no daughters.
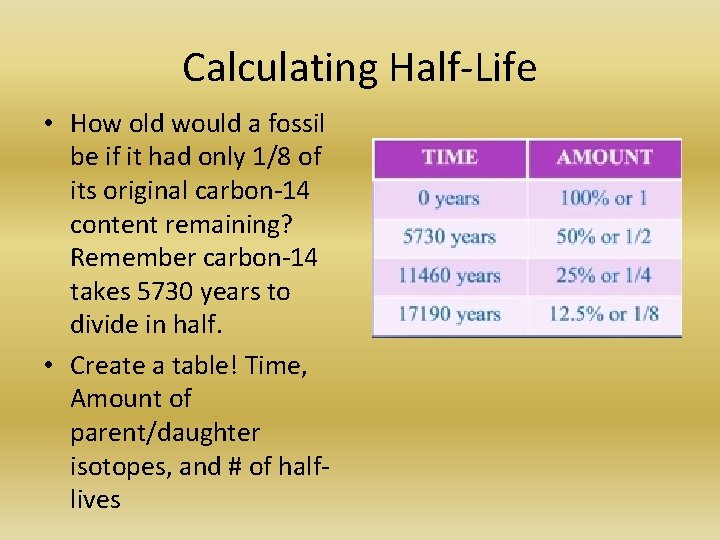
Calculating Half-Life • How old would a fossil be if it had only 1/8 of its original carbon-14 content remaining? Remember carbon-14 takes 5730 years to divide in half. • Create a table! Time, Amount of parent/daughter isotopes, and # of halflives

Problem #1 • The half-life of radon 222 is 3. 8 days. How much of a 100 g sample is left after 15. 2 days? Answer: 6. 25 grams Time Amount 0 Days 100 grams 3. 8 50 grams 7. 6 25 grams 11. 4 12. 5 grams 15. 2 6. 25 grams
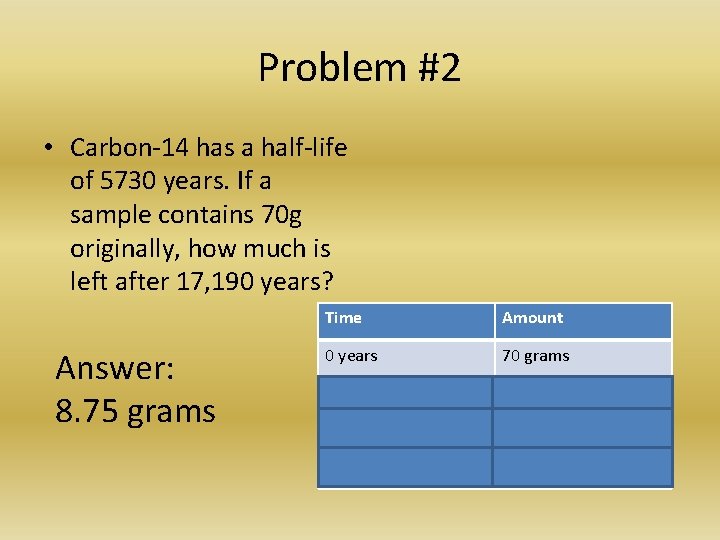
Problem #2 • Carbon-14 has a half-life of 5730 years. If a sample contains 70 g originally, how much is left after 17, 190 years? Answer: 8. 75 grams Time Amount 0 years 70 grams 5730 years 35 grams 11, 460 years 17. 5 grams 17, 190 years 8. 75 grams

Problem #3 • How much of a 500 g sample of potassium-42 is left after 62 hours? The half-life of potassium-42 is 12. 4 hours. Time Amount 0 hours 500 grams 12. 4 hours 250 grams 24. 8 hours 125 grams 37. 2 hours 62. 5 grams 49. 6 hours 31. 25 grams 62 hours 15. 625 grams Answer: 15. 625 grams
- Slides: 13