Genetic Testing for Melanoma Risk Stratification Sancy Leachman















- Slides: 26

Genetic Testing for Melanoma Risk Stratification Sancy Leachman, MD, Ph. D Assistant Professor Huntsman Cancer Institute and the Department of Dermatology University of Utah Health Sciences Center Pacific Dermatologic Association August 9, 2008 1

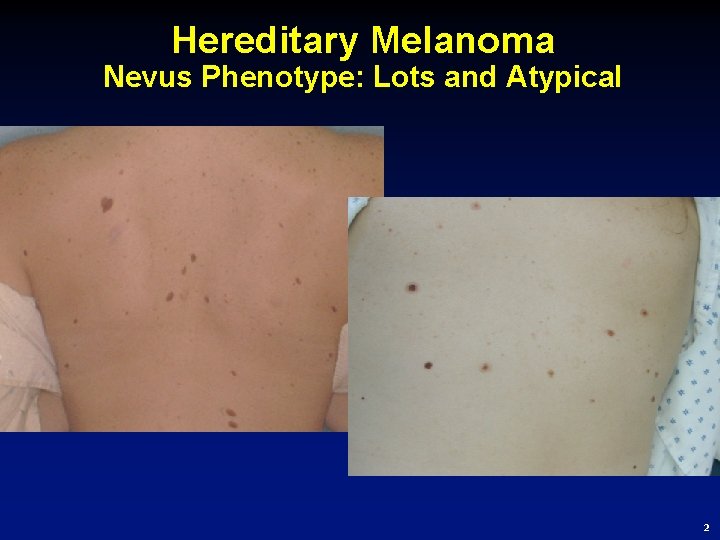
Hereditary Melanoma Nevus Phenotype: Lots and Atypical 2

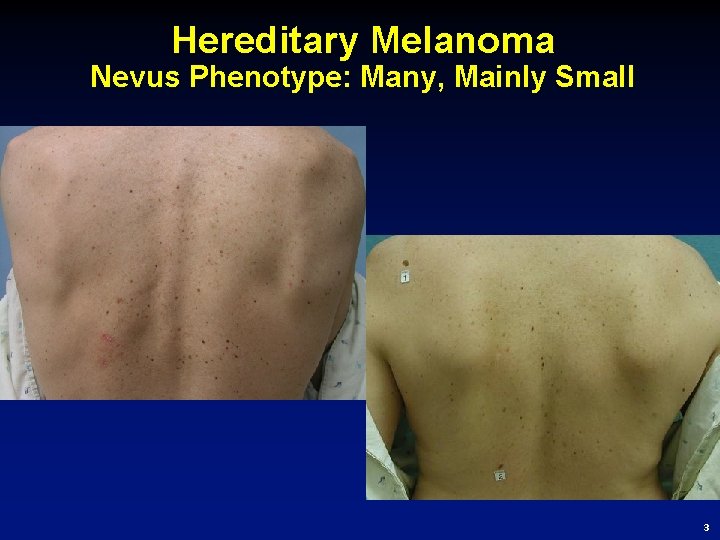
Hereditary Melanoma Nevus Phenotype: Many, Mainly Small 3

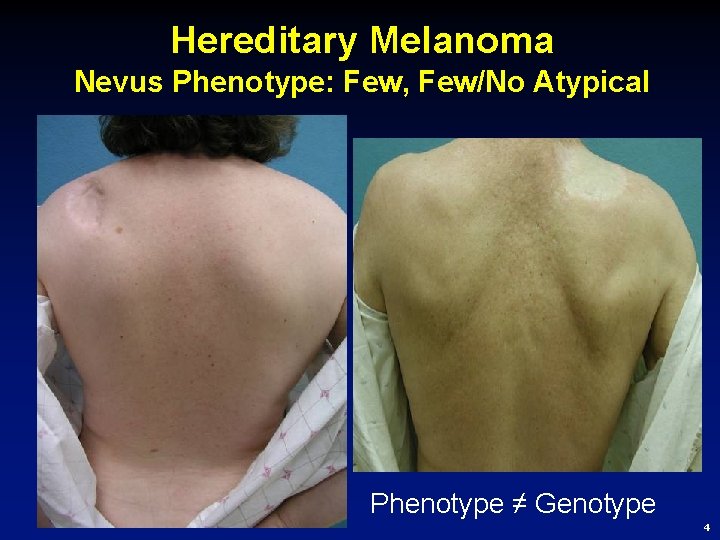
Hereditary Melanoma Nevus Phenotype: Few, Few/No Atypical Phenotype ≠ Genotype 4

Other Phenotypic Markers “Constitutional” Risk 5

Wild-Type and Homozygous “R” Variant MC 1 R 6

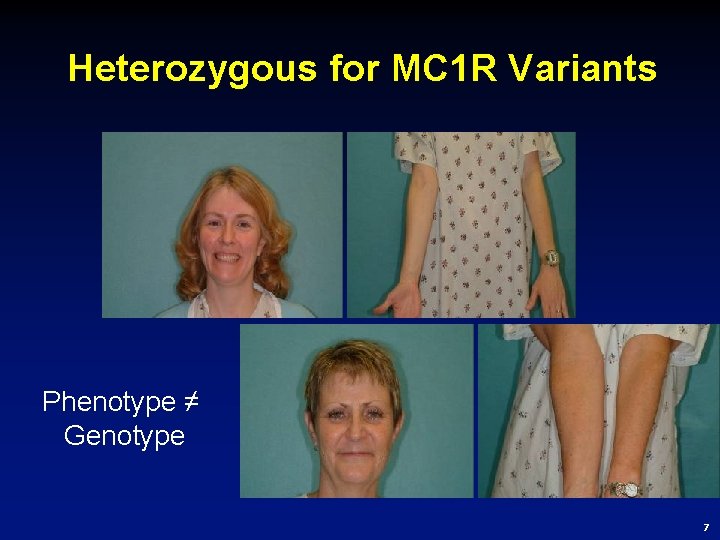
Heterozygous for MC 1 R Variants Phenotype ≠ Genotype 7

• Relative Factor M e m b e r Risk Estimation Family History is Greatest Risk o f m e l a n o m a f a m i l y Kefford RF, et al. J Clin Oncol. 1999; 17: 3245 -3251. 8

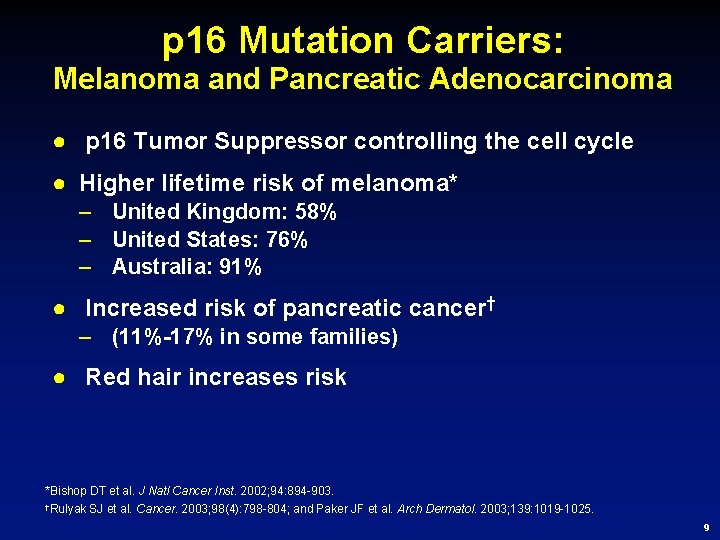
p 16 Mutation Carriers: Melanoma and Pancreatic Adenocarcinoma ● p 16 Tumor Suppressor controlling the cell cycle ● Higher lifetime risk of melanoma* – United Kingdom: 58% – United States: 76% – Australia: 91% ● Increased risk of pancreatic cancer† – (11%-17% in some families) ● Red hair increases risk *Bishop DT et al. J Natl Cancer Inst. 2002; 94: 894 -903. †Rulyak SJ et al. Cancer. 2003; 98(4): 798 -804; and Paker JF et al. Arch Dermatol. 2003; 139: 1019 -1025. 9

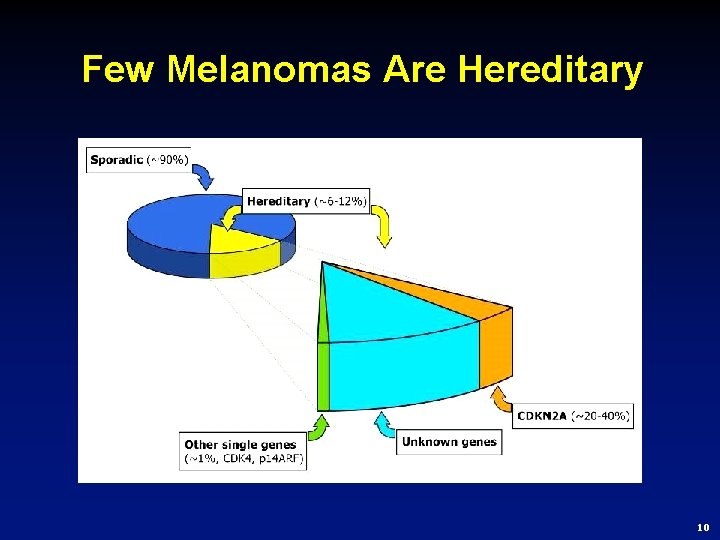
Few Melanomas Are Hereditary 10

Case Presentation ● A dermatologist from Kentucky contacted our genetic counseling group to discuss how to perform genetic testing on a patient: – 64 y/o nurse practitioner with dysplastic nevus syndrome, a personal history of melanoma, no children – No known family history of melanoma or pancreatic cancer – Patient wishes to have p 16 genetic testing performed on a self-pay basis – Patient is interested in participating in available research protocols for melanoma 11

Should Clinical Genetic Testing be Offered to This Patient? 1. Yes 2. No 3. Maybe 12

Issues for Consideration ● Statistically, the patient has only between a 0. 2%-2. 0% risk of carrying a p 16 mutation (Aitken et al, and Begg et al) ● The patient does NOT have features suggestive of elevated risk for carriage of a p 16 mutation (Hansen et al. Lancet Oncology. 2004) – Multiple primary melanomas – Two or more other family members with melanoma – Family member with pancreatic cancer ● Because she has already had melanoma, there is little chance that a positive or negative test result will alter prevention, early detection, management, or follow-up recommendations ● She expressed the desire for testing and willingness to pay ● Knowledge of status may provide psychological benefit to her because of her curiosity and professional background ● If p 16 -positive (unlikely), other family members could be tested and pancreatic cancer screening (if available) could be offered ● Research protocols are available to p 16 mutation-tested individuals 13

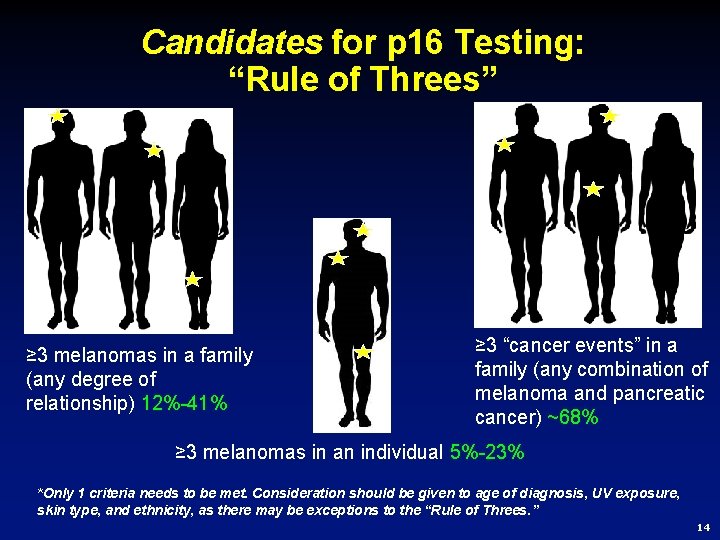
Candidates for p 16 Testing: “Rule of Threes” ≥ 3 melanomas in a family (any degree of relationship) 12%-41% ≥ 3 “cancer events” in a family (any combination of melanoma and pancreatic cancer) ~68% ≥ 3 melanomas in an individual 5%-23% *Only 1 criteria needs to be met. Consideration should be given to age of diagnosis, UV exposure, skin type, and ethnicity, as there may be exceptions to the “Rule of Threes. ” 14

Should Clinical Genetic Testing be Offered to This Patient? 1. Yes 2. No 3. Maybe Answer: Based on typical criteria used to evaluate a patient for genetic testing, the answer is “NO. ” However, as with all areas of medicine, there is “art” involved. 15

Case Presentation ● 32 y/o woman with numerous clinically atypical nevi ● No personal history of melanoma ● Confirmed family history of invasive melanoma in 2 of 6 siblings, and 2 paternal uncles. ● Her father died from metastatic pancreatic carcinoma ● She has 3 children (ages 10, 8, and 6 years) ● Two children have clinically atypical nevi ● She is NOT interested in participating in an available research protocol for familial melanoma ● She wishes to have p 16 genetic testing performed 16

Should Clinical Genetic Testing be Offered to This Patient? 1. Yes 2. No 3. Maybe 17

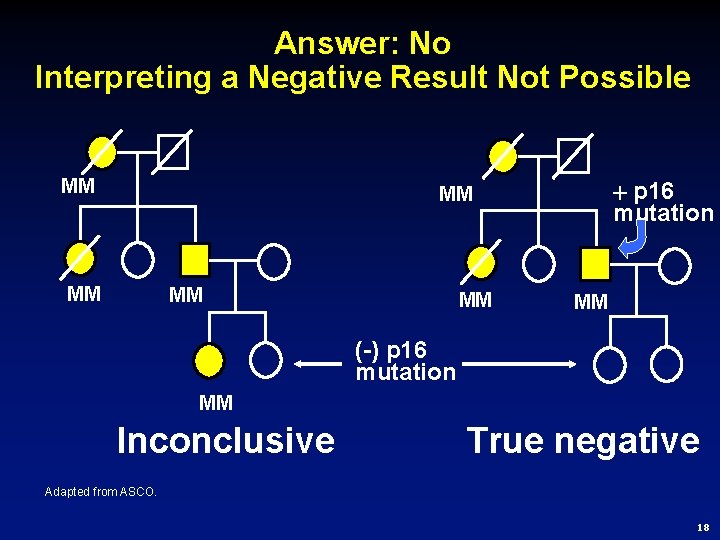
Answer: No Interpreting a Negative Result Not Possible MM p 16 mutation MM MM MM (-) p 16 mutation MM Inconclusive True negative Adapted from ASCO. 18

Should an Affected Member of the Family be Tested? 1. Yes 2. No 3. Maybe 19

Issues for Consideration ● Statistically, the patient’s family has a greater than 50% risk of carrying a p 16 mutation ● Carriers in her family are also likely to be predisposed to pancreatic cancer ● She expressed the desire for testing ● If she is not interested in clinical research, there will be little change in management ● Knowledge of status may provide psychological benefit, especially if negative ● Knowledge of status may permit lifestyle change and rigorous adherence to prevention and early detection strategies in her children if positive ● Summary: Benefit of knowledge, little to no risk ● A family member would be tested in our institution if desired 20

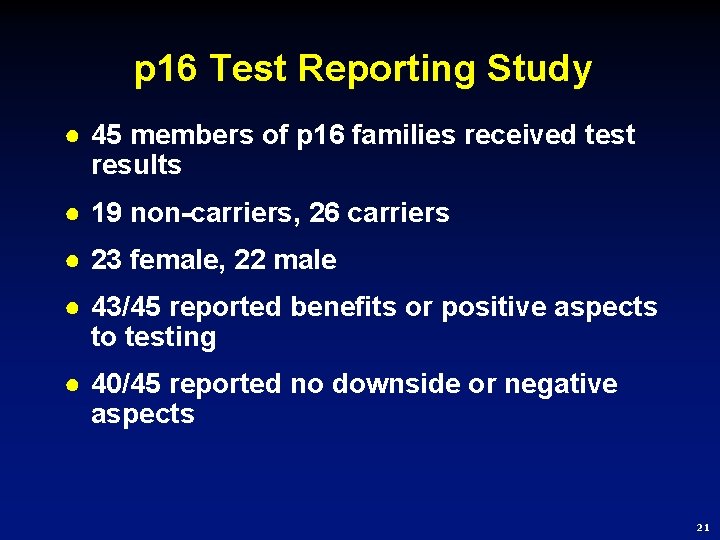
p 16 Test Reporting Study ● 45 members of p 16 families received test results ● 19 non-carriers, 26 carriers ● 23 female, 22 male ● 43/45 reported benefits or positive aspects to testing ● 40/45 reported no downside or negative aspects 21

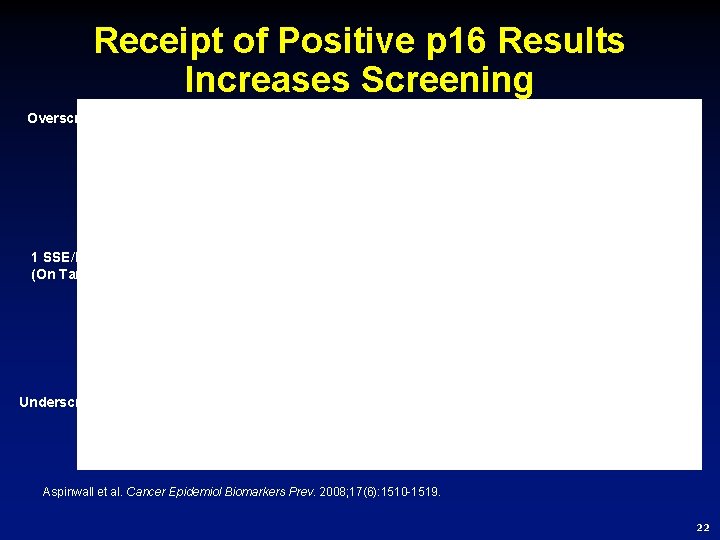
Receipt of Positive p 16 Results Increases Screening Overscreeners P <. 0003 n. s. 1 SSE/Month (On Target) P <. 023 Underscreeners Aspinwall et al. Cancer Epidemiol Biomarkers Prev. 2008; 17(6): 1510 -1519. 22

How Should it be Done? ● Identify high-risk patients ● Get Help First Time Around!! § Refer to a research protocol § Consult a clinical genetic testing center • Find a local center: www. nsgc. org • www. cancer. gov (National Cancer Institute) • Huntsman Cancer Institute: wendy. kohlmann@hci. utah. edu 23

Where is Testing Performed? ● Clinical U. S. genetic laboratories offering p 16 genetic testing (some will assist with obtaining insurance approvals) § Find details at www. genetests. org § Current CLIA certified laboratories • Gene. DX (USA) • Myriad Genetic Laboratories (USA) • Yale University School of Medicine (USA) 24

How Much Does it Cost? ● Approximately $750 for first test ● Site-specific testing about $385 ● 70% who go through pre-authorization receive 90% coverage on average 25

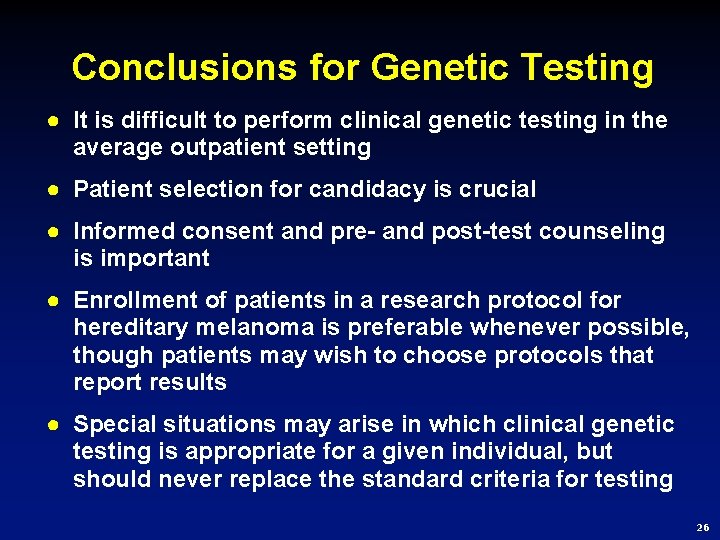
Conclusions for Genetic Testing ● It is difficult to perform clinical genetic testing in the average outpatient setting ● Patient selection for candidacy is crucial ● Informed consent and pre- and post-test counseling is important ● Enrollment of patients in a research protocol for hereditary melanoma is preferable whenever possible, though patients may wish to choose protocols that report results ● Special situations may arise in which clinical genetic testing is appropriate for a given individual, but should never replace the standard criteria for testing 26