Genitourinary tumours prostate and non prostate Patricia Walcott












- Slides: 39

Genitourinary tumours: prostate and non prostate Patricia Walcott, BSc (Hons), MSc Medical Science Liaison Medical Ipsen Ltd The following contains scientific information on unapproved indications. This is a summary of the selected genitourinary tumour data presented at ESOU and is not all encompassing April 2016 UK/URO 08412

ESOU 2016: Prostate Cancer Androgen Receptor Pathway Inhibitors in locally advanced PCA : Latest results D. Pfister, Cologne (DE) Rational of neoadjuvant therapy 2 challenges in treatment of high risk disease • Local control of the primary tumour • Systemic control of microscopic metastatic disease Primary goal of neoadjuvant therapy • Downstaging locally advanced PCa to facilitate resection • In-vivo observation of treatment response • Improvement of long-term survival A systematic review and meta-analysis of randomised trials of neoadjuvant hormone therapy for localised and locally advanced prostate carcinoma. Shelley MD and al. , Cancer Treat, 2009; 35: 9 – 17 • Analysis of 10 randomised trial on NHT in locally advanced PCA • Significant improvement of: − positive surgical margins RR: 0, 49 p<0. 00001 − organ confinement RR: 1, 63 p<0. 001 − lymph node invasion RR: 0, 49 p<0. 02 • BUT: no benefit in overall or cancer specific survival Androgen Receptor Pathway Inhibitors in locally advanced PCA: Latest Results Presented D. Pfister, Cologne (DE) at ESOU 16 15 -17 Jan 2016 UK/URO 08412 2

Drawbacks of neoadjuvant ADT: biological response and resistance might be the reason for non-improvement of long-term outcome Androgen Receptor Pathway Inhibitors in locally advanced PCA: Latest Results Presented D. Pfister, Cologne (DE) at ESOU 16 15 -17 Jan 2016 UK/URO 08412 3

• Neoadjuvant ADT for 0, <3, 3 to 6, 6 to 9 months prior to RP • Comparison of biopsy and radical prostatectomy specimens – Intraprostatic androgen levels and gene expression analysis of androgen related genes Results • • • Significant reduction of intraprostatic testosterone and DHT by 75% Significant alteration of androgen dependent genes Significant downregulation of several AR-responsive genes No alterations in AR of PSA-genes No significant reduction in AR and PSA expression Androgen Receptor Pathway Inhibitors in locally advanced PCA: Latest Results Presented D. Pfister, Cologne (DE) at ESOU 16 15 -17 Jan 2016 UK/URO 08412 4

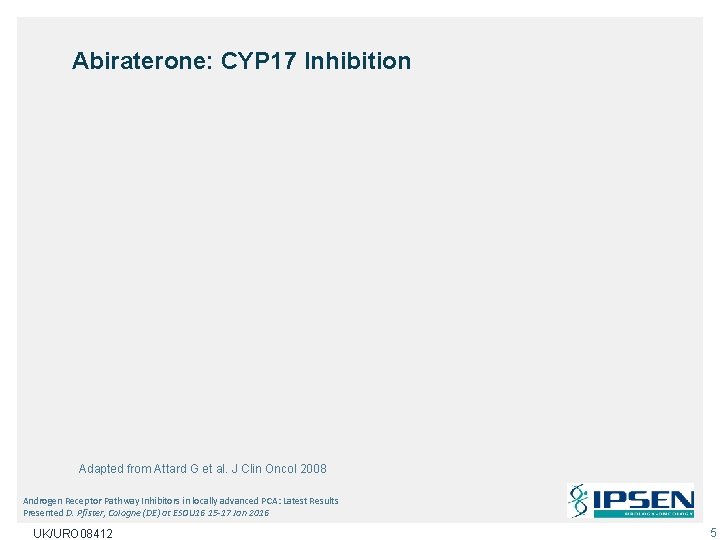
Abiraterone: CYP 17 Inhibition Adapted from Attard G et al. J Clin Oncol 2008 Androgen Receptor Pathway Inhibitors in locally advanced PCA: Latest Results Presented D. Pfister, Cologne (DE) at ESOU 16 15 -17 Jan 2016 UK/URO 08412 5

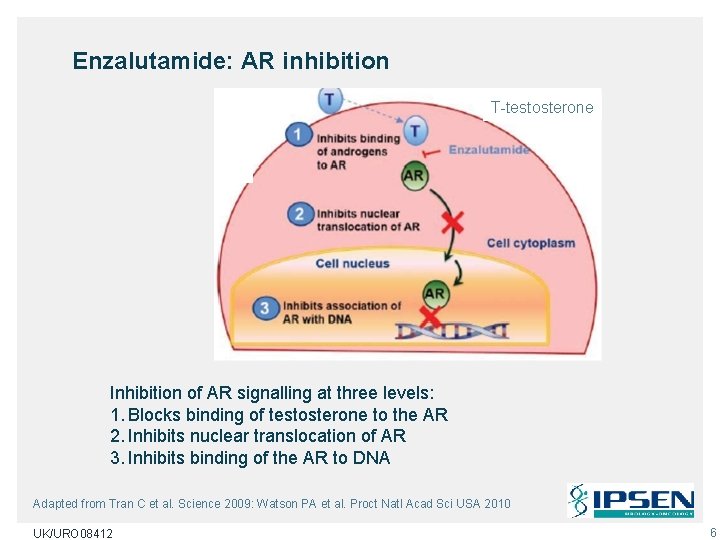
Enzalutamide: AR inhibition T-testosterone Inhibition of AR signalling at three levels: 1. Blocks binding of testosterone to the AR 2. Inhibits nuclear translocation of AR 3. Inhibits binding of the AR to DNA Adapted from Tran C et al. Science 2009: Watson PA et al. Proct Natl Acad Sci USA 2010 UK/URO 08412 6

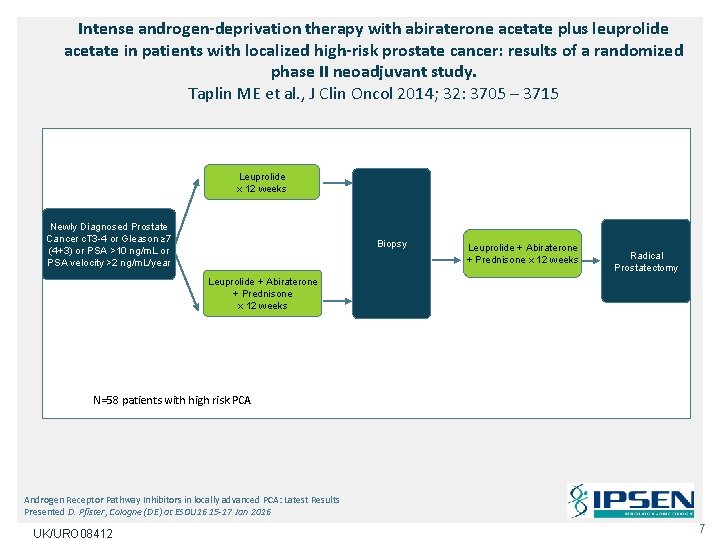
Intense androgen-deprivation therapy with abiraterone acetate plus leuprolide acetate in patients with localized high-risk prostate cancer: results of a randomized phase II neoadjuvant study. Taplin ME et al. , J Clin Oncol 2014; 32: 3705 – 3715 Leuprolide x 12 weeks Newly Diagnosed Prostate Cancer c. T 3 -4 or Gleason ≥ 7 (4+3) or PSA >10 ng/m. L or PSA velocity >2 ng/m. L/year Biopsy Leuprolide + Abiraterone + Prednisone x 12 weeks Radical Prostatectomy Leuprolide + Abiraterone + Prednisone x 12 weeks N=58 patients with high risk PCA Androgen Receptor Pathway Inhibitors in locally advanced PCA: Latest Results Presented D. Pfister, Cologne (DE) at ESOU 16 15 -17 Jan 2016 UK/URO 08412 7

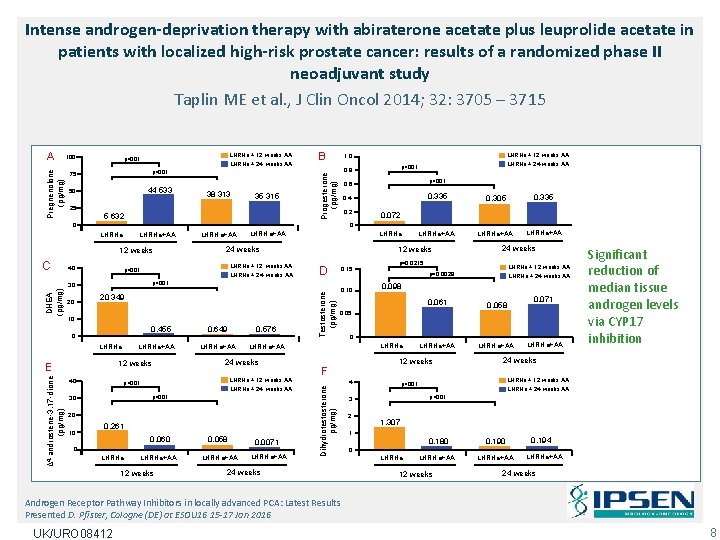
Intense androgen-deprivation therapy with abiraterone acetate plus leuprolide acetate in patients with localized high-risk prostate cancer: results of a randomized phase II neoadjuvant study Taplin ME et al. , J Clin Oncol 2014; 32: 3705 – 3715 100 LHRHa + 12 weeks AA p<001 LHRHa + 24 weeks AA 75 p<001 50 44 533 38 313 35 315 25 5 632 B LHRHa+AA 12 weeks 40 0. 2 0. 305 0. 335 LHRHa+AA 0. 072 LHRHa+AA 12 weeks 24 weeks LHRHa + 12 weeks AA LHRHa + 24 weeks AA D 0. 15 10 0. 455 LHRHa+AA 12 weeks E 40 0. 649 0. 576 LHRHa+AA 24 weeks LHRHa + 12 weeks AA p<001 LHRHa + 24 weeks AA p<001 30 20 0. 261 0. 060 0. 058 0 LHRHa+AA 12 weeks LHRHa+AA 0. 0071 LHRHa+AA Testosterone (pg/mg) 20 349 0. 10 LHRHa + 12 weeks AA p=0. 0029 LHRHa + 24 weeks AA 0. 098 0. 061 0. 058 LHRHa+AA 0. 071 0 LHRHa 12 weeks F 4 24 weeks p=0. 0215 0. 05 Dihydrotestosterone pg/mg) DHEA (pg/mg) 0. 335 0. 4 p<001 0 Δ 4 -androstene-3, 17 -dione (pg/mg) LHRHa+AA p<001 30 10 p<001 0. 6 0 LHRHa 20 LHRHa + 24 weeks AA p<001 0. 8 0 C LHRHa + 12 weeks AA 1. 0 Progesterone (pg/mg) Pregnenolone (pg/mg) A 24 weeks LHRHa + 12 weeks AA p<001 LHRHa + 24 weeks AA p<001 3 2 LHRHa+AA Significant reduction of median tissue androgen levels via CYP 17 inhibition 1. 307 1 0. 180 0. 190 LHRHa+AA 0 LHRHa 12 weeks 0. 194 LHRHa+AA 24 weeks Androgen Receptor Pathway Inhibitors in locally advanced PCA: Latest Results Presented D. Pfister, Cologne (DE) at ESOU 16 15 -17 Jan 2016 UK/URO 08412 8

Additional results: • Still high percentage of p. T 3, p. N 1 and positive surgical margins • Still high percentage of residual tumour burden even after 24 weeks of neoadjuvant treatment • No significant side effects of treatment • No negative impact on surgical morbidity UK/URO 08412 9

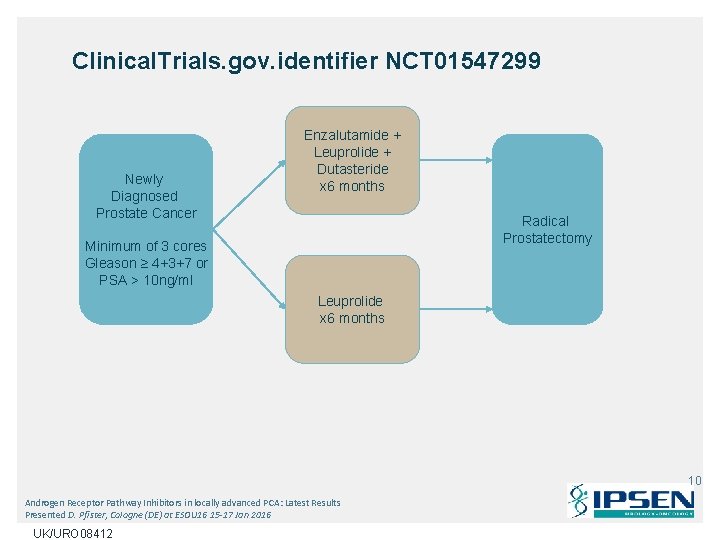
Clinical. Trials. gov. identifier NCT 01547299 Newly Diagnosed Prostate Cancer Enzalutamide + Leuprolide + Dutasteride x 6 months Radical Prostatectomy Minimum of 3 cores Gleason ≥ 4+3+7 or PSA > 10 ng/ml Leuprolide x 6 months 10 Androgen Receptor Pathway Inhibitors in locally advanced PCA: Latest Results Presented D. Pfister, Cologne (DE) at ESOU 16 15 -17 Jan 2016 UK/URO 08412

Summary • No general indication for neoadjuvant ADT in locally advanced PCA • Individual decision in large tumours to facilitate local treatment • No AR pathways inhibitors Ø Feasibility studies positive Ø No oncological outcome data Ø Still significant tumour burden after neoadjuvant therapy Ø Far away from clinical routine Androgen Receptor Pathway Inhibitors in locally advanced PCA: Latest Results Presented D. Pfister, Cologne (DE) at ESOU 16 15 -17 Jan 2016 UK/URO 08412 11

Intermittent hormone therapy: Not a standard for many urologists and oncologists. Why? F. Calais Da Silva, Lisbon (PT) • In Real Life patients do stop taking medication when: Ø Feel well Ø Secondary effects Ø Economics • But still low PSA and no clinical regression • The basic idea for intermittent hormonal therapy is the fact that long-term castration stimulates prostate cell apoptosis • After an average period of 24 months, the tumour relapses, characterised by a castrate-dependent state of growth • Experimantal data indicate that castrate dependent progression may begin early after castration, coinciding with the cessation of androgen-induced differentiation of stem cells UK/URO 08412 12

• It has been suggested that stopping castration prior to progression would mean that any subsequent tumour growth would be solely sustained by the proliferation of androgen-dependent stem cells • The stem cells should therefore be susceptible once again to androgen withdrawal • Thus, intermittent androgen deprivation (IAD) could delay the emergence of the androgen-independent clone • This rationale has been developed mainly through models Ø Shionoggi mouse by Akakura Ø With a possible better result Ø But it may be significantly different to tumour behaviour in men • Other possible benefits of IAD include the preservation of Qo. L in off treatment period, possible long-term benefits include bone protection and/or a protective effect against the metabolic syndrome • A reduction in treatment cost Intermittent hormone therapy: Not a standard for many urologists and oncologists. Why? Presented F. Calais Da Silva, Lisbon (PT) at ESOU 16 15 -17 Jan 2016 UK/URO 08412 13

• PSA anxiety – Medical – Patient • Lack of information • No change • Controversial Intermittent hormone therapy: Not a standard for many urologists and oncologists. Why? Presented F. Calais Da Silva, Lisbon (PT) at ESOU 16 15 -17 Jan 2016 UK/URO 08412 14

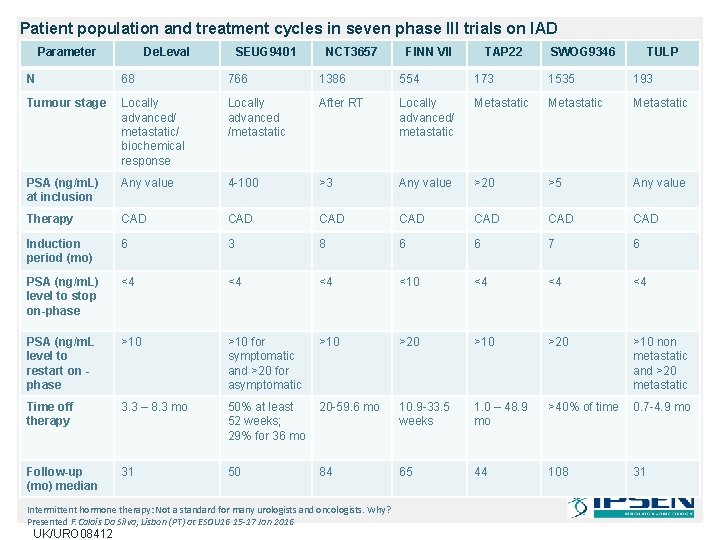
Patient population and treatment cycles in seven phase III trials on IAD Parameter De. Leval SEUG 9401 NCT 3657 FINN VII TAP 22 SWOG 9346 TULP N 68 766 1386 554 173 1535 193 Tumour stage Locally advanced/ metastatic/ biochemical response Locally advanced /metastatic After RT Locally advanced/ metastatic Metastatic PSA (ng/m. L) at inclusion Any value 4 -100 >3 Any value >20 >5 Any value Therapy CAD CAD Induction period (mo) 6 3 8 6 6 7 6 PSA (ng/m. L) level to stop on-phase <4 <4 <4 <10 <4 <4 <4 PSA (ng/m. L level to restart on phase >10 for symptomatic and >20 for asymptomatic >10 >20 >10 non metastatic and >20 metastatic Time off therapy 3. 3 – 8. 3 mo 50% at least 52 weeks; 29% for 36 mo 20 -59. 6 mo 10. 9 -33. 5 weeks 1. 0 – 48. 9 mo >40% of time 0. 7 -4. 9 mo Follow-up (mo) median 31 50 84 65 44 108 31 Intermittent hormone therapy: Not a standard for many urologists and oncologists. Why? Presented F. Calais Da Silva, Lisbon (PT) at ESOU 16 15 -17 Jan 2016 UK/URO 08412

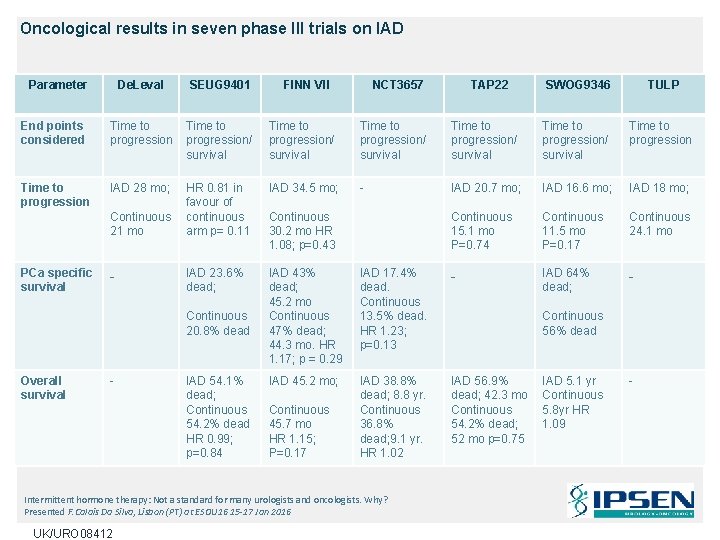
Oncological results in seven phase III trials on IAD Parameter De. Leval SEUG 9401 End points considered Time to progression/ survival Time to progression IAD 28 mo; HR 0. 81 in favour of continuous arm p= 0. 11 IAD 34. 5 mo; - IAD 23. 6% dead; IAD 43% dead; 45. 2 mo Continuous 47% dead; 44. 3 mo. HR 1. 17; p = 0. 29 IAD 45. 2 mo; Continuous 21 mo PCa specific survival - Continuous 20. 8% dead Overall survival - IAD 54. 1% dead; Continuous 54. 2% dead HR 0. 99; p=0. 84 FINN VII NCT 3657 SWOG 9346 TULP Time to progression/ survival Time to progression IAD 20. 7 mo; IAD 16. 6 mo; IAD 18 mo; Continuous 15. 1 mo P=0. 74 Continuous 11. 5 mo P=0. 17 Continuous 24. 1 mo IAD 17. 4% dead. Continuous 13. 5% dead. HR 1. 23; p=0. 13 - IAD 64% dead; - IAD 38. 8% dead; 8. 8 yr. Continuous 36. 8% dead; 9. 1 yr. HR 1. 02 IAD 56. 9% dead; 42. 3 mo Continuous 54. 2% dead; 52 mo p=0. 75 Continuous 30. 2 mo HR 1. 08; p=0. 43 Continuous 45. 7 mo HR 1. 15; P=0. 17 Intermittent hormone therapy: Not a standard for many urologists and oncologists. Why? Presented F. Calais Da Silva, Lisbon (PT) at ESOU 16 15 -17 Jan 2016 UK/URO 08412 TAP 22 Continuous 56% dead IAD 5. 1 yr Continuous 5. 8 yr HR 1. 09 -

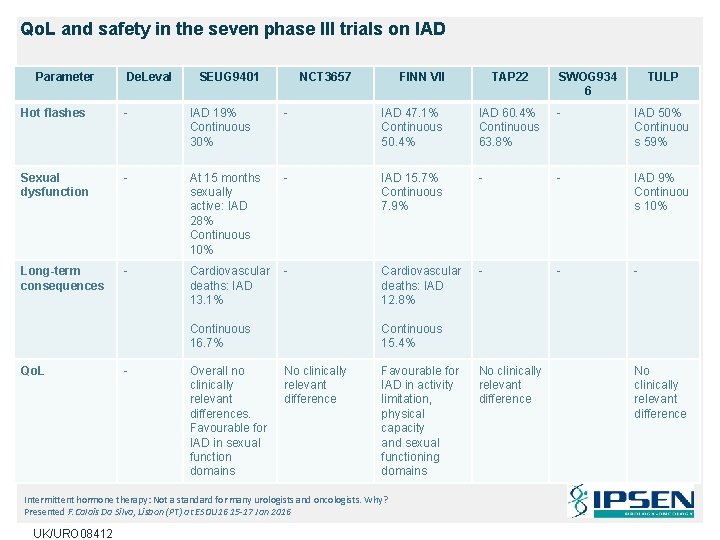
Qo. L and safety in the seven phase III trials on IAD Parameter De. Leval SEUG 9401 NCT 3657 FINN VII TAP 22 SWOG 934 6 TULP Hot flashes - IAD 19% Continuous 30% - IAD 47. 1% Continuous 50. 4% IAD 60. 4% Continuous 63. 8% - IAD 50% Continuou s 59% Sexual dysfunction - At 15 months sexually active: IAD 28% Continuous 10% - IAD 15. 7% Continuous 7. 9% - - IAD 9% Continuou s 10% Long-term consequences - Cardiovascular deaths: IAD 13. 1% - Cardiovascular deaths: IAD 12. 8% - - - Continuous 16. 7% Qo. L - Overall no clinically relevant differences. Favourable for IAD in sexual function domains Continuous 15. 4% No clinically relevant difference Favourable for IAD in activity limitation, physical capacity and sexual functioning domains Intermittent hormone therapy: Not a standard for many urologists and oncologists. Why? Presented F. Calais Da Silva, Lisbon (PT) at ESOU 16 15 -17 Jan 2016 UK/URO 08412 No clinically relevant difference

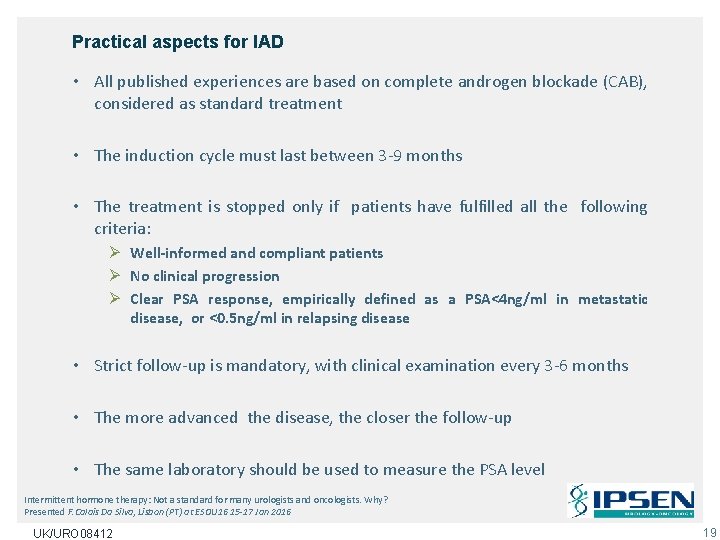
Practical aspects for IAD • All published experiences are based on complete androgen blockade (CAB), considered as standard treatment • The induction cycle must last between 3 -9 months • The treatment is stopped only if patients have fulfilled all the following criteria: Ø Well-informed and compliant patients Ø No clinical progression Ø Clear PSA response, empirically defined as a PSA<4 ng/ml in metastatic disease, or <0. 5 ng/ml in relapsing disease • Strict follow-up is mandatory, with clinical examination every 3 -6 months • The more advanced the disease, the closer the follow-up • The same laboratory should be used to measure the PSA level Intermittent hormone therapy: Not a standard for many urologists and oncologists. Why? Presented F. Calais Da Silva, Lisbon (PT) at ESOU 16 15 -17 Jan 2016 UK/URO 08412 19

• Treatment is resumed when the patient reaches either a clinical progression, or PSA above a predetermined, empirically fixed, threshold • Usually 4 -10 ng/ml in non-metastatic or 10 -20 ng/ml in metastatic cases. The same treatment is used for at least 3 -6 months • Subsequent cycles of treatment are based on the same rules until the first sign is seen of a castrate-resistant status • The best population to consider for IAD has still to be fully characterised • However, the most important factor seems to be the patient’s response to the first cycle of IAD, the PSA level response, initial PSA • We are waiting for one randomised trial SEUG 9901 to mature to publication with 1544 patients and statistical power and Qo. L to give more information UK/URO 08412 20

Conclusion • Seven extensively described phase 3 trials randomising 4675 patients to IAD vs continuous ADT • The induction periods ranged from 3 -8 months; in all but one trial, the PSA level designated for ADT discontinuation was 4 ng/ml. Mean follow-up ranged from 40 -108 months • Collectively, these trials support the concept that, mainly in metastatic cases, IAD can produce oncologic results similar to continuous ADT • In terms of overall survival, the hazard ratios for IAD and continuous ADT were very similar (range: 0. 98 -1. 08) • The Qo. L benefit of IAD appears to be modest at best • With IAD, Qo. L is likely influenced by the duration of the off-treatment periods and by the rate of testosterone recovery Intermittent hormone therapy: Not a standard for many urologists and oncologists. Why? Presented F. Calais Da Silva, Lisbon (PT) at ESOU 16 15 -17 Jan 2016 UK/URO 08412 21

Take home message • Intermittent androgen-deprivation therapy (ADT) can produce oncologic results similar (not inferior, as defined by some trials) to those of continuous ADT but with potentially better tolerability • Better stratification is needed in terms of prognostic parameters such as disease extension and Gleason score • It must be used in some patients Intermittent hormone therapy: Not a standard for many urologists and oncologists. Why? Presented F. Calais Da Silva, Lisbon (PT) at ESOU 16 15 -17 Jan 2016 UK/URO 08412 22

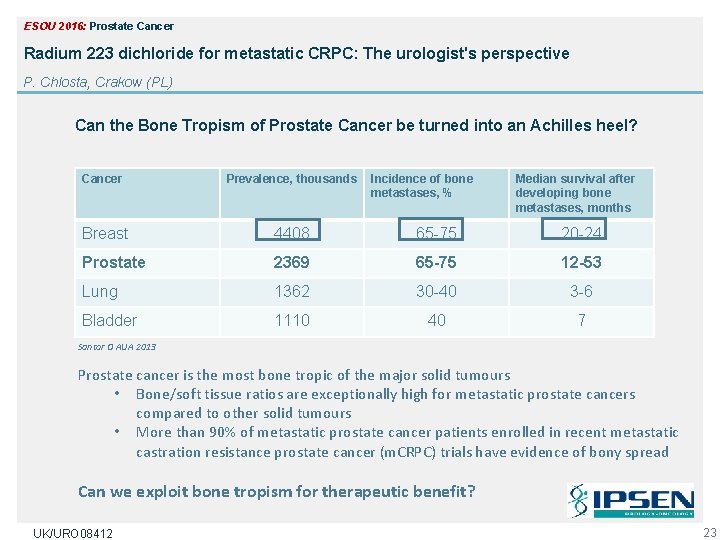
ESOU 2016: Prostate Cancer Radium 223 dichloride for metastatic CRPC: The urologist's perspective P. Chlosta, Crakow (PL) Can the Bone Tropism of Prostate Cancer be turned into an Achilles heel? Cancer Prevalence, thousands Incidence of bone metastases, % Median survival after developing bone metastases, months Breast 4408 65 -75 20 -24 Prostate 2369 65 -75 12 -53 Lung 1362 30 -40 3 -6 Bladder 1110 40 7 Santor O AUA 2013 Prostate cancer is the most bone tropic of the major solid tumours • Bone/soft tissue ratios are exceptionally high for metastatic prostate cancers compared to other solid tumours • More than 90% of metastatic prostate cancer patients enrolled in recent metastatic castration resistance prostate cancer (m. CRPC) trials have evidence of bony spread Can we exploit bone tropism for therapeutic benefit? UK/URO 08412 23

Radium-223 mechanism of action From Shore ND et al Urology, 2015: 85: 717 -724 Radium 223 dichloride for metastatic CRPC: The urologist’s perspective Presented P. Chlosta, Crakow (PL) at ESOU 16 15 -17 Jan 2016 UK/URO 08412 24

• Skeletal related events (SRE) represent a dramatic evolution of bone metastatic CRPC, with an estimated OS of 7 -10 months after first SRE. PCa is a biologically heterogeneous disease, so targeting osteoblasts rather than PCa cells is an appealing option • Radium-223 is a radioelement which is captured by osteoblasts and emits alpha radiation. The tissue penetration of alpha radiation is very low (<0. 1 mm), thus limiting toxicity on other cells than osteoblasts • The key results come from the ALSYMPCA study Primary end point: OS Key secondary end points • • Time to first symptomatic skeletal event (SSE) Changes in the PSA and alkaline phosphatase levels Safety Qo. L assessments SSEs composed the need to administer EBRT for bone pain New symptomatic pathologic bone fracture (vertebral or non-vertebral) Spinal cord compression Tumour related orthopaedic surgical intervention Radium 223 dichloride for metastatic CRPC: The urologist’s perspective Presented P. Chlosta, Crakow (PL) at ESOU 16 15 -17 Jan 2016 UK/URO 08412 25

ALSYMPCA (ALpharadin in SYMptomatic Prostate Cancer) Phase 3 Study Design PATIENTS TREATMENT STRATIFICATION • Confirmed Symptomatic CRPC • ≥ 2 bone metastases • No known visceral metastases • Post-docetaxel, unfit for docetaxel, or refused docetaxel • Total ALP: < 220 U/L vs ≥ 220 U/L • Bisphonate use: Yes vs No • Prior docetaxel: Yes vs No RANDOMIZED 6 injections at 4 -week intervals 2: 1 Planned follow-up is 3 years Radium-223 (50 k. Bq/kg) + Best standard of care Placebo (saline) + Best standard of care N=921 Parker C, Nilsson S, Heinrich D and al. N Engl J Med. 2013 Jul 18; 369(3): 213 -23 Radium 223 dichloride for metastatic CRPC: The urologist’s perspective Presented P. Chlosta, Crakow (PL) at ESOU 16 15 -17 Jan 2016 UK/URO 08412 26

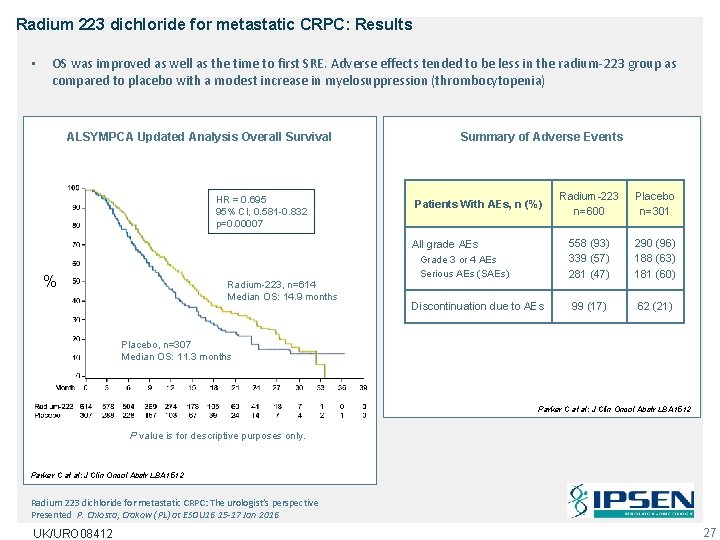
Radium 223 dichloride for metastatic CRPC: Results • OS was improved as well as the time to first SRE. Adverse effects tended to be less in the radium-223 group as compared to placebo with a modest increase in myelosuppression (thrombocytopenia) ALSYMPCA Updated Analysis Overall Survival HR = 0. 695 95% CI, 0. 581 -0. 832 p=0. 00007 Summary of Adverse Events Patients With AEs, n (%) All grade AEs % Radium-223, n=614 Median OS: 14. 9 months Grade 3 or 4 AEs Serious AEs (SAEs) Discontinuation due to AEs Radium-223 n=600 Placebo n=301 558 (93) 339 (57) 281 (47) 290 (96) 188 (63) 181 (60) 99 (17) 62 (21) Placebo, n=307 Median OS: 11. 3 months Parker C at al: J Clin Oncol Abstr LBA 1512 P value is for descriptive purposes only. Parker C at al: J Clin Oncol Abstr LBA 1512 Radium 223 dichloride for metastatic CRPC: The urologist’s perspective Presented P. Chlosta, Crakow (PL) at ESOU 16 15 -17 Jan 2016 UK/URO 08412 27

ALSYMPCA Conclusions In CRPC patients with bone metastases • Only one compound, radium-223 significantly prolongs overall survival Ø P value = 0. 00185; HR = 0. 695; 95% CI, 0. 552 -0. 875 • Radium-223 significantly prolonged time to first clinically relevant SRE Ø P value = 0. 00046; HR = 0. 610; 95% CI 0. 461 -0. 807 • Radum-223 was very well tolerated UK/URO 08412 28

Radium 223 dichloride for metastatic CRPC: Combination • Interestingly, the effects of ra-223 on SRE were potentiated by bisphonates Combine Effective Agents Time to SSE in patients With CRPC and Bone Metastases Ra 223 + bisphonates 100 Radium-223 (n=250): 19. 6 Placebo (n=124): 10. 2 HR 0. 49, p<0. 001 60 40 80 60 40 20 20 0 0 3 4 8 12 16 20 24 28 32 Months Radium-223 (n=364): 11. 8 Placebo (n=183): 8. 4 HR 0. 77, p=0. 068 100 Patients Without SSE, % 80 Patients Without SSE, % Ra 223 3 4 8 12 16 20 24 28 32 Months Sartor, Lancet Oncol 2014 The message was conveyed that urologists should cooperate with oncologists and become more involved in the management of new active agents in the treatment of HSPC and CRPC Radium 223 dichloride for metastatic CRPC: The urologist’s perspective Presented P. Chlosta, Crakow (PL) at ESOU 16 15 -17 Jan 2016 UK/URO 08412 29

Overall conclusions • Urologist can effectively manage advance prostate cancer • Radium-223 joins immunotherapy, novel hormonal agents and CHTH • Multidisciplinary coordination while monitoring patient during and after treatment • After decades of effort, a bone targeted approach has been demonstrated in phase III to prolong survival • Many new challenges exist, but how to combine effective agents for maximal benefit is one of the next clear areas of focus Radium 223 dichloride for metastatic CRPC: The urologist’s perspective Presented P. Chlosta, Crakow (PL) at ESOU 16 15 -17 Jan 2016 UK/URO 08412 30

Bladder Cancer UK/URO 08412

ESOU 2016: Bladder Cancer Alternative adjuvant treatments for the NMIBC in the BCG shortage era J Palou, Barcelona (ES) • Bacille Calmette-Guerin (BCG) is a well established treatment for intermediate and high risk non-muscle invasive bladder cancer • In patients with high risk tumours associated to CIS in the absence of BCG it is recommended to undergo a radical cystectomy • In terms of medical treatments, potential treatments are summarised BCG: an established treatment • BCG is a well established treatment for intermediate and high risk non-muscle invasive bladder cancer • It is not perfect, since there are recurrences and progression, but is the most effective treatment in patients with CIS and/or high grade bladder cancer UK/URO 08412 32

Clinical practice recommendations for the management of non-muscle invasive bladder cancer Lamm D et al Eur J Urol Suppl 7 2008 Alternative adjuvant treatments for the NMIBC in the BCG shortage era Presented J Palou, Barcelona (ES) at ESOU 16 15 -17 Jan 2016 UK/URO 08412 33

The problem… In July 2012: Sanofi-Pasteur announced that they were halting production of Immuncyst, the Connaught strain of BCG, after inspectors found mould in the sterile manufacturing area of their Toronto plant following a previous flood What has been the problem with such a BCG shortage? Patients are receiving and still in some countries, suboptimal courses or no BCG, that may result in higher recurrence rates and, for the very highest risk cases, higher progression rates Alternative adjuvant treatments for the NMIBC in the BCG shortage era Presented J Palou, Barcelona (ES) at ESOU 16 15 -17 Jan 2016 UK/URO 08412 34

Treatment options in BCG failure • • • Mitomycin: only 19% disease free at 3 years Valrubicin: complete response of 21% Interferon alfa 2 b: nothing Gemcitabine: only 50% complete response at 3 months Docetaxel: 22% free of disease at 3 years Paclitaxel: 50% response at 2 years DAT: Device assisted therapy • The availability of DAT is a possibility with promising results in intermediate risk, high risk and in BCG failures • Local availability of some DAT will vary from country to country and by hospital • The combination of EMDA or hyperthermia with mitomycin offer better results UK/URO 08412 36

Alternative adjuvant treatments for the NMIBC in the BCG shortage era: Summary • Local microwave (RF) induced hyperthermia with MMC (SYNERGO, RITE) have produced promising results , but more scientific evidence is needed and there is an increase in side effects as compared to MMC alone. Chemotherapy and intravesical hyperthermia • • • Systematic review: 22 studies. SYNERGO Only 2 RCT 59% lower recurrence with C-HT than with MMC only 87. 6% bladder preservation Inconclusive in regards to recurrence and progression time Indications: • High grade patients with recurrent tumours, no candidates to radical cystectomy (RC) • High risk patients with recurrence and with contraindication for BCG Alternative adjuvant treatments for the NMIBC in the BCG shortage era Presented by J. Palou, Barcelona (ES) at ESOU 16 15 -17 Jan 2016 UK/URO 08412 37

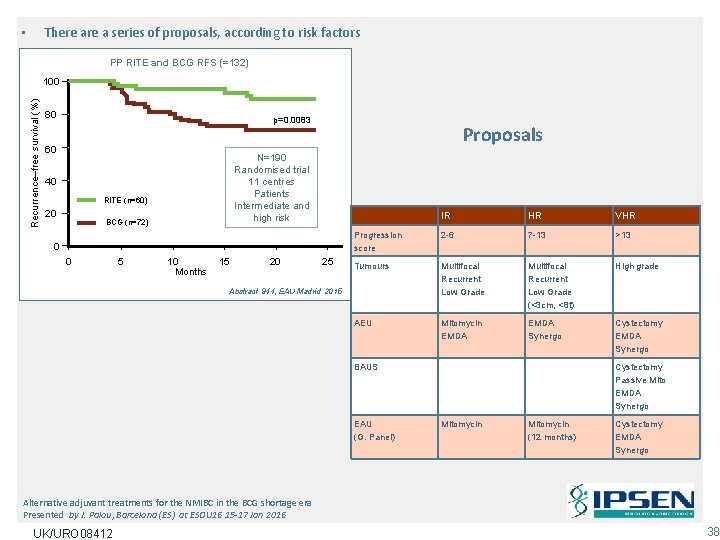
There a series of proposals, according to risk factors • PP RITE and BCG RFS (=132) Recurrence–free survival (%) 100 80 p=0. 0083 60 N=190 Randomised trial 11 centres Patients Intermediate and high risk 40 RITE (n=60) 20 Proposals BCG (n=72) 0 0 5 10 Months 15 20 25 IR HR VHR Progression score 2 -6 7 -13 >13 Tumours Multifocal Recurrent Low Grade (<3 cm, <8 t) High grade AEU Mitomycin EMDA Synergo Cystectomy EMDA Synergo Abstract 944, EAU Madrid 2015 BAUS EAU (G. Panel) Cystectomy Passive Mito EMDA Synergo Mitomycin (12 months) Cystectomy EMDA Synergo Alternative adjuvant treatments for the NMIBC in the BCG shortage era Presented by J. Palou, Barcelona (ES) at ESOU 16 15 -17 Jan 2016 UK/URO 08412 38

Therapeutic Options in High-risk Non-muscle-invasive Bladder Cancer During the Current Worldwide Shortage of Bacille Calmette-Guerin Hugh Mostafid, Joan Palou Redorta, Richard Sylvester, J. Alfred Witjes 1 - No maintenance 2 - 1/3 of the dose 3 - IR: mitomycin + maintenance 4 - Chemohyperthermia in high risk 5 - Gemcitabine 6 - Radical Cystectomy • Discussion suggested that on-going trials with PD-1/PD-L 1 agents might profoundly change the landscape Alternative adjuvant treatments for the NMIBC in the BCG shortage era Presented by J. Palou, Barcelona (ES) at ESOU 16 15 -17 Jan 2016 UK/URO 08412

Conclusions • No specific treatment for NMIBC due to BCG shortage • Different variables are going to influence the final decision • The combined decision of the clinician and the patient will determine the most theoretically beneficial treatment Alternative adjuvant treatments for the NMIBC in the BCG shortage era Presented by J. Palou, Barcelona (ES) at ESOU 16 15 -17 Jan 2016 UK/URO 08412 40

Questions? UK/URO 08412
 Patricia walcott
Patricia walcott Tongue rough edges
Tongue rough edges Norline metivier
Norline metivier Xiv derek walcott meaning
Xiv derek walcott meaning Aisha walcott
Aisha walcott Kristen walcott
Kristen walcott Chapter 29 the child with a genitourinary condition
Chapter 29 the child with a genitourinary condition Chapter 29 the child with a genitourinary condition
Chapter 29 the child with a genitourinary condition Genitourinary & stds
Genitourinary & stds Male genitourinary anatomy
Male genitourinary anatomy Nursing care of male patients with genitourinary disorders
Nursing care of male patients with genitourinary disorders Dieu veut des soldats fideles
Dieu veut des soldats fideles Function of prostate gland
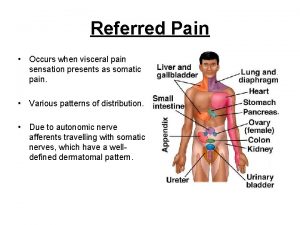
Function of prostate gland Prostate referred pain
Prostate referred pain Prostate
Prostate Normal weight of prostate
Normal weight of prostate Prostate
Prostate Viscerosomatic reflex
Viscerosomatic reflex Pirad 4 lesion prostate
Pirad 4 lesion prostate Benign and malignant tumors
Benign and malignant tumors Irm de prostate
Irm de prostate Prostate pathology
Prostate pathology Prostate histology
Prostate histology Tuip prostate
Tuip prostate Alfazusin
Alfazusin Base of prostate gland
Base of prostate gland Prostate cancer staging
Prostate cancer staging Prostate pathology
Prostate pathology Prostate mri pi rads
Prostate mri pi rads Spongy urethra histology
Spongy urethra histology Mdv3100 prostate cancer
Mdv3100 prostate cancer Laprp
Laprp Prostate
Prostate Prostate cancer tnm classification
Prostate cancer tnm classification Male reproductive system
Male reproductive system Prostate
Prostate Hormonothérapie cancer prostate
Hormonothérapie cancer prostate Adenocarcinome prostate
Adenocarcinome prostate Bahasa medis penis
Bahasa medis penis Irm prostate
Irm prostate