Genetic Polymorphism in Drug Metabolism CYP 450 Isoenzymes
Genetic Polymorphism in Drug Metabolism – CYP 450 Isoenzymes Presented by: Lahari Paladugu (Pharm. D 09 -10) Presented on: February 7, 2014
What is Genetic Polymorphism? (1) The existence together of many forms of DNA sequences at a locus within the population. (2) A discontinuous genetic variation that results in different forms or types of individuals among the members of a single species.

Genetic Polymorphism & Drug Metabolism • inter-individual variation of drug effects • Genetic polymorphisms of drug-metabolizing enzymes give rise to distinct subgroups in the population that differ in their ability to perform certain drug biotransformation reactions. • Polymorphisms are generated by mutations in the genes for these enzymes, which cause decreased, increased, or absent enzyme expression or activity by multiple molecular mechanisms.
Drug Metabolism • The metabolism of drugs and other xenobiotics into more hydrophilic metabolites is essential for their elimination from the body, as well as for termination of their biological and pharmacological activity. • Drug metabolism or biotransformation reactions are classified as either phase I functionalization reactions or phase II biosynthetic (conjugation reactions).

Drug Metabolism (cont. ) • The enzyme systems involved in the biotransformation of drugs are localized primarily in the liver. • Other organs with significant metabolic capacity include the GI tract, kidneys, and lungs. • These biotransformation reactions are carried out by CYPs (cytochrome CYP 450 isoforms) and by a variety of transferases.
Drug Metabolism (cont. ) • Pathways of drug metabolism are classified as either: • Phase I reactions: oxidation, reduction, hydrolysis • Phase II reactions: acetylation, glucuronidation, sulfation, methylation • Both types of reactions convert relatively lipid soluble drugs into relatively inactive and more water soluble metabolites, allowing for more efficient systemic elimination.

Polymorphisms • Genetic differences in drug metabolism are the result of genetically based variation in alleles for genes that code for enzymes responsible for the metabolism of drugs. • In polymorphisms, the genes contain abnormal pairs or multiples or abnormal alleles leading to altered enzyme function. • Differences in enzyme activity occur at different rates according to racial group.
Single Nucleotide Polymorphisms (SNPs) • Single changes in one allele of a gene responsible for a variety of metabolic processes including enzymatic metabolism. • The combination of alleles encoding the gene determines the activity and effectiveness of the enzyme. • The overall function of the enzyme is the phenotype of enzyme function. • Phenotype: the observable physical or biochemical characteristics determined by both genetic makeup and environmental influences
• Poor metabolizers • two defective alleles (ex: CYP 2 D 6*4/*5 and CYP 2 D 6*4/*4) • Combination of alleles including one resulting in no enzyme (ex: CYP 2 D 6*5 and CYP 2 D 6*4 deletion) • Intermediate metabolizers • Heterozygous – having only one wild type allele and one defective allele • Normal metabolizers • Carry wild type alleles (ex: CYP 2 D 6*1/*3). • Wild type alleles encode genes for normal enzyme function

• Extensive metabolizers • Carry one wild type and one amplified gene • ex: CYP 2 D 6*1/*2, CYP 2 D 6*A/*1 a, and CYP 2 D 6*1 A/*5 • Ultra-rapid metabolizers • Carry two or more copies of amplified gene • ex: CYP 2 D 6*2/*3

• Genetic changes may inactivate or reduce enzyme activity leading to increase in the substrate drug. • Genetic duplication may increase enzyme activity resulting in lower levels of substrate drug.
Inhibitors & Inducers • Polymorphisms affect drug interactions by altering the effect of inhibitors and inducers on the enzyme. • results in an exaggerated effect or minimal effect on the substrate • Inhibitor: An enzyme inhibitor is a molecule, which binds to enzymes and decreases their activity. • Inducer: An enzyme inducer is a type of drug that increases the metabolic activity of an enzyme either by binding to the enzyme and activating it, or by increasing the expression of the gene coding for the enzyme.

Extensive Metabolizers - Inhibitors • Extensive metabolizer ----- level of substrate drug is normally low due to rapid metabolism by the enzyme. • An inhibitor to the enzyme will inhibit the extensive metabolism and cause significant elevations in the substrate drug. • Effect of inhibitors is much greater in an EM inc. level of substrate levels

Poor Metabolizers - Inhibitors • In a poor metabolizer, the level of substrate drug remains high because the metabolism of the substrate is much less than normal. • When an inhibitor is added, the additional inhibition of metabolism is not much greater than is already occurring in the PM. • The effect of inhibitor is less in a PM than in normal metabolizers. • The drug interaction might not occur.
Extensive Metabolizers - Inducers • Level of substrate drug is lower than in a normal metabolizer due to rapid metabolism. • The addition of an inducer does not cause a greater difference in the level of substrate because the metabolism is already increased greatly. • The drug interaction might not occur.
Poor Metabolizers - Inducers • Level of substrate drug is higher than expected in normal metabolizer because of the lower metabolism of substrate. • The addition of inducer will cause a signification increase in the metabolism of the substrate much lower level of substrate than expected in a normal metabolizer. • Drug interaction may occur to a greater extent. • Drug interaction may result in substrate levels similar to those of normal metabolizers.
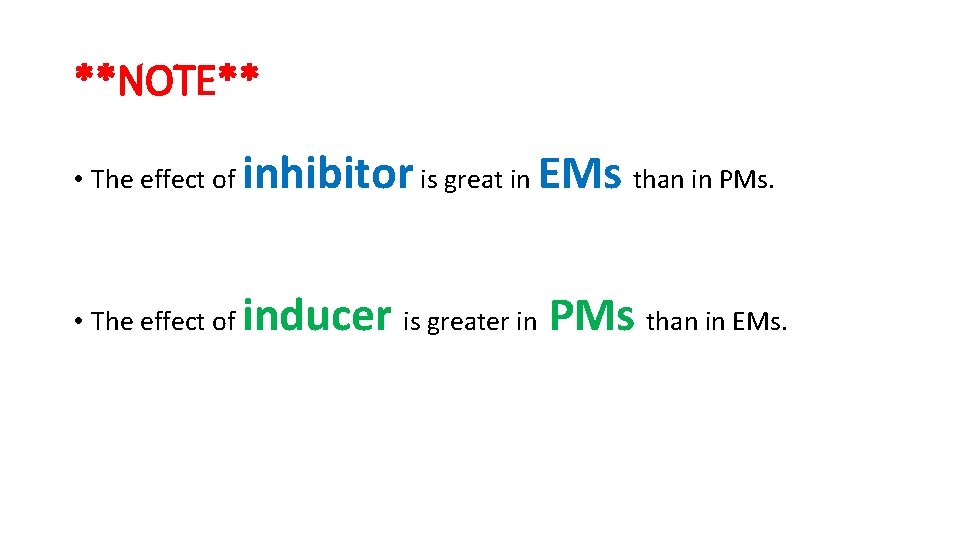
**NOTE** • The effect of inhibitor is great in EMs than in PMs. • The effect of inducer is greater in PMs than in EMs.
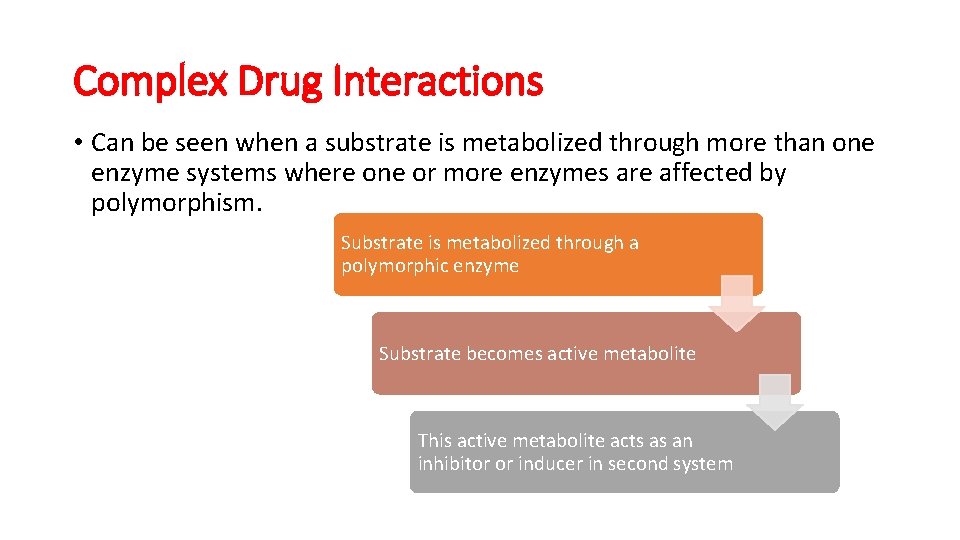
Complex Drug Interactions • Can be seen when a substrate is metabolized through more than one enzyme systems where one or more enzymes are affected by polymorphism. Substrate is metabolized through a polymorphic enzyme Substrate becomes active metabolite This active metabolite acts as an inhibitor or inducer in second system

Genetic Polymorphisms in Genes that Can Influence Drug Metabolism – CYP 450 Isoforms

Phase I Enzymes Enzyme Substrate Clinical Consequence CYP 1 A 1 Benzopyrine, phenacetin Inc. or dec. cancer risk CYP 1 A 2 Acetaminophen, amonafide, caffeine, paraxanthine, ethoxyresorufin, Decreased theophylline metabolism propranolol, fluvoxamine CYP 1 B 1 Estrogen metabolites Possible inc. cancer risk CYP 2 A 6 Coumarin, nicotine, halothane Dec. nicotine metabolism and cigarette addiction CYP 2 B 6 Cyclophosphamide, aflatozin, mephenytoin Significance unknown CYP 2 C 8 Retinoic acid, paclitaxel Significance uknown CYP 2 C 9 Tolbutamide, warfarin, phenytoin, NSAIDS Anticoagulant effect on warfarin CYP 2 C 19 Mephenytoin, omeprazole, hexobarbital, mephobartibal, propranolol, proquanil, phenytoin Peptic ulcer response to omeprazole CYP 2 D 6 Betablockers, antidepressants, antipsychotics, codeine, debrisoquin, dextromethorphan, encainide, flecanide, fluoxetine, guanoxan, methxyamphetamine, phenacetin, propafenone, sparteine Tardive dyskinesia from antipsychotics; narcotic side effects, efficacy and dependency, imipramine dose requirement; beta blocker effects
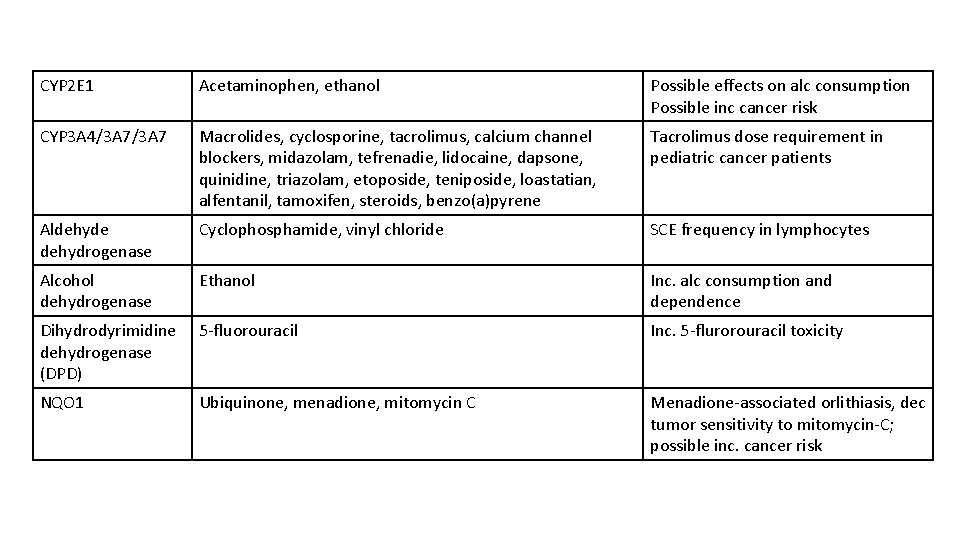
CYP 2 E 1 Acetaminophen, ethanol Possible effects on alc consumption Possible inc cancer risk CYP 3 A 4/3 A 7 Macrolides, cyclosporine, tacrolimus, calcium channel blockers, midazolam, tefrenadie, lidocaine, dapsone, quinidine, triazolam, etoposide, teniposide, loastatian, alfentanil, tamoxifen, steroids, benzo(a)pyrene Tacrolimus dose requirement in pediatric cancer patients Aldehyde dehydrogenase Cyclophosphamide, vinyl chloride SCE frequency in lymphocytes Alcohol dehydrogenase Ethanol Inc. alc consumption and dependence Dihydrodyrimidine dehydrogenase (DPD) 5 -fluorouracil Inc. 5 -flurorouracil toxicity NQO 1 Ubiquinone, menadione, mitomycin C Menadione-associated orlithiasis, dec tumor sensitivity to mitomycin-C; possible inc. cancer risk
P 450 Enzymes in Drug Metabolism • The polymorphic P 450 (CYP) enzyme superfamily is the most important system involved in the biotransformation of many endogenous and exogenous substances including drugs, toxins, and carcinogens. • Genotyping for CYP polymorphisms provides important genetic information that help to understand the effects of xenobiotics on human body. • For drug metabolism, the most important polymorphisms are those of the genes coding for CYP 2 C 9, CYP 2 C 19, CYP 2 D 6, and CYP 3 A 4/5, which can result in therapeutic failure or severe adverse reactions.
CYTOCHROME P 4502 D 6 • Most extensively studied polymorphic drug metabolizing enzyme • Debrisoquin --- marked hypotension • Impaired ability to hydroxylate, and therefore, inactivate debrisoquin • 5 -10% of white subjects have relative deficiency in ability to oxidize debrisoquin • Also have impaired ability to metabolize the antiarrhythmic and oxytocic drug sparteine • PM lower urinary concentration, higher plasma concentrations • Subjects inherited two copies of a gene or genes that encoded an enzyme with either decreased CYP 2 D 6 activity or no activity at all • Prominent in East African population – frequency as high as 29%

CYTOCHROME P 4502 C SUBFAMILY • Accounts for approximately 18% of the CYP content in the liver • Catalyzes roughly 20% of the CYP-mediated metabolism of drugs CYP 2 C 19 • Study using mephenytoin as probe drug determined that individuals can be segregated into EMs and PMs. • PM trait is autosomal recessive – present in 3 -5% of Caucasians & 12 -23% of Asian populations

CYP 2 C 19 (cont. ) • Also catalyzes the metabolism of several proton pump inhibitors (i. e. omeprazole), diazepam, thalidomide, and some barbiturates. • Responsible for inactivation or propranolol and metabolic activation of antimalarial drug proquanil.

CYP 2 C 19 & Diazepam • Diazepam is demethylated by CYP 2 C 19 • Plasma diazepam half-life is longer in individuals who are homozygous for the defective CYP 2 C 19*2 allele compared to those who are homozygous for the wild type allele. • Half-life of the desmethyldiazepam metabolite is also longer in CYP 2 C 19 poor metabolizers. • High frequency in Asian population. • Diazepam induced toxicity may occur as a result of slower metabolism – careful dosing in Asian population.
CYP 2 C 9 • Major CYP 2 C subfamily member in the liver • Primarily responsible for the oxidative metabolism of important compounds – warfarin, phenytoin, tolbutamide, glipizide, losartan, etc. • 6 different polymorphisms – CYP 2 C 9*1, *2, *3, *4, *5, *6 • CYP 2 C 9*1 – wild type allele, CYP 2 C 9*2 -*6 – variants • Variants *2 and *3 alleles are common in Caucasians (≈35%) • CYP 2 C 9*2 and *3 alleles associated with significant reduction in the metabolism and clearance of selected CYP 2 C 9 substrates

CYP 2 C 9 & Warfarin • Polymorphisms linked to both toxicity and dosage requirements for optimal anticoagulation with warfarin. • *2 and *3 variants – higher risk of acute bleeding complications than patients with *1 wild type genotype. • Require 15 -30% lower maintenance dose of warfarin to achieve target INR • Patients with variant CYP 2 C 9 genotype take a median of 95 days longer to achieve stable dosing compared to wildtype group

Dihydropyrimide Dehydrogenase • Metabolism of antineoplastic agent fluorouracil. • In the 1980 s, fatal CNS toxicity developed in several patients after treatment with standard doses fluorouracil. • Patients had inherited deficiency of dihyropyrimidine dehydrogenase. • DPD metabolizes fluorouracil and endogenous pyrimidines. • Severe fluorouracil toxicity occurs when DPD activity < 100 pmol/min/mg protein. • 3% of population carries heterozygous mutations that inactivate DPD and 1% are homozygous for the inactivating mutations. • Heterozygous individuals do not exhibit no phenotype until challenged with fluorouracil.
CYTOCHROME P 4503 A SUBFAMILY • CYP 3 A subfamily plays a critical role in the metabolism of more drugs than any other phase I enzyme. • Expressed in liver and small intestine • Contribute to oral absorption, first-pass, and systemic metabolism • Expression is highly inducible – enzyme activity influence by factors such as variable homeostatic control mechanisms, up- or downregulation by environment factors, and polymorphisms.

CYP 3 A 4 • More than 30 SNPs have been identified for CYP 3 A 4 gene • Unlike other P 450 s, there is no evidence for deleted or null allele for CYP 3 A 4. • The most common variant in CYP 3 A 4, CYP 3 A 4*1 B is an A 392 G transition in the promoter region referred to as the nifedipine response element. • One study shows that this variant may be associated with a slower clearance of cyclosporine. • This is a rather controversial finding.

CYP 3 A 5 • Polymorphically expressed in adults in about 10 -20% in Caucasians, 33% in Japanese, and 55% in African Americans. • The variable CYP 3 A 5*3 is a result of improper m. RNA splicing and reduced translation of functional protein. • CYP 3 A 5 is the primary extra-hepatic CYP 3 A isoform, its polymorphic expression has been implicated in disease risk and the metabolism of endogenous steroids or drug in tissues other than liver. • CYP 3 A 5 has been linked to tacrolimus dose requirements to maintain adequate immunosuppression in solid organ transplant patients.

CYP 3 A 7 • Expressed in fetal liver during development • Hepatic expression is generally down-regulated after birth, but the CYP 3 A 7 protein has been detected in some adults • Increased CYP 3 A 7 expression has been associated with the replacement of 60 nucleotide fragment of the CYP 3 A 7 promoter with the corresponding region form of the CYP 3 A 4 promoter (CYP 3 A 7*1 C allele. ) • This promoter swap results in increased gene expression of the pregnane X receptor response element.

• PXR signaling serves as a central regulator of inducible CYP 3 A 4 expression as well as several other genes involved in drug detoxification. • Polymorphisms in PXR suggest observed variability in CYP 3 A 4 enzymatic activity may be due to, in part, inherited differences in the upstream signaling proteins that control induction of gene expression.
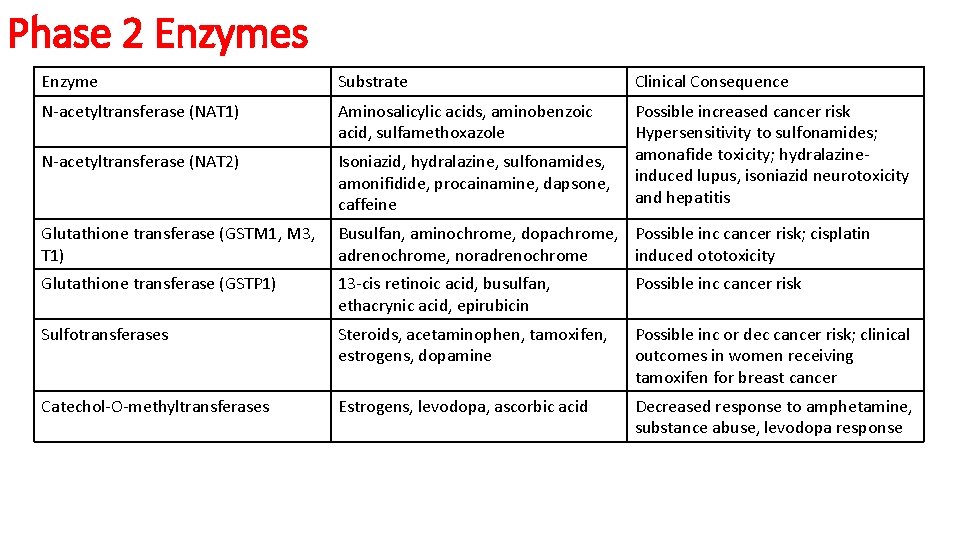
Phase 2 Enzymes Enzyme Substrate Clinical Consequence N-acetyltransferase (NAT 1) Aminosalicylic acids, aminobenzoic acid, sulfamethoxazole N-acetyltransferase (NAT 2) Isoniazid, hydralazine, sulfonamides, amonifidide, procainamine, dapsone, caffeine Possible increased cancer risk Hypersensitivity to sulfonamides; amonafide toxicity; hydralazineinduced lupus, isoniazid neurotoxicity and hepatitis Glutathione transferase (GSTM 1, M 3, T 1) Busulfan, aminochrome, dopachrome, Possible inc cancer risk; cisplatin adrenochrome, noradrenochrome induced ototoxicity Glutathione transferase (GSTP 1) 13 -cis retinoic acid, busulfan, ethacrynic acid, epirubicin Possible inc cancer risk Sulfotransferases Steroids, acetaminophen, tamoxifen, estrogens, dopamine Possible inc or dec cancer risk; clinical outcomes in women receiving tamoxifen for breast cancer Catechol-O-methyltransferases Estrogens, levodopa, ascorbic acid Decreased response to amphetamine, substance abuse, levodopa response
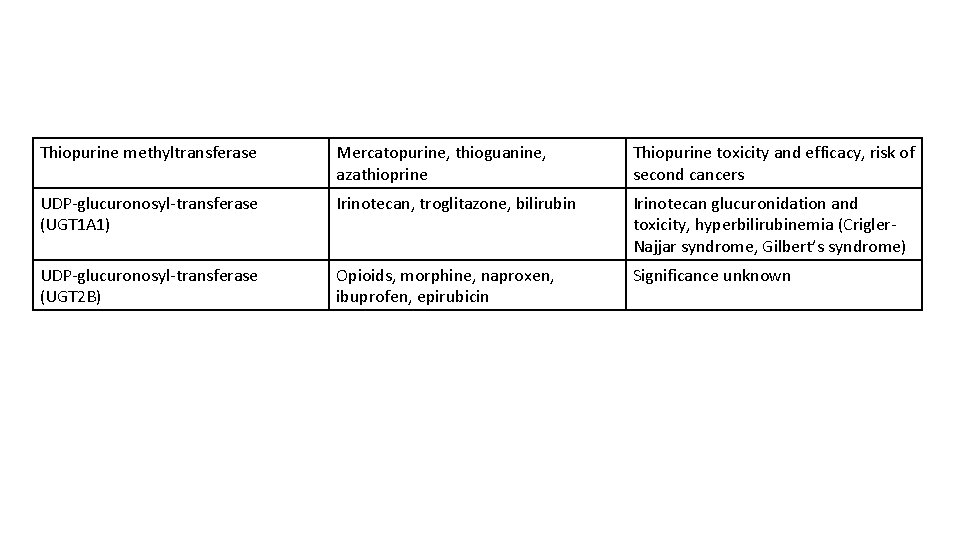
Thiopurine methyltransferase Mercatopurine, thioguanine, azathioprine Thiopurine toxicity and efficacy, risk of second cancers UDP-glucuronosyl-transferase (UGT 1 A 1) Irinotecan, troglitazone, bilirubin Irinotecan glucuronidation and toxicity, hyperbilirubinemia (Crigler. Najjar syndrome, Gilbert’s syndrome) UDP-glucuronosyl-transferase (UGT 2 B) Opioids, morphine, naproxen, ibuprofen, epirubicin Significance unknown

N-ACETYLTRANSFERASE • N-acetylation of isoniazid to acetylisoniazid • Individuals are slow or rapid acetylators • Ethnic variation is seen • Slow acetylation: Japanese (10%), Chinese (20%), Caucasians (60%) • NAT 2 protein is the specific protein isoform that acetylates isoniazid. • 27 unique NAT 2 alleles identified • NAT 2*4 is the wild type allele • NAT 2 alleles containing the G 191 A, T 341 C, A 434 C, G 590 A, and/or G 857 A missense associated substitutions are associated with slow acetylator phenotype.

References • Shargel, Leon. Chapter 12 – Pharmacogenetics. Applied Biopharmaceutics and Pharmacokinetics, 5 th edition. Ebook. • Shargel, Leon. Comprehensive Pharmacy Review, 7 th Edition. Philadelphia: Lipincott- William & Wilkins, 2010. Print. Pages 430 -433. • David B. Troy, Paul Beringer. Remington: The Science and Practice of Pharmacy, 21 st Edition. Pages 1230 – 1239. • Brunton, Laurence. Chabner, Bruce. Knollman, Bjorn. Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 12 th edition. Pages 124 -130. • • • http: //www. biology-online. org/dictionary/Genetic_polymorphism http: //en. wikipedia. org/wiki/Drug_metabolism http: //www. medscape. com/viewarticle/444804_5 http: //www. ncbi. nlm. nih. gov/pmc/articles/PMC 1934960/ http: //dmd. aspetjournals. org/content/29/4/570. full
- Slides: 38