GEOLOGIC CARBON CYCLE Textbook chapter 5 6 14

GEOLOGIC CARBON CYCLE • Textbook chapter 5, 6 & 14 • Global carbon cycle • Long-term stability and feedback

Geological carbon cycle Weathering of rocks Sediment burial Williams and Follows (2011) Volcanic degassing

Volcanic degassing • Volcano • Hydrothermal vents • Very approximate carbon flux ~ 0. 04 GTC/year • Small carbon source relative to human emission, air-sea CO 2 exchange, biological productivity • BUT it is dominant over long timescales ~ millions of years+
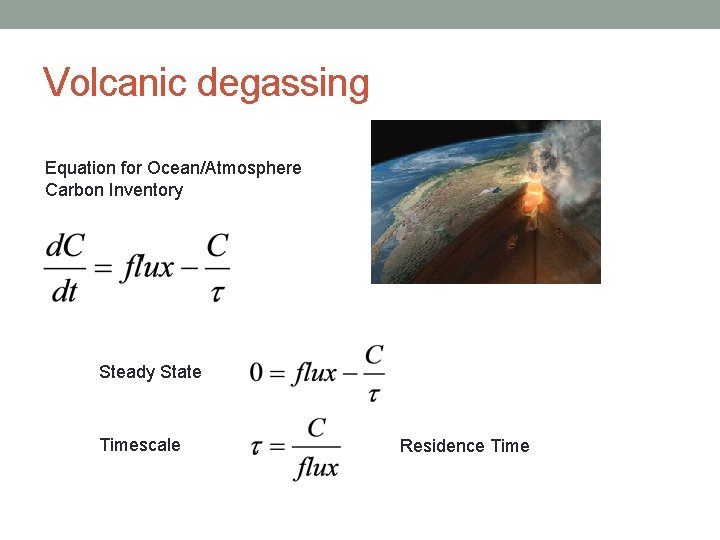
Volcanic degassing Equation for Ocean/Atmosphere Carbon Inventory Steady State Timescale Residence Time
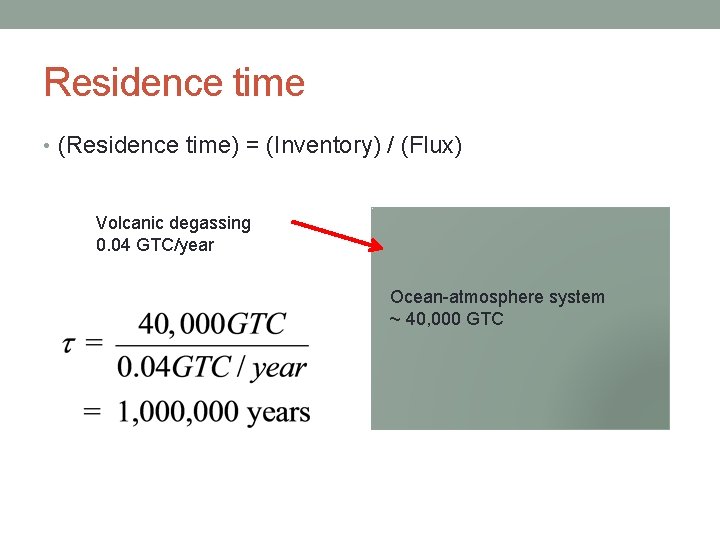
Residence time • (Residence time) = (Inventory) / (Flux) Volcanic degassing 0. 04 GTC/year Ocean-atmosphere system ~ 40, 000 GTC
Weathering • Physical Weathering = mechanical breakdown of rocks • Erosion • Formation of sediments • Chemical Weathering = chemical breakdown • Salinity • Some nutrients (silicate, phosphate) • Alkalinity (Ca 2+)

Weathering of Carbonate Rocks 1. Carbon dioxide is removed from the atmosphere by dissolving in water and forming carbonic acid CO 2 + H 2 O -> H 2 CO 3 (carbonic acid) 2. Carbonic acid is used to weather rocks (e. g. rain), yielding bicarbonate ions, other ions, and clays, which are dumped into ocean (e. g. river runoff) H 2 CO 3 + H 2 O + silicate minerals -> HCO 3 - + cations (Ca++, Fe++, Na+, etc. ) + clays 3. Calcium carbonate is precipitated from calcium and bicarbonate ions in seawater by marine organisms like coral, coccolithophores, foraminifera Ca++ + 2 HCO 3 - -> Ca. CO 3 + CO 2 + H 2 O (form both calcite and aragonite) the carbon is now stored on the seafloor in layers of limestone
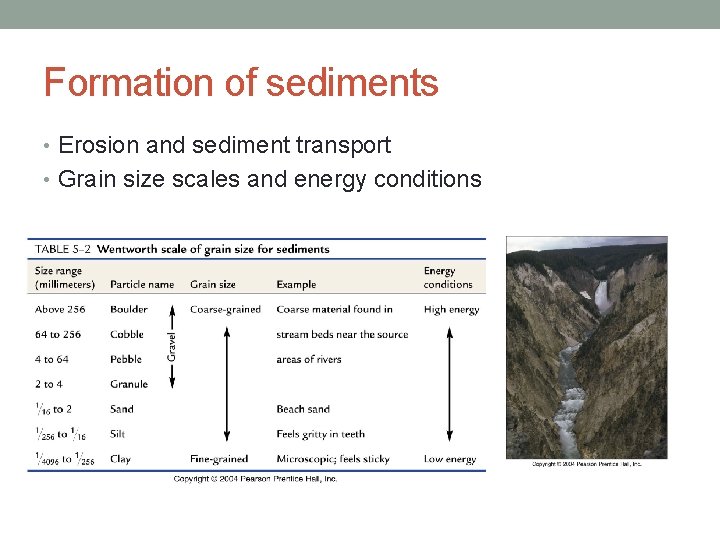
Formation of sediments • Erosion and sediment transport • Grain size scales and energy conditions
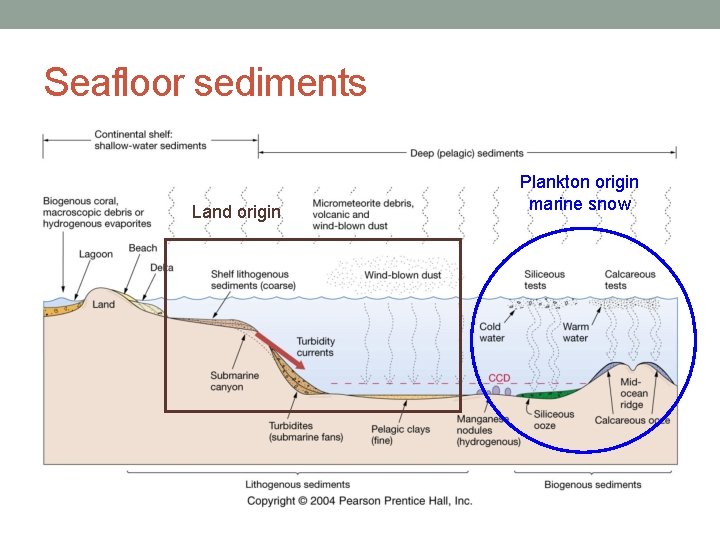
Seafloor sediments Land origin Plankton origin marine snow
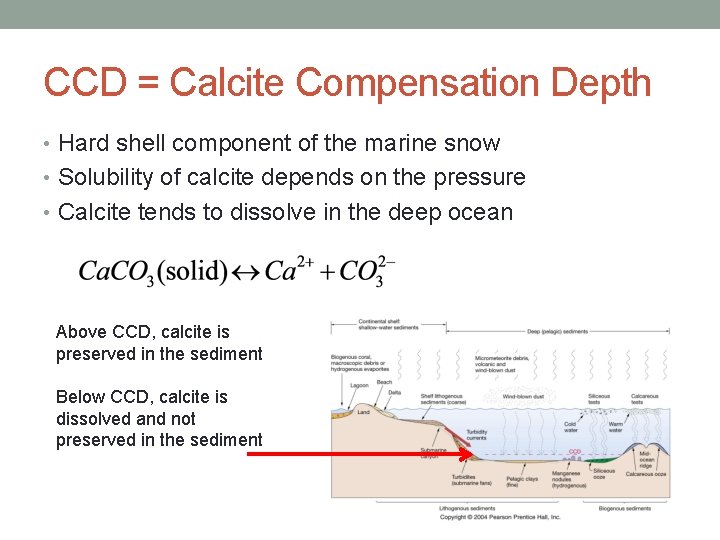
CCD = Calcite Compensation Depth • Hard shell component of the marine snow • Solubility of calcite depends on the pressure • Calcite tends to dissolve in the deep ocean Above CCD, calcite is preserved in the sediment Below CCD, calcite is dissolved and not preserved in the sediment
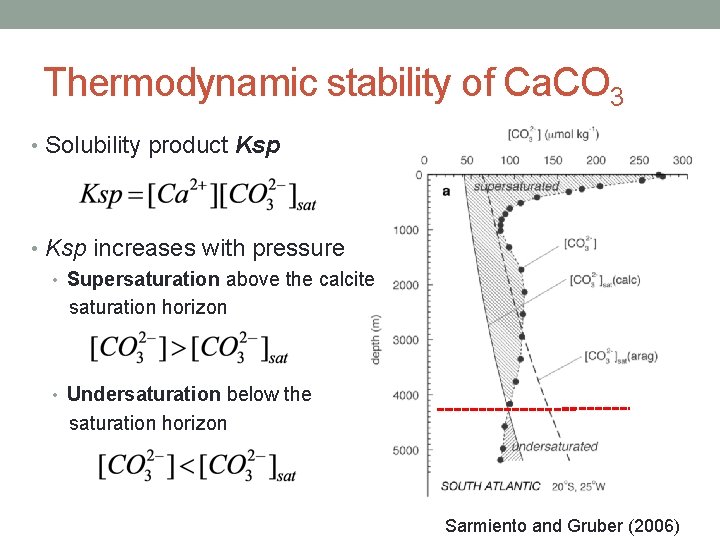
Thermodynamic stability of Ca. CO 3 • Solubility product Ksp • Ksp increases with pressure • Supersaturation above the calcite saturation horizon • Undersaturation below the saturation horizon Sarmiento and Gruber (2006)
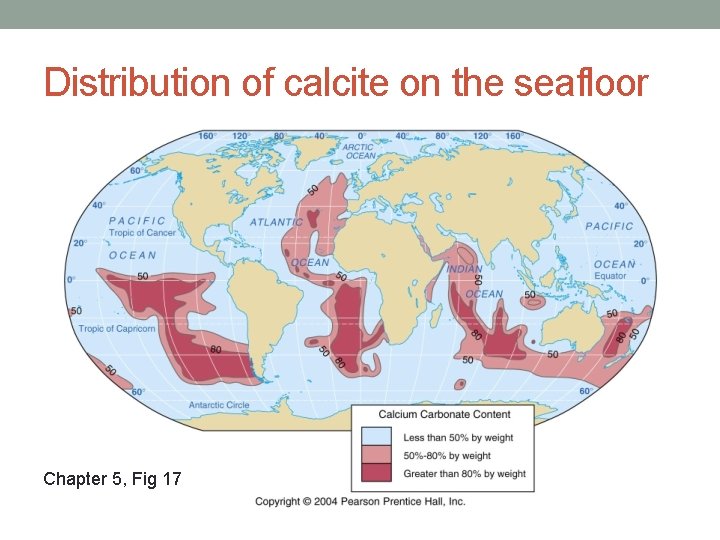
Distribution of calcite on the seafloor Chapter 5, Fig 17
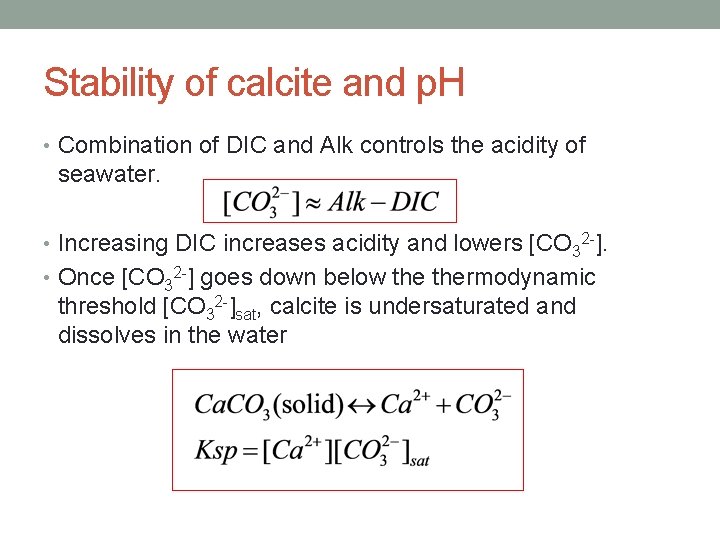
Stability of calcite and p. H • Combination of DIC and Alk controls the acidity of seawater. • Increasing DIC increases acidity and lowers [CO 32 -]. • Once [CO 32 -] goes down below thermodynamic threshold [CO 32 -]sat, calcite is undersaturated and dissolves in the water
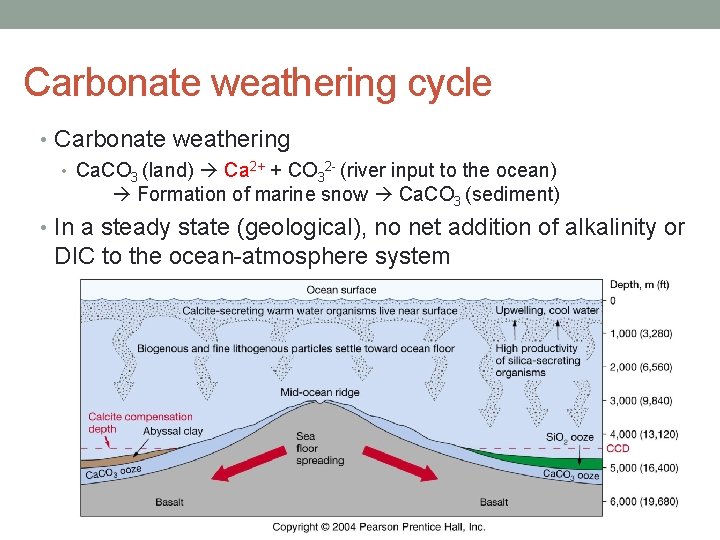
Carbonate weathering cycle • Carbonate weathering • Ca. CO 3 (land) Ca 2+ + CO 32 - (river input to the ocean) Formation of marine snow Ca. CO 3 (sediment) • In a steady state (geological), no net addition of alkalinity or DIC to the ocean-atmosphere system
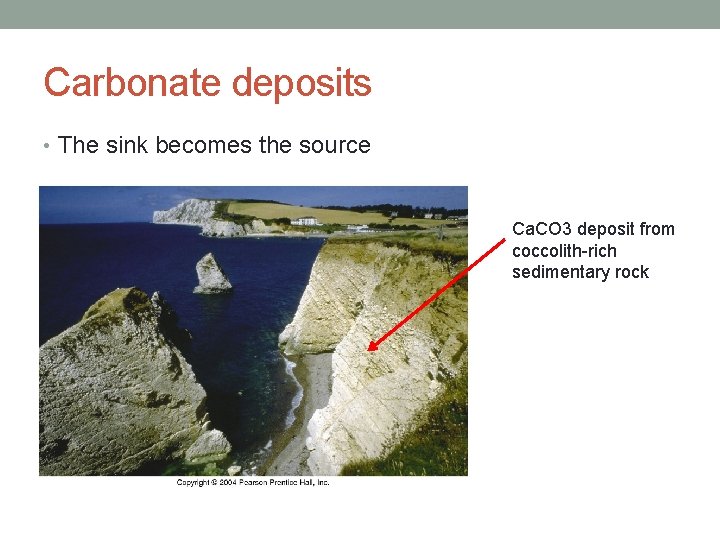
Carbonate deposits • The sink becomes the source Ca. CO 3 deposit from coccolith-rich sedimentary rock
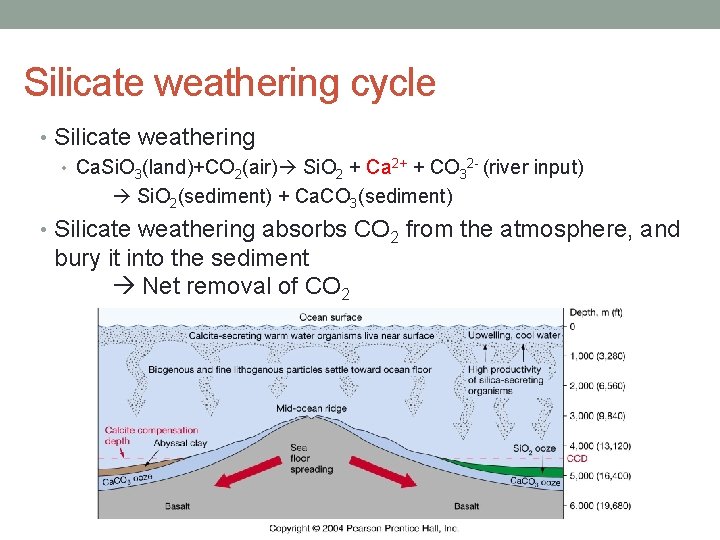
Silicate weathering cycle • Silicate weathering • Ca. Si. O 3(land)+CO 2(air) Si. O 2 + Ca 2+ + CO 32 - (river input) Si. O 2(sediment) + Ca. CO 3(sediment) • Silicate weathering absorbs CO 2 from the atmosphere, and bury it into the sediment Net removal of CO 2
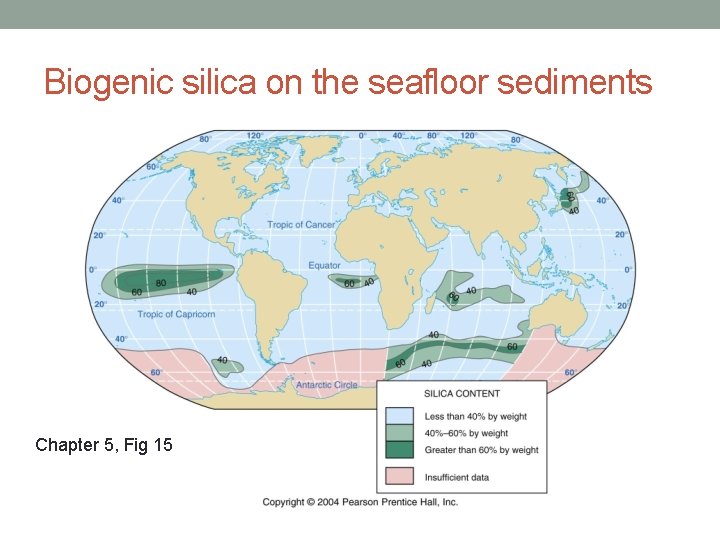
Biogenic silica on the seafloor sediments Chapter 5, Fig 15
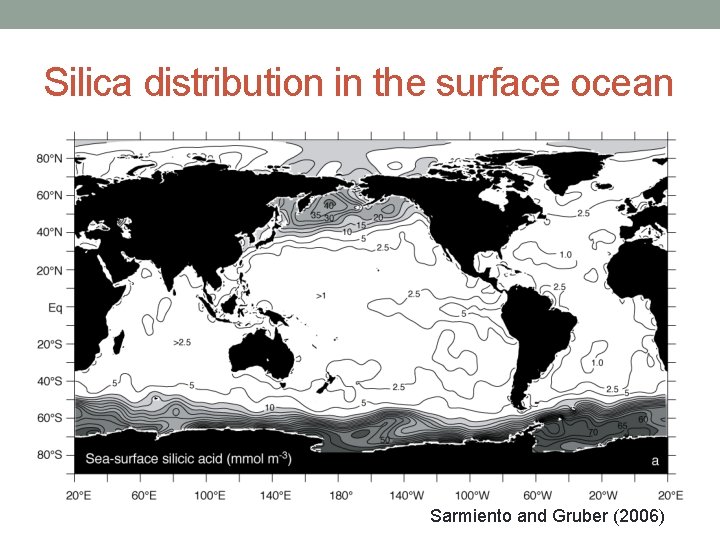
Silica distribution in the surface ocean Sarmiento and Gruber (2006)
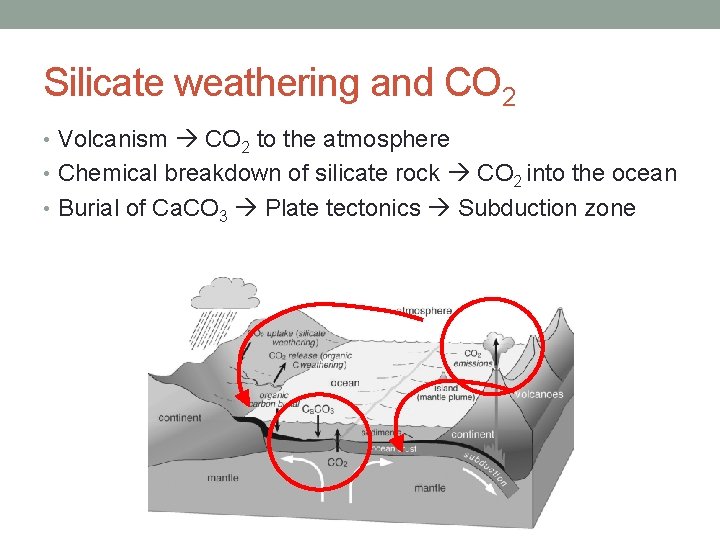
Silicate weathering and CO 2 • Volcanism CO 2 to the atmosphere • Chemical breakdown of silicate rock CO 2 into the ocean • Burial of Ca. CO 3 Plate tectonics Subduction zone
Faint young sun paradox • Sagan and Mullen (1972): In the early Earth, the solar energy input was only about 70% of today but the climate was as warm as today. • Long-term stability of the Earth’s climate system • Temperature remained within 0°C and 100°C

Weathering-CO 2 feedback • Hypothesis: The speed of rocks’ chemical breakdown partly depends on the temperature. • Cold climate tends to slow down chemical weathering • Slow-down of silicate weathering cannot balance volcanic CO 2 flux • Climate warms up due to increased atmospheric CO 2 • Weathering-CO 2 feedback tends to stabilize the climate temperature over millions of years • Is this sufficient to explain the early Earth’s warmth? Rosing et al. , (2010) Nature: ongoing debate
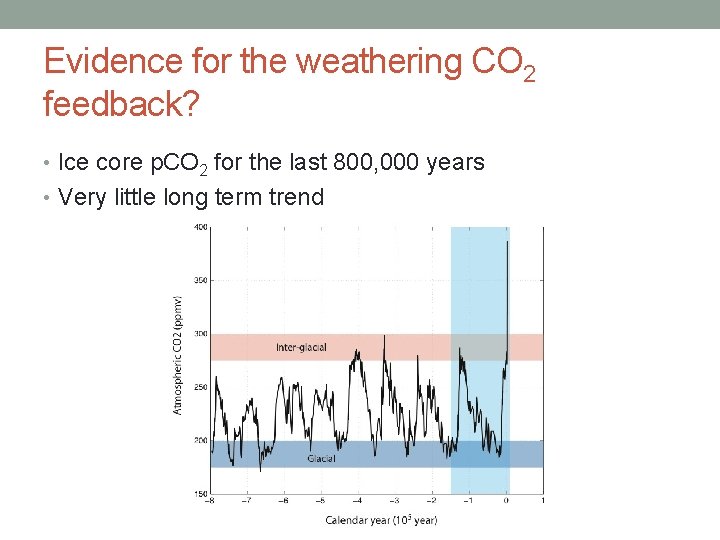
Evidence for the weathering CO 2 feedback? • Ice core p. CO 2 for the last 800, 000 years • Very little long term trend
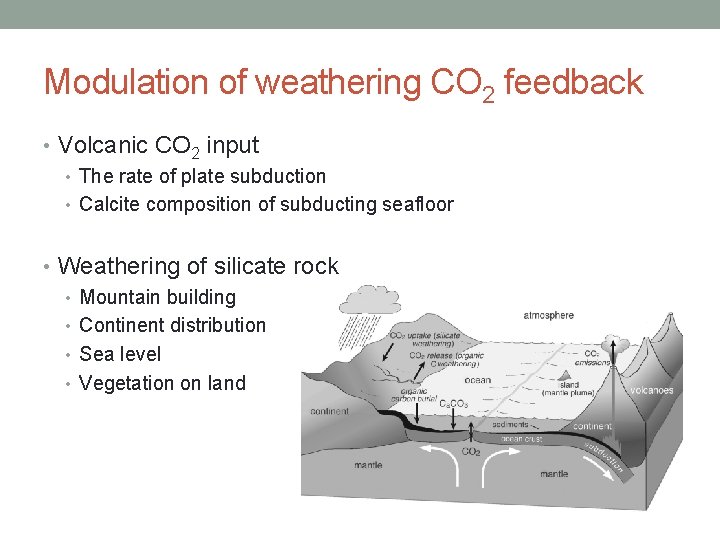
Modulation of weathering CO 2 feedback • Volcanic CO 2 input • The rate of plate subduction • Calcite composition of subducting seafloor • Weathering of silicate rock • Mountain building • Continent distribution • Sea level • Vegetation on land
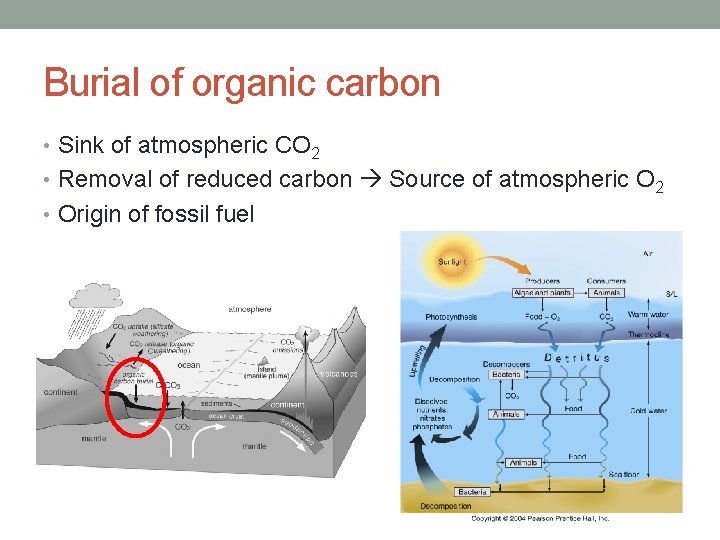
Burial of organic carbon • Sink of atmospheric CO 2 • Removal of reduced carbon Source of atmospheric O 2 • Origin of fossil fuel
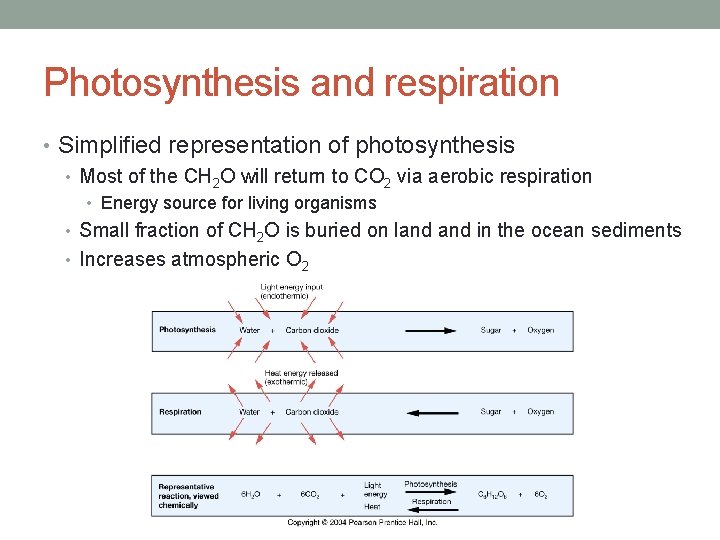
Photosynthesis and respiration • Simplified representation of photosynthesis • Most of the CH 2 O will return to CO 2 via aerobic respiration • Energy source for living organisms • Small fraction of CH 2 O is buried on land in the ocean sediments • Increases atmospheric O 2
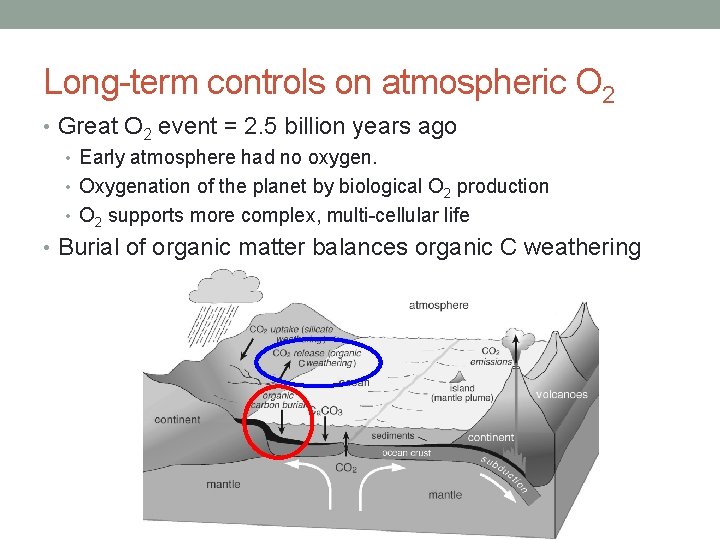
Long-term controls on atmospheric O 2 • Great O 2 event = 2. 5 billion years ago • Early atmosphere had no oxygen. • Oxygenation of the planet by biological O 2 production • O 2 supports more complex, multi-cellular life • Burial of organic matter balances organic C weathering

Organic Carbon-O 2 feedback • Hypothesis: Burial of organic carbon depends on the oxygen content of the deep ocean • If atmospheric O 2 gets low, deep water goes anoxic • Aerobic respiration stops and the respiration of organic matter decreases • More organic matter are preserved in the sediment • Increases atmospheric O 2 • This feedback can potentially stabilize the atmospheric oxygen • No quantitative model/theory yet
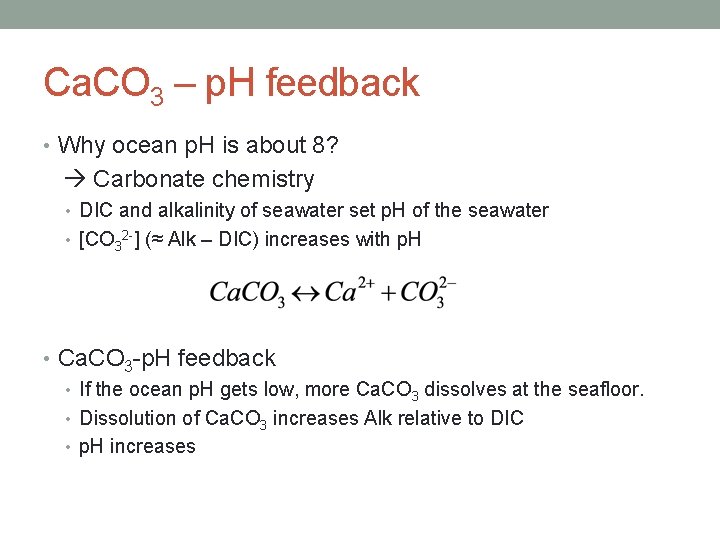
Ca. CO 3 – p. H feedback • Why ocean p. H is about 8? Carbonate chemistry • DIC and alkalinity of seawater set p. H of the seawater • [CO 32 -] (≈ Alk – DIC) increases with p. H • Ca. CO 3 -p. H feedback • If the ocean p. H gets low, more Ca. CO 3 dissolves at the seafloor. • Dissolution of Ca. CO 3 increases Alk relative to DIC • p. H increases
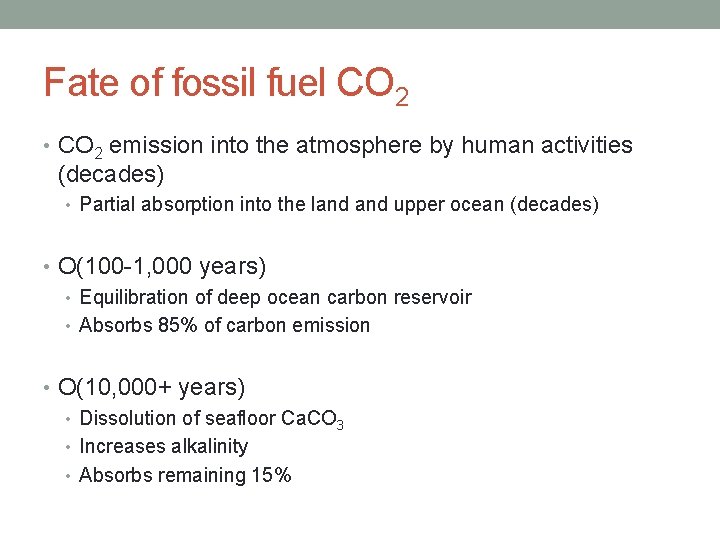
Fate of fossil fuel CO 2 • CO 2 emission into the atmosphere by human activities (decades) • Partial absorption into the land upper ocean (decades) • O(100 -1, 000 years) • Equilibration of deep ocean carbon reservoir • Absorbs 85% of carbon emission • O(10, 000+ years) • Dissolution of seafloor Ca. CO 3 • Increases alkalinity • Absorbs remaining 15%
Theme III: long-term carbon-climate relation • Three stabilizing mechanisms for temperature, CO 2, alkalinity and p. H of the seawater • Operates over plate tectonic timescales, providing stability to the climate and biogeochemical cycles • Weathering-CO 2 feedback • Silicate weathering • Organic Carbon-O 2 feedback • Preservation of organic matter on the seafloor • Ca. CO 3 -p. H feedback • Preservation of Ca. CO 3 on the seafloor
Changing mood of carbon cycle • O(10 -1 k years) Ocean carbon cycle acts as a sink of carbon and heat, moderating the climate change • O(100 k years) Ocean carbon cycle seems to act as an amplifier of the glacial-interglacial climate change • O(1 million years) Ocean carbon cycle seems to stabilize the climate and cycling of elements through the three feedbacks… • Further reading: D. Archer (2010) “The Global Carbon Cycle”, Princeton University Press.
- Slides: 31