Genetics and epigenetics of NAFLD and NASH Sameh













- Slides: 35

Genetics and epigenetics of NAFLD and NASH Sameh Lashen Lecturer of Hepatology University of Alexandria


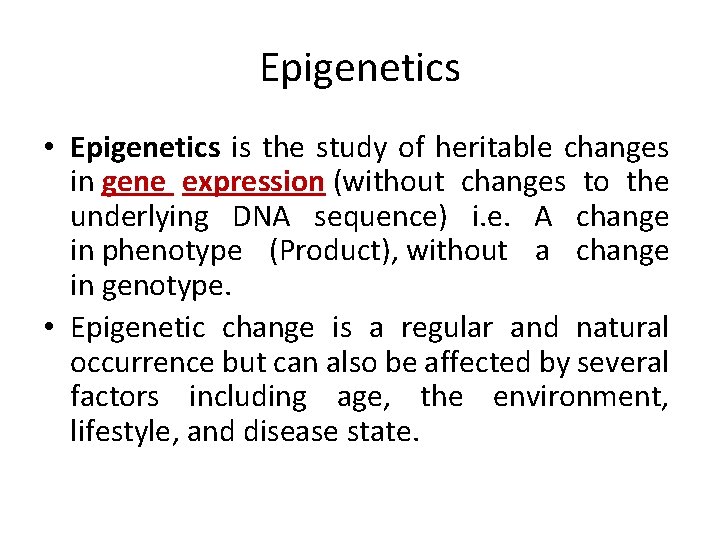
Epigenetics • Epigenetics is the study of heritable changes in gene expression (without changes to the underlying DNA sequence) i. e. A change in phenotype (Product), without a change in genotype. • Epigenetic change is a regular and natural occurrence but can also be affected by several factors including age, the environment, lifestyle, and disease state.



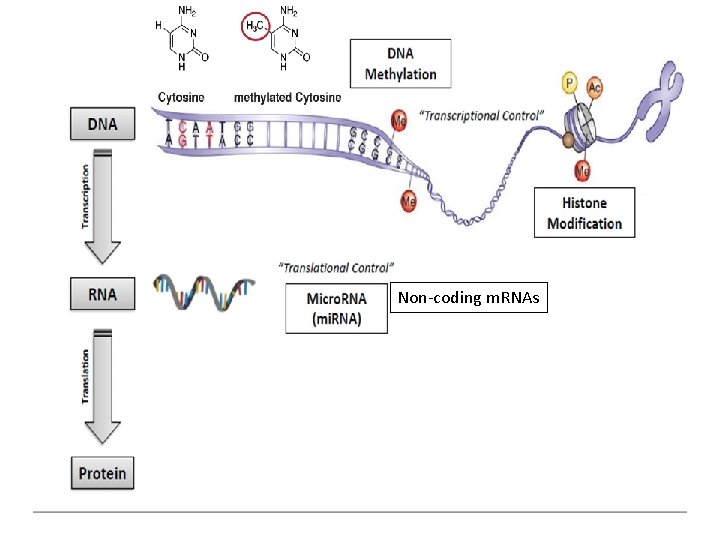
Non-coding m. RNAs

s e g n a h c c i t ne e G Non-coding mi. RNAs Environmental factors


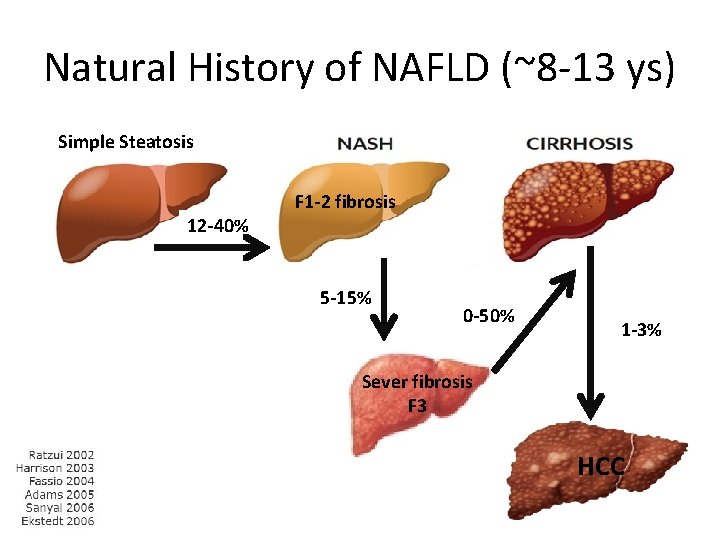
Natural History of NAFLD (~8 -13 ys) Simple Steatosis 12 -40% F 1 -2 fibrosis 5 -15% 0 -50% 1 -3% Sever fibrosis F 3 HCC

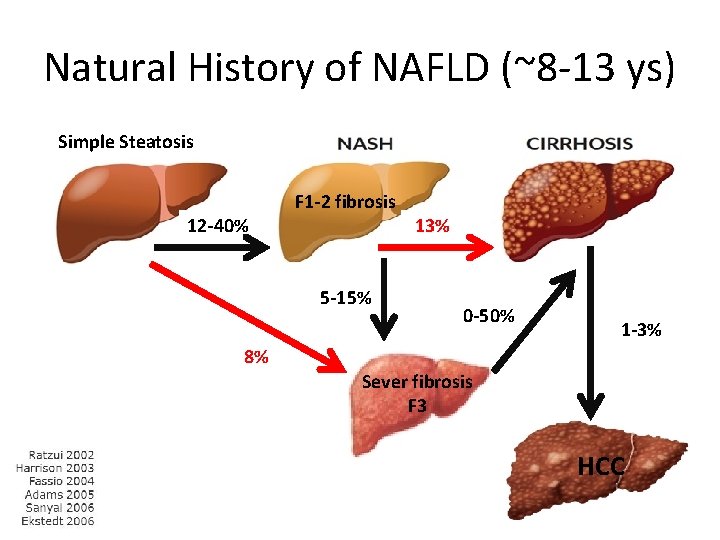
Natural History of NAFLD (~8 -13 ys) Simple Steatosis 12 -40% F 1 -2 fibrosis 5 -15% 13% 0 -50% 1 -3% 8% Sever fibrosis F 3 HCC

Natural History of NAFLD (~8 -13 ys) Simple Steatosis 12 -40% F 1 -2 fibrosis 5 -15% 13% 0 -50% 1 -3% 8% ? ? 0. 04 -0. 3% Sever fibrosis F 3 ? ? % HCC

1 st degree relatives

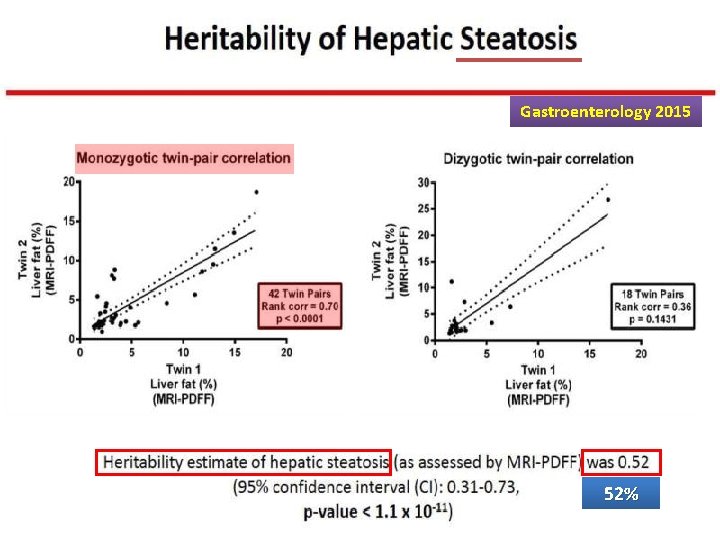
Gastroenterology 2015 52%

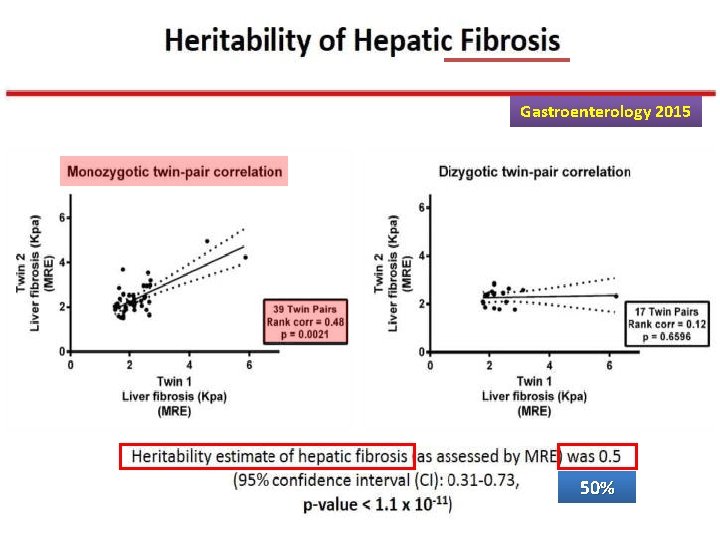
Gastroenterology 2015 50%

Heritability of NAFLD Overall, these data suggest that approximately half of hepatic fat content variability is explained by genetic and epigenetic factors, which also determine the risk of metabolic disease and hepatic fibrosis.

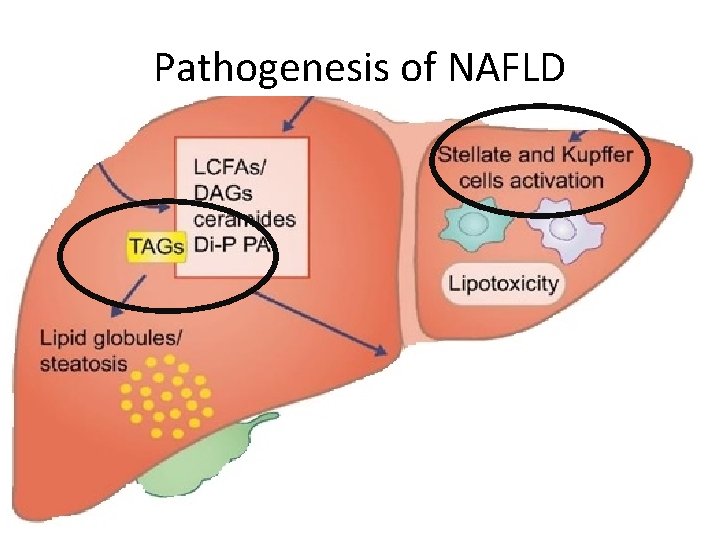
Pathogenesis of NAFLD

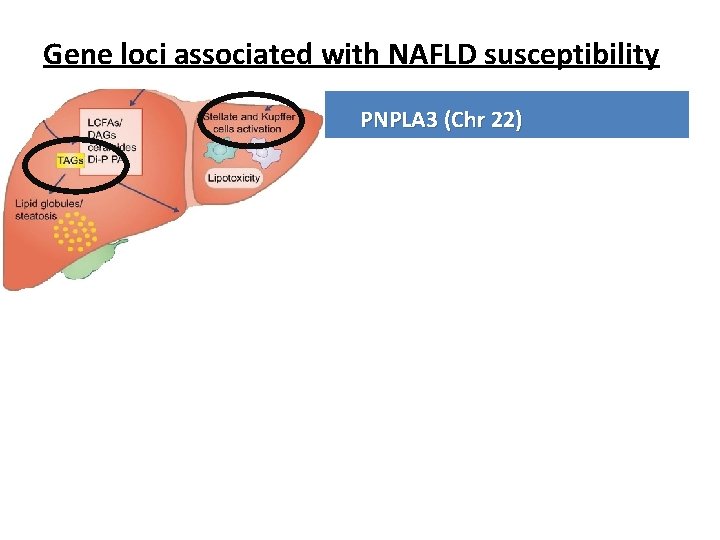
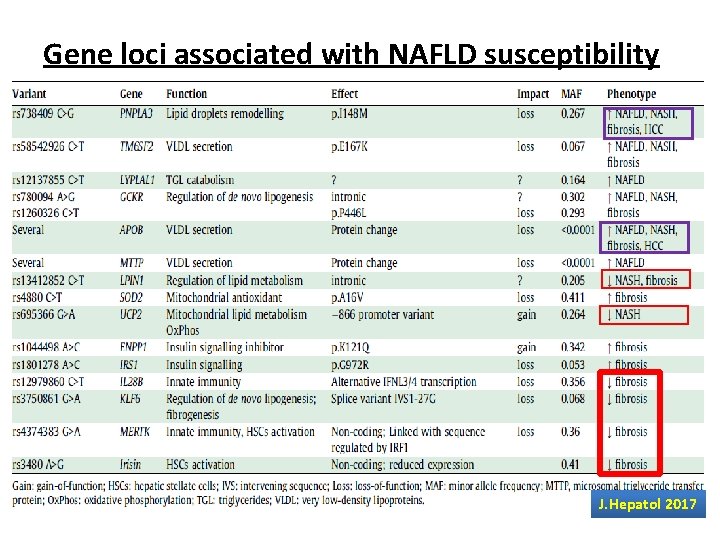
Gene loci associated with NAFLD susceptibility Gene PNPLA 3 (Chr 22) Normal Code for protein involved in lipid hydrolysis function (hydrolase activity for TG) Mutation Loss of function [ Isoleucin Methionin at 148] changes Molecular Entrapment of TG inside hepatocytes and stellate effect cells hepatic injury and fibrosis progression Clinical In homozygous, NAFLD progression and X 10 impact increase in HCC risk

Gene loci associated with NAFLD susceptibility Gene Trans-membrane 6 superfamily member 2 (TM 6 SF 2)… (Chr 19) Normal Protein which incorporates Apo-lipoprotein B 100 function +TG into VLDV push outside the liver Mutation Loss of function changes Molecular Entrapment of TG inside the liver effect Low circulating lipoproteins Clinical NAFLD progression impact Low risk of cardiovascular diseases

Gene loci associated with NAFLD susceptibility Gene Membrane bound O-acyl-transferase domain containing 7 (MBOAT 7) Normal Protein involved in phosphatidylinositol supply to function hepatocytes Mutation Loss of function changes Molecular reduced levels of phosphatidyl-inositol containing arachidonic acid in hepatocytes and the circulation effect Clinical impact Higher risk of NAFL , NASH and fibrosis. Higher risk of progression to HCC. J. Hepatol 2017

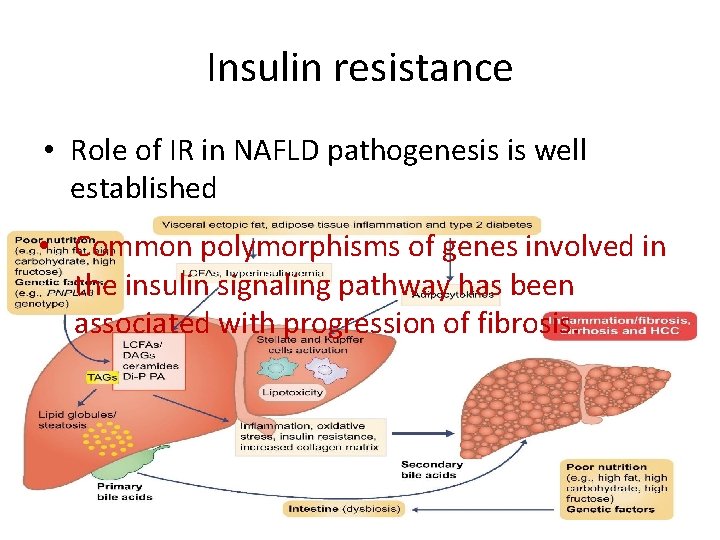
Insulin resistance • Role of IR in NAFLD pathogenesis is well established • Common polymorphisms of genes involved in the insulin signaling pathway has been associated with progression of fibrosis.

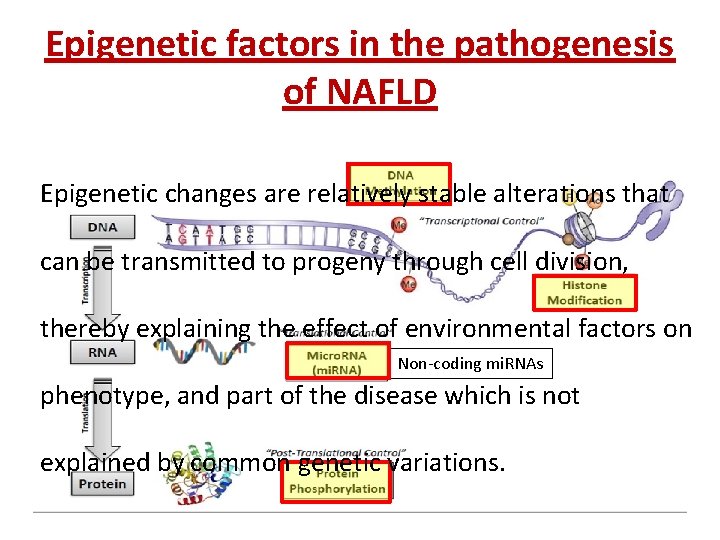
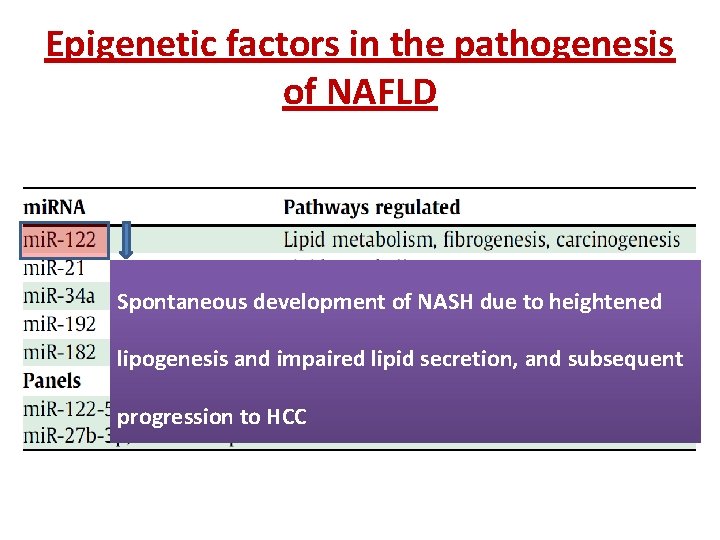
Epigenetic factors in the pathogenesis of NAFLD Epigenetic changes are relatively stable alterations that can be transmitted to progeny through cell division, thereby explaining the effect of environmental factors on Non-coding mi. RNAs phenotype, and part of the disease which is not explained by common genetic variations.

Epigenetic factors in the pathogenesis of NAFLD A classic demonstration of the role of epigenetic factors in modulating an individual’s susceptibility to NAFLD is represented by the effect of intrauterine exposure to high-fat diet (HFD) in inbred mouse strains

Epigenetic factors in the pathogenesis of NAFLD This not only worsen visceral fat accumulation and insulin resistance after birth, but it also results in more severe hepatic fat accumulation and the development of NASH.

Epigenetic factors in the pathogenesis of NAFLD The same effect occurs in human where liver damage due to fetal exposure to high fat diet interferes with cell cycle regulation in hepatocytes because of p 21 induction by methylation.

Epigenetic factors in the pathogenesis of NAFLD In line with these data, increased hepatic methylation of PPAR-γ, involved in mitochondrial biogenesis of enzymes involved in oxidative phosphorylation insulin resistance, and NAFLD progression.

Epigenetic factors in the pathogenesis of NAFLD Hypo-methylation of fibrogenic TGF-b 1 and PDGF-a has been associated with rapid progression of NAFLD

Epigenetic factors in the pathogenesis of NAFLD Spontaneous development of NASH due to heightened lipogenesis and impaired lipid secretion, and subsequent progression to HCC

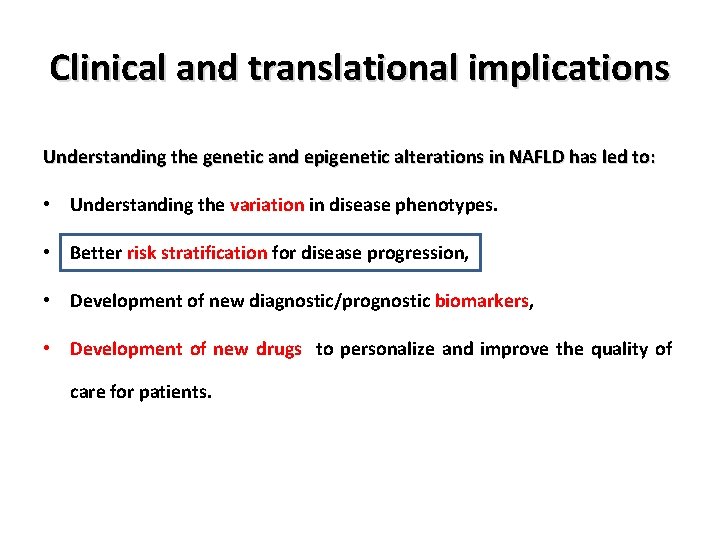
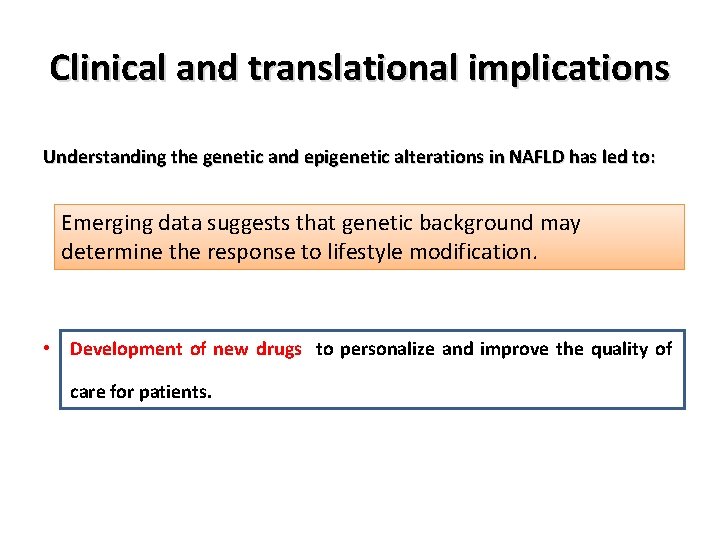
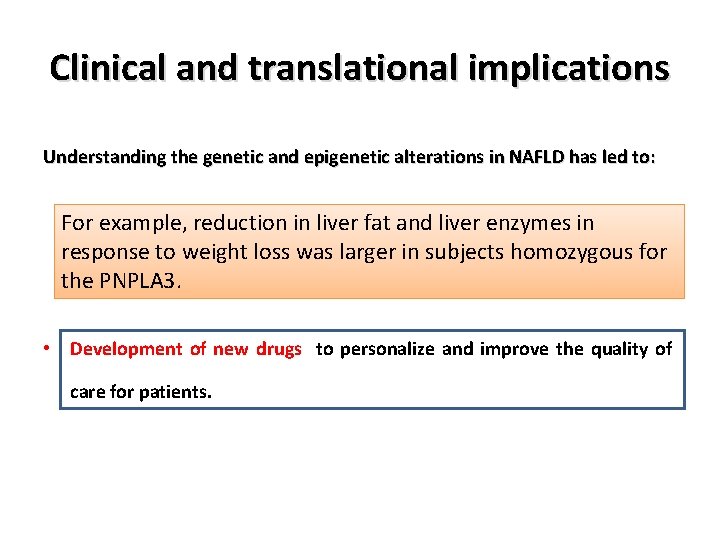
Clinical and translational implications Understanding the genetic and epigenetic alterations in NAFLD has led to: • Understanding the variation in disease phenotypes. • Better risk stratification for disease progression, • Development of new diagnostic/prognostic biomarkers, • Development of new drugs to personalize and improve the quality of care for patients.

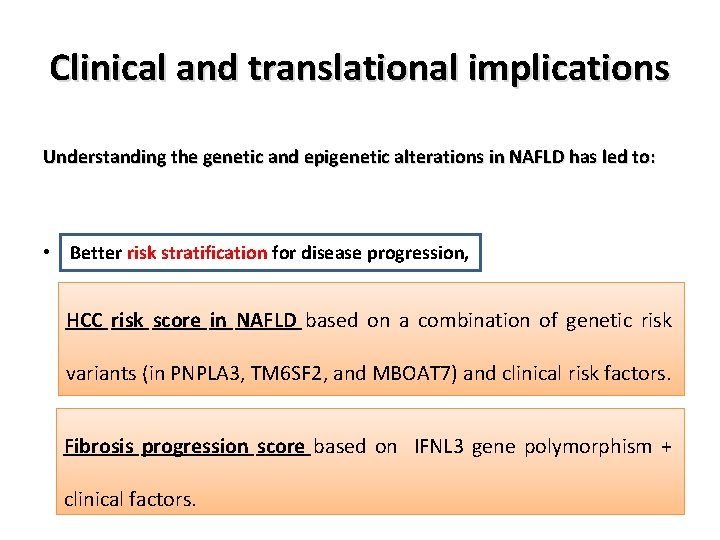
Clinical and translational implications Understanding the genetic and epigenetic alterations in NAFLD has led to: • Understanding the variation in disease phenotypes. • Better risk stratification for disease progression, • Development of new diagnostic/prognostic biomarkers, HCC risk score in NAFLD based on a combination of genetic risk • Development of new drugs to personalize and improve the quality of variants (in PNPLA 3, TM 6 SF 2, and MBOAT 7) and clinical risk factors. care for patients. Fibrosis progression score based on IFNL 3 gene polymorphism + clinical factors.

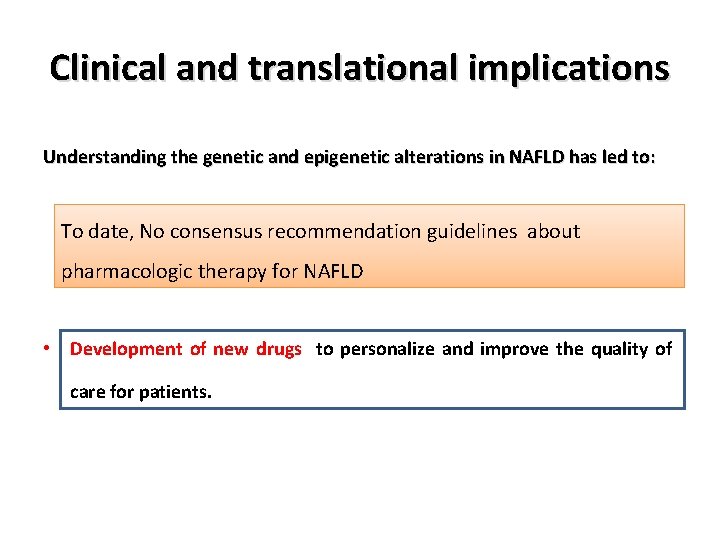
Clinical and translational implications Understanding the genetic and epigenetic alterations in NAFLD has led to: • Understanding the variation in disease phenotypes. To date, No consensus recommendation guidelines about • Better risk stratification for disease progression, pharmacologic therapy for NAFLD • Development of new diagnostic/prognostic biomarkers, • Development of new drugs to personalize and improve the quality of care for patients.

Clinical and translational implications Understanding the genetic and epigenetic alterations in NAFLD has led to: • Understanding the variation in disease phenotypes. Emerging data suggests that genetic background may • determine the response to lifestyle modification. Better risk stratification for disease progression, • Development of new diagnostic/prognostic biomarkers, • Development of new drugs to personalize and improve the quality of care for patients.

Clinical and translational implications Understanding the genetic and epigenetic alterations in NAFLD has led to: • Understanding the variation in disease phenotypes. For example, reduction in liver fat and liver enzymes in • response to weight loss was larger in subjects homozygous for Better risk stratification for disease progression, the PNPLA 3. • Development of new diagnostic/prognostic biomarkers, • Development of new drugs to personalize and improve the quality of care for patients.

Clinical and translational implications Understanding the genetic and epigenetic alterations in NAFLD has led to: • Understanding the variation in disease phenotypes. More recently, down-regulation of the mutated PNPLA 3 holds promise for the treatment of liver diseases • protein Better risk stratification for disease progression, associated with steato-hepatitis. • Development of new diagnostic/prognostic biomarkers, • Development of new drugs to personalize and improve the quality of care for patients.


Thank You
 Sameh elnikety
Sameh elnikety Histone modification epigenetics
Histone modification epigenetics What is epigenetics
What is epigenetics Elisabetta bugianesi
Elisabetta bugianesi Nafld summit 2019
Nafld summit 2019 Nafld
Nafld Nafld
Nafld Brewer and nash model
Brewer and nash model To my valentine by ogden nash
To my valentine by ogden nash Nash-evenwicht
Nash-evenwicht Pure-strategy nash equilibria
Pure-strategy nash equilibria Equilibrio de nash
Equilibrio de nash Ottimo paretiano teoria dei giochi
Ottimo paretiano teoria dei giochi Meeliftersgedrag economie
Meeliftersgedrag economie Almost essential
Almost essential Nash gleichgewicht
Nash gleichgewicht Cystic hygroma
Cystic hygroma Nash evenwicht
Nash evenwicht Contoh zhahir
Contoh zhahir Equilibrio de nash
Equilibrio de nash Joan nash
Joan nash Théorie des jeux
Théorie des jeux Stag hunt vs prisoner's dilemma
Stag hunt vs prisoner's dilemma Dominant strategy economics
Dominant strategy economics Nash equilibrium example
Nash equilibrium example Frank jelly
Frank jelly Esercizi equilibrio di nash
Esercizi equilibrio di nash Neurona motora
Neurona motora Solucion del ejercicio
Solucion del ejercicio Nash-evenwicht
Nash-evenwicht Dominant strategy
Dominant strategy Subgame perfect nash equilibrium
Subgame perfect nash equilibrium Adam nash designer baby
Adam nash designer baby Pure-strategy nash equilibria
Pure-strategy nash equilibria Pure strategy nash equilibrium
Pure strategy nash equilibrium Var
Var