GENERIC DRUGS PRODUCT DEVELOPMEN GENERIC DRUG A drug















- Slides: 20

GENERIC DRUGS PRODUCT DEVELOPMEN

GENERIC DRUG A drug product that is comparable to brand/innovator drug in dosage form, strength, route of administration, quality and performance characteristics, and intended use. It should contain the same active ingredients as the original formulation

WHAT ARE GENERIC DRUGS ? They are drugs which have the same chemical composition as branded drugs are and sold under their chemical name. For example paracetamol , a pain killer , is the generic name for branded drugs like Crocin and Calpol. The market situation is a little different in India than the USA or other developed nations. In USA , when a new drug is launched only the company that holds the FDA patent are legally allowed to set the drug , thus giving them market monopoly. In India however there were no patent laws till 2005 ; which meant that anyone could replicate any drug in India without legal ramifications. This led to the trend of branded and generic drugs which has 99. 5% of the countrys generic drug share

BASIC DIFFERENCE BETWEEN GENERIC AND BRAND NAME DRUGS • Generic drugs are copies of brand-name drugs that have exactly the same dosage, intended use, effects, side effects, route of administration, risks, safety, and strength as the original drug. In other words, their pharmacological effects are exactly the same as those of their brand-name counterparts. • The brand name of a medication is the name given by the company that makes the drug and is usually easy to say for sales and marketing purposes. The generic name, on the other hand, is the name of the active ingredient.

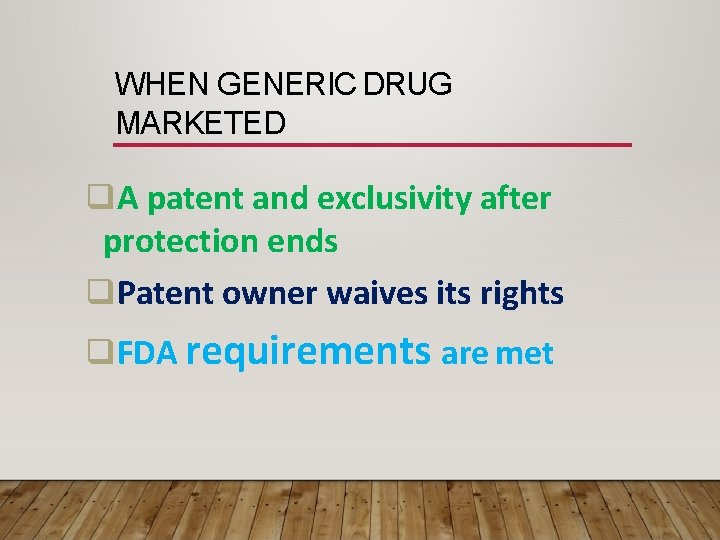
WHEN GENERIC DRUG MARKETED A patent and exclusivity after protection ends Patent owner waives its rights FDA requirements are met

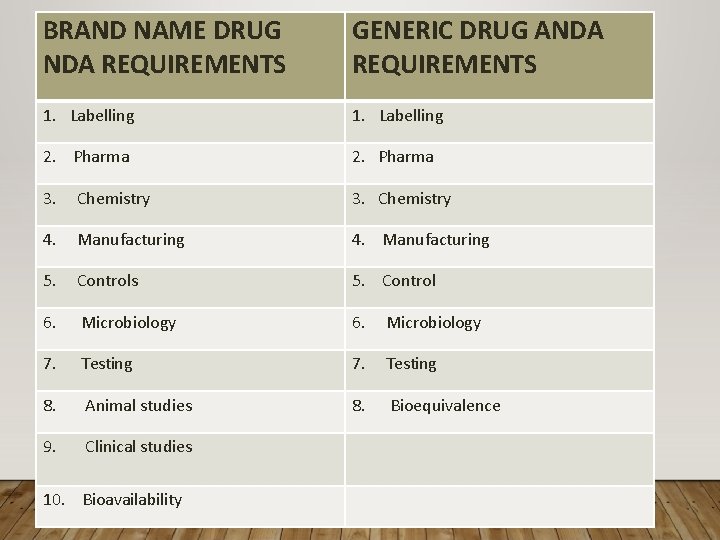
BRAND NAME DRUG NDA REQUIREMENTS GENERIC DRUG ANDA REQUIREMENTS 1. Labelling 2. Pharma 3. Chemistry 3. Chemistry 4. Manufacturing 4. Manufacturing 5. Controls 5. Control 6. Microbiology 7. Testing 8. Animal studies 8. Bioequivalence 9. Clinical studies 10. Bioavailability

WHERE ARE THEY AVAILABLE? Generic drugs are sold everywhere including our local chemist. To buy them one simply has to ask for generic version of a branded drug though they don’t have them for all medicines The department of pharmaceuticals of the government is responsible for promoting generic drugs but they have not done a very good job

After the expiry of the patent or marketing rights of the patent drug , generic drugs are marketed. They are comparable to brand drug in dosage form , strength , route of administration , quality and performance characteristics , and intended use. Generic drugs are available at affordable prices with maintaining quality. . These Generic formulations balance public interest as critical disease like cancer , AIDS etc

PRODUCT DEVELOPMENT PRODUCT : A product is something sold by an enterprise to its customers. PRODUCT DEVELOPMENT : Product development is the set of activities beginning with the perception of a market opportunity and ending in the production , sale and delivery of a product.

THE PRODUCT DEVELOPMENT PROCESS A process is a sequences of steps that transforms a set of inputs into a set of outputs A product development process is the sequence of steps or activities that an enterprise employs to conceive, design, and commercialize a product. Some organizations define and follow a precise and detailed product development process. While others may not even be able to describe their processes

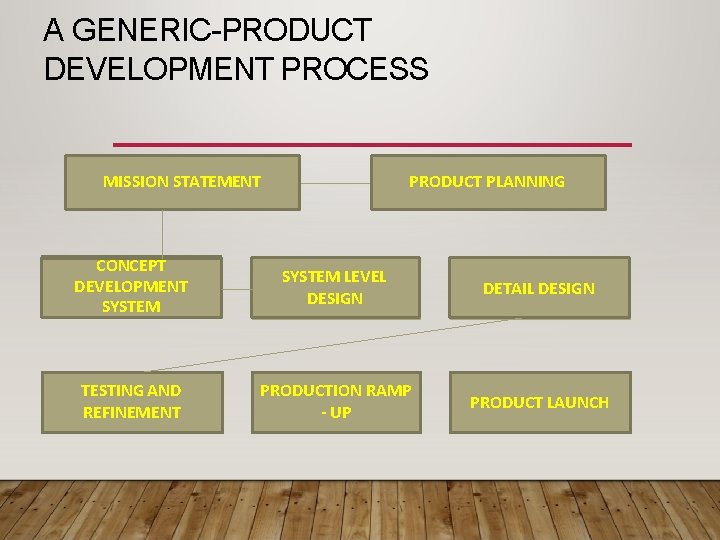
A GENERIC PRODUCT DEVELOPMENT PROCESS We will consider here a generic product development that can be used in a market pull- situation. The input of the process is a mission statement and the output of the process is the product launch MISSION STATEMENT : identifies the target market for the product , provides a basic functional description of the product , and specifies the business goals of the effort ; results from well executed product planning phase PRODUCT LAUNCH : Occures when the product becomes available for purchase in the market place

A GENERIC-PRODUCT DEVELOPMENT PROCESS MISSION STATEMENT PRODUCT PLANNING CONCEPT DEVELOPMENT SYSTEM LEVEL DESIGN DETAIL DESIGN TESTING AND REFINEMENT PRODUCTION RAMP - UP PRODUCT LAUNCH

CONCEPT DEVELOPMENT & APPROVAL Generic drug product manufacturers must formulate a drug product that will have the same therapeutic efficacy and clinical performance as their brand-name counterpart. Safety, efficacy and therapeutic equivalence of such products early compared to the innovator or brand name drug product for obtaining marketing approval The needs of the target market are identified , alternative product concepts are generated and evaluated , and a single development is selected for further development A concept is the description of the form , function and features of a product and is usually accompanied by a set of specifications , an analysis of competitive products , and an economic justification of the project.

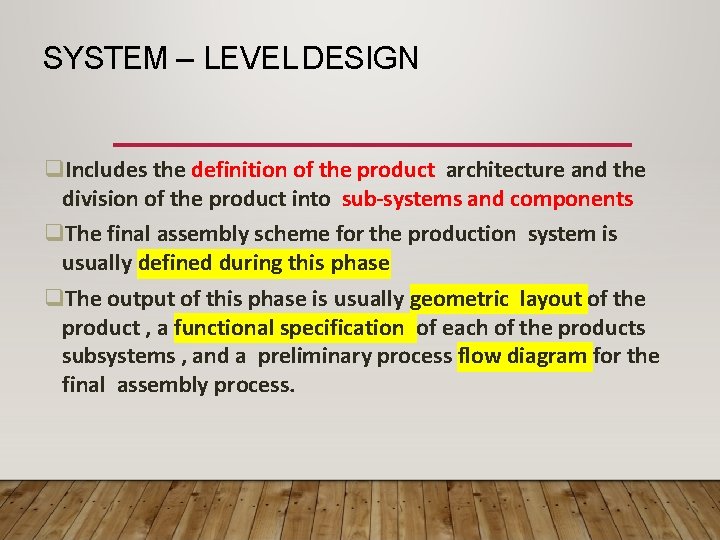
SYSTEM – LEVEL DESIGN Includes the definition of the product architecture and the division of the product into sub-systems and components The final assembly scheme for the production system is usually defined during this phase The output of this phase is usually geometric layout of the product , a functional specification of each of the products subsystems , and a preliminary process flow diagram for the final assembly process.

REASON BEHIND DEVELOPMENT Brand drugs are the drugs which are protected by the patent. �In 2002 about 47%of prescription drug product are generic versions while 53% innovator product. �Generic products growth is 19% in 1984 and 50% in 2004. �Every year about 4 billions dollars business potential exists for next 4 years due to patent expiry. 3

DETAIL DESIGN Includes the complete specification of the geometry , materials , and tolerance of all the unique parts in the product and the identification of all the standard parts to be purchased from suppliers. A process plan is established and tooling is designed for each part to be fabricated within the production system The output of this phase documentation for the product. is the control

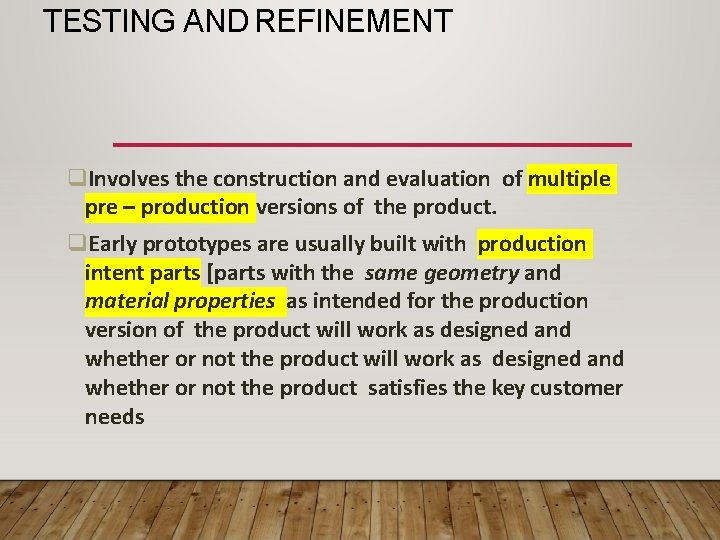
TESTING AND REFINEMENT Involves the construction and evaluation of multiple pre – production versions of the product. Early prototypes are usually built with production intent parts [parts with the same geometry and material properties as intended for the production version of the product will work as designed and whether or not the product satisfies the key customer needs

Later prototypes are usually built with parts supplied by the intended production process but may not be assembled using the intended final assembly process. Later prototypes are extensively evaluated internally and are also typically tested by customers in their own use environment. The goal of the beta prototypes is usually to answer questions about performance and reliability in order to identify necessary changes for the final product.

PRODUCTION RAMP - UP Ramp up is a term used in economics and business to describe an increase in firm production ahead of anticipated increases in product demand. Alternatively, ramp up describes the period from completed initial product development to maximum capacity utilization, characterized by product and process experimentation and improvements. Ramp up in the first sense often occurs when a company strikes a deal with a distributor, retailer, or producer, which will substantially increase product demand.

THANK YOU
 Training and developmen
Training and developmen Exhausted drug meaning
Exhausted drug meaning Examples of generic goals and product-specific goals
Examples of generic goals and product-specific goals Generic rectangle method
Generic rectangle method Generic software vs custom software
Generic software vs custom software Biopharmaceutic considerations in drug product design
Biopharmaceutic considerations in drug product design Target product profile definition
Target product profile definition Perkalian vektor cross dan dot
Perkalian vektor cross dan dot Product mix and product line
Product mix and product line Sifat dot product
Sifat dot product Overview definition
Overview definition Product life cycle kotler
Product life cycle kotler Gnp and gdp
Gnp and gdp Mkt 600
Mkt 600 Core product augmented product
Core product augmented product Proses produksi gabungan
Proses produksi gabungan Dot vs cross product
Dot vs cross product Product range vs product mix
Product range vs product mix Average product of labor
Average product of labor First screen template feasibility analysis
First screen template feasibility analysis What is the starting material for synthesis of salbutamol
What is the starting material for synthesis of salbutamol