Generalized instrumentation system Sensor Measurand Primary Sensing element





- Slides: 31

Generalized instrumentation system Sensor Measurand Primary Sensing element Variable Conversion element Signal processing Output display Perceptible output

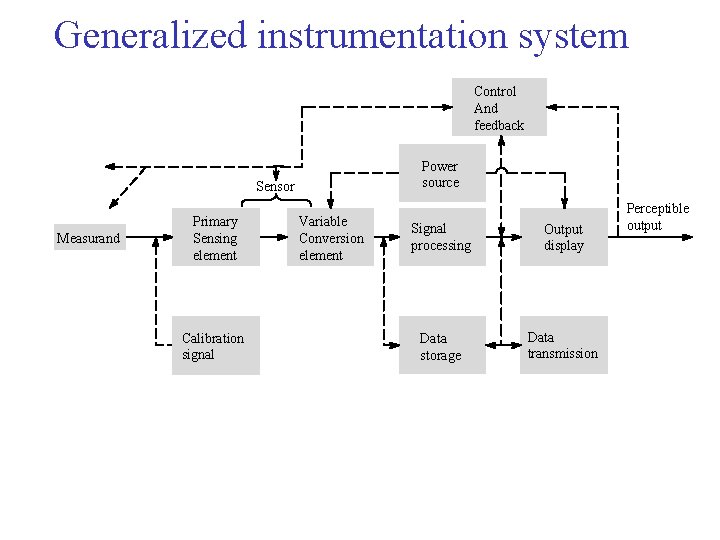
Generalized instrumentation system Control And feedback Power source Sensor Measurand Primary Sensing element Calibration signal Variable Conversion element Signal processing Output display Data storage Data transmission Perceptible output

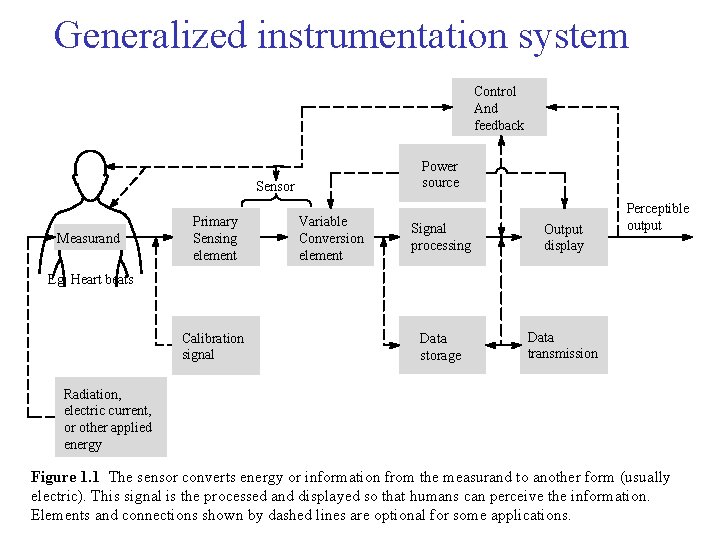
Generalized instrumentation system Control And feedback Power source Sensor Measurand Primary Sensing element Variable Conversion element Signal processing Output display Data storage Data transmission Perceptible output Eg. Heart beats Calibration signal Radiation, electric current, or other applied energy Figure 1. 1 The sensor converts energy or information from the measurand to another form (usually electric). This signal is the processed and displayed so that humans can perceive the information. Elements and connections shown by dashed lines are optional for some applications.

Measurand: Physical quantity • • Biopotential Pressure Flow Dimensions (imaging) Displacement (velocity, acceleration, force) Impedance Temperature Chemical Concentration

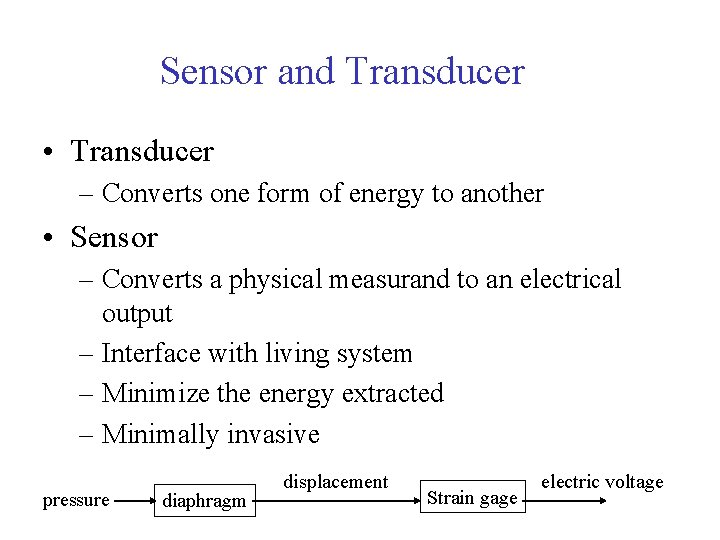
Sensor and Transducer • Transducer – Converts one form of energy to another • Sensor – Converts a physical measurand to an electrical output – Interface with living system – Minimize the energy extracted – Minimally invasive pressure diaphragm displacement Strain gage electric voltage

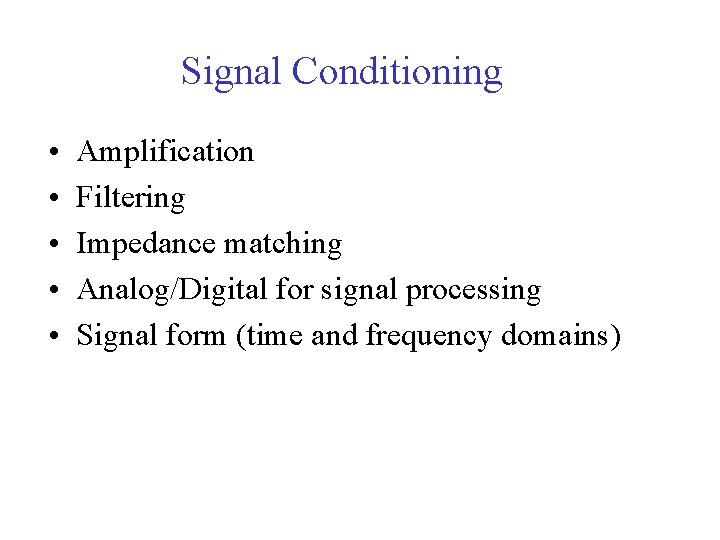
Signal Conditioning • • • Amplification Filtering Impedance matching Analog/Digital for signal processing Signal form (time and frequency domains)

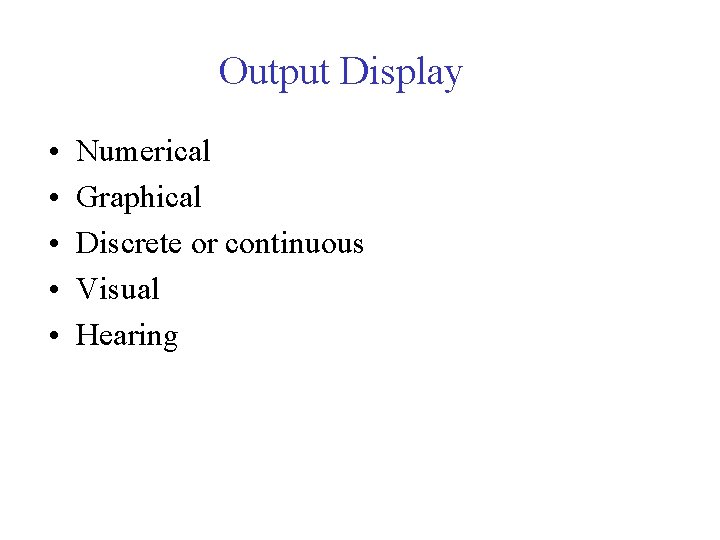
Output Display • • • Numerical Graphical Discrete or continuous Visual Hearing

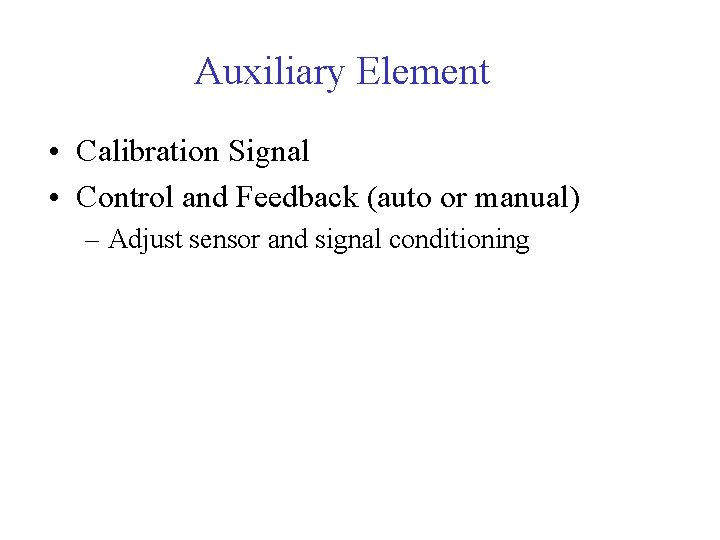
Auxiliary Element • Calibration Signal • Control and Feedback (auto or manual) – Adjust sensor and signal conditioning

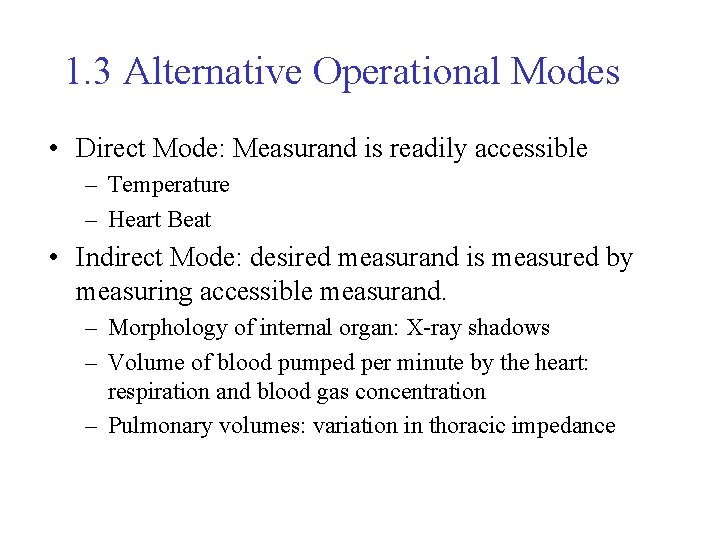
1. 3 Alternative Operational Modes • Direct Mode: Measurand is readily accessible – Temperature – Heart Beat • Indirect Mode: desired measurand is measured by measuring accessible measurand. – Morphology of internal organ: X-ray shadows – Volume of blood pumped per minute by the heart: respiration and blood gas concentration – Pulmonary volumes: variation in thoracic impedance

Characteristics of Instrument Performance Two classes of characteristics are used to evaluated and compare new instrument • Static Characteristics: describe the performance for dc or very low frequency input. • Dynamic Characteristics: describe the performance for ac and high frequency input.

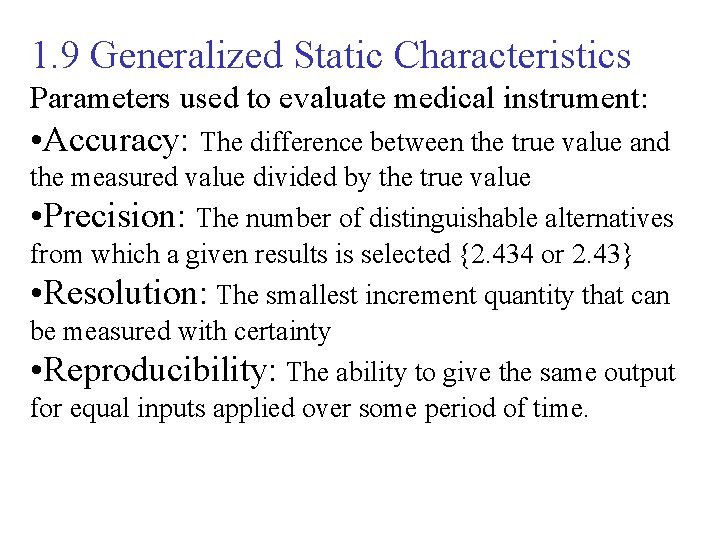
1. 9 Generalized Static Characteristics Parameters used to evaluate medical instrument: • Accuracy: The difference between the true value and the measured value divided by the true value • Precision: The number of distinguishable alternatives from which a given results is selected {2. 434 or 2. 43} • Resolution: The smallest increment quantity that can be measured with certainty • Reproducibility: The ability to give the same output for equal inputs applied over some period of time.

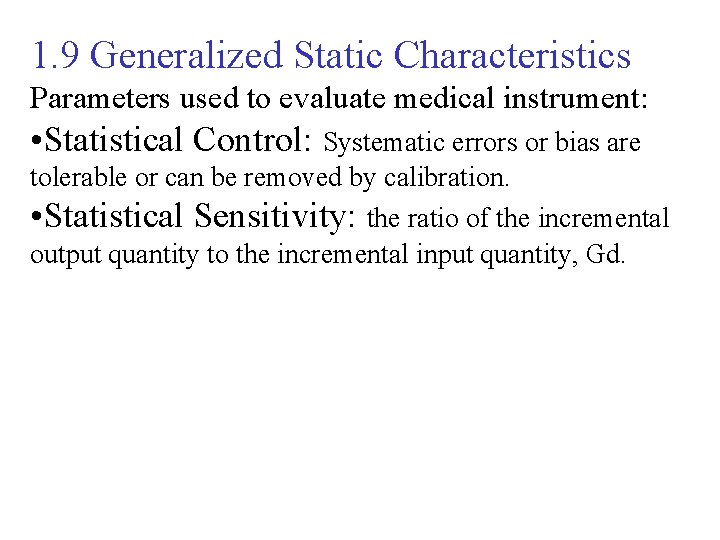
1. 9 Generalized Static Characteristics Parameters used to evaluate medical instrument: • Statistical Control: Systematic errors or bias are tolerable or can be removed by calibration. • Statistical Sensitivity: the ratio of the incremental output quantity to the incremental input quantity, Gd.

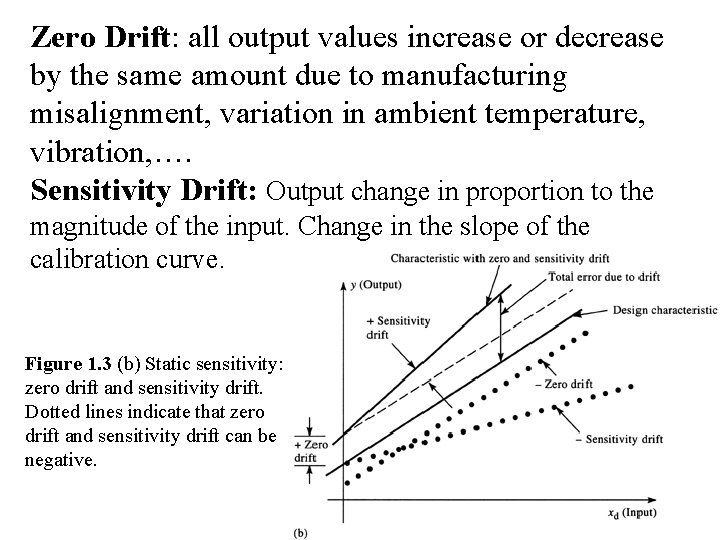
Zero Drift: all output values increase or decrease by the same amount due to manufacturing misalignment, variation in ambient temperature, vibration, …. Sensitivity Drift: Output change in proportion to the magnitude of the input. Change in the slope of the calibration curve. Figure 1. 3 (b) Static sensitivity: zero drift and sensitivity drift. Dotted lines indicate that zero drift and sensitivity drift can be negative.

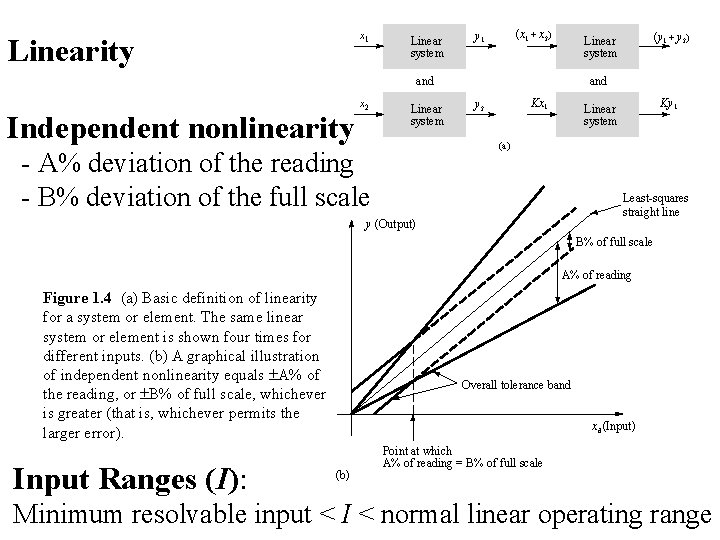
x 1 Linearity Linear system (x 1 + x 2) y 1 and Independent nonlinearity x 2 Linear system (y 1 + y 2) Linear system and Kx 1 y 2 Ky 1 Linear system (a) - A% deviation of the reading - B% deviation of the full scale Least-squares straight line y (Output) B% of full scale A% of reading Figure 1. 4 (a) Basic definition of linearity for a system or element. The same linear system or element is shown four times for different inputs. (b) A graphical illustration of independent nonlinearity equals A% of the reading, or B% of full scale, whichever is greater (that is, whichever permits the larger error). Input Ranges (I): Overall tolerance band xd (Input) (b) Point at which A% of reading = B% of full scale Minimum resolvable input < I < normal linear operating range

Example A linear system described by the following equation y=2 x+3. Find the overall tolerance band for the system if the input range is 0 to 10 and its independent nonlinearity is 0. 5% deviation of the full scale and 1. 5% deviation of the reading.

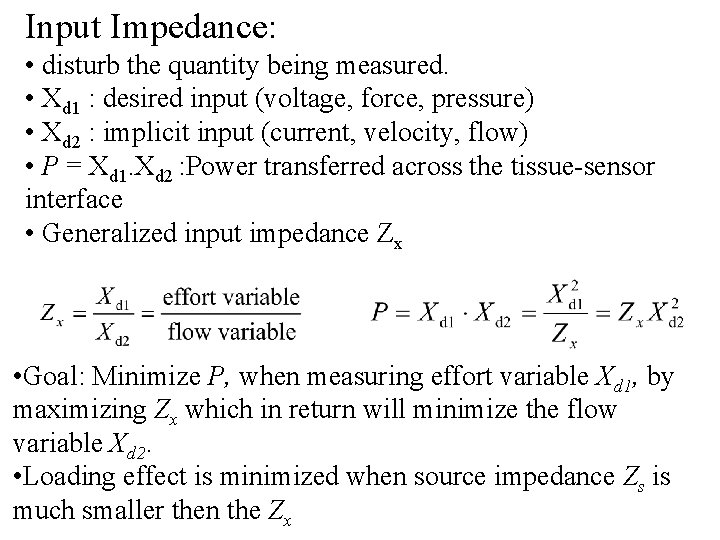
Input Impedance: • disturb the quantity being measured. • Xd 1 : desired input (voltage, force, pressure) • Xd 2 : implicit input (current, velocity, flow) • P = Xd 1. Xd 2 : Power transferred across the tissue-sensor interface • Generalized input impedance Zx • Goal: Minimize P, when measuring effort variable Xd 1, by maximizing Zx which in return will minimize the flow variable Xd 2. • Loading effect is minimized when source impedance Zs is much smaller then the Zx

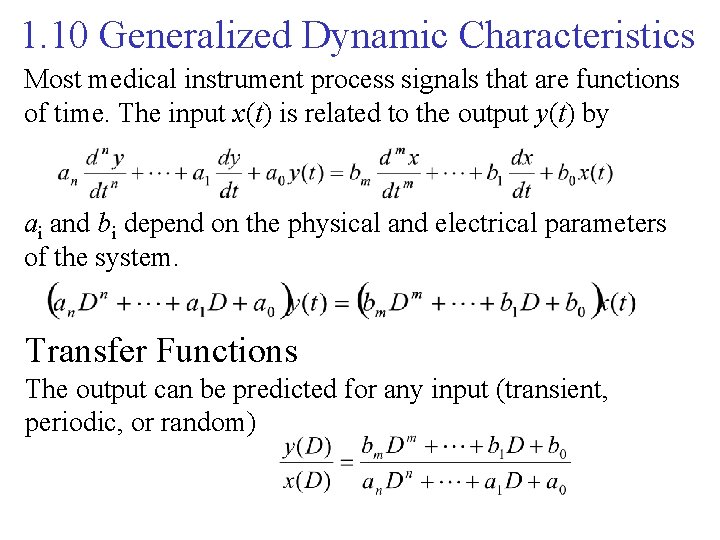
1. 10 Generalized Dynamic Characteristics Most medical instrument process signals that are functions of time. The input x(t) is related to the output y(t) by ai and bi depend on the physical and electrical parameters of the system. Transfer Functions The output can be predicted for any input (transient, periodic, or random)

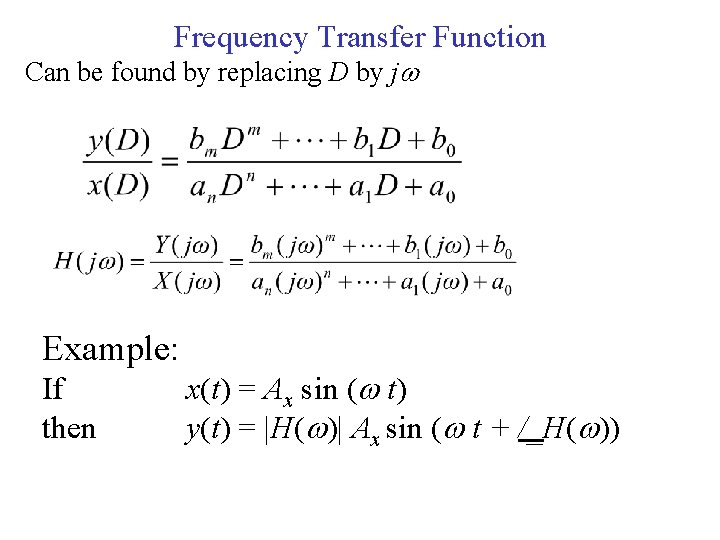
Frequency Transfer Function Can be found by replacing D by j Example: If then x(t) = Ax sin ( t) y(t) = |H( )| Ax sin ( t + /_H( ))

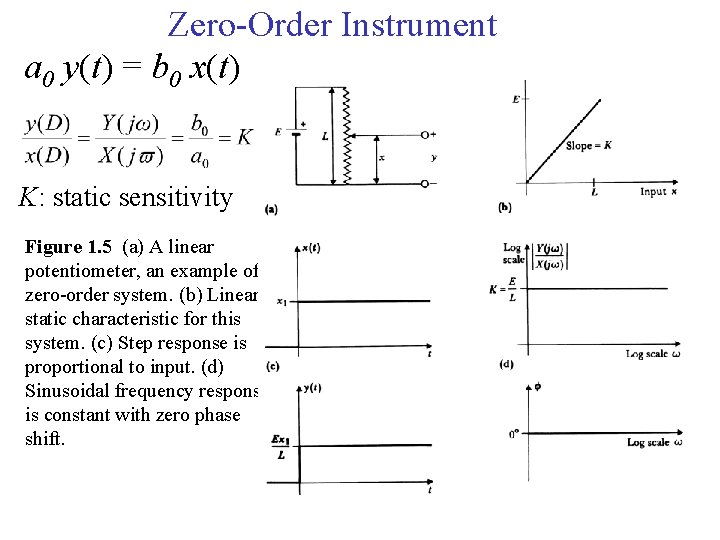
Zero-Order Instrument a 0 y(t) = b 0 x(t) K: static sensitivity Figure 1. 5 (a) A linear potentiometer, an example of a zero-order system. (b) Linear static characteristic for this system. (c) Step response is proportional to input. (d) Sinusoidal frequency response is constant with zero phase shift.

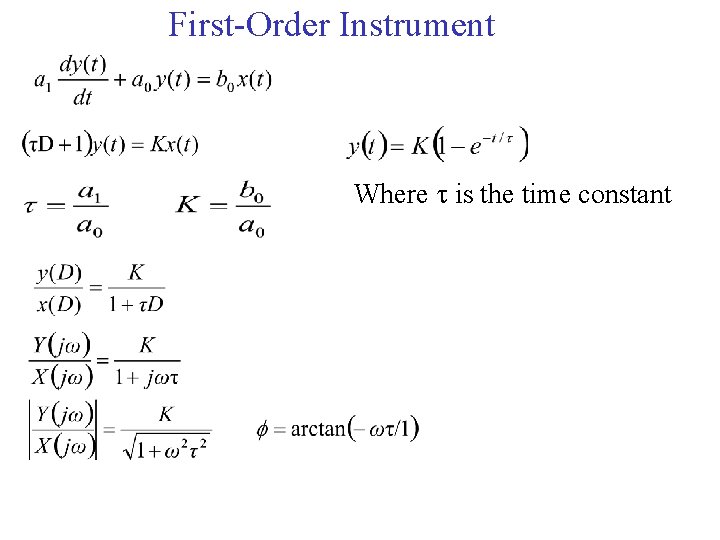
First-Order Instrument Where is the time constant

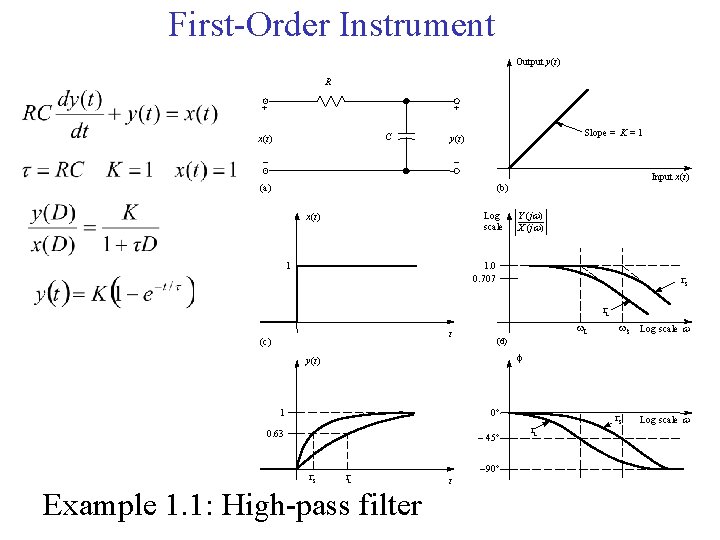
First-Order Instrument Output y(t) R + + C x(t) Slope = K = 1 y(t) - - Input x(t) (a) (b) Log scale x(t) 1 Y (j ) X (j ) 1. 0 0. 707 S L t (c) w. L Log scale (d) f y(t) 0° 1 0. 63 - 45° S w. S L Example 1. 1: High-pass filter -90° t L S Log scale

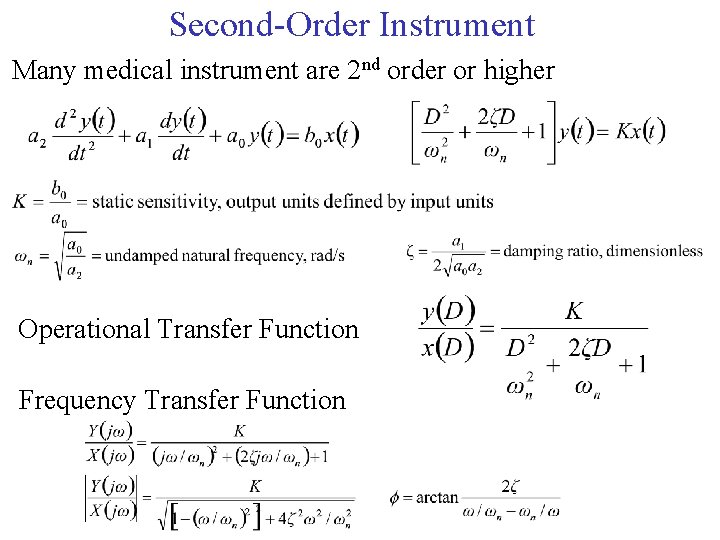
Second-Order Instrument Many medical instrument are 2 nd order or higher Operational Transfer Function Frequency Transfer Function

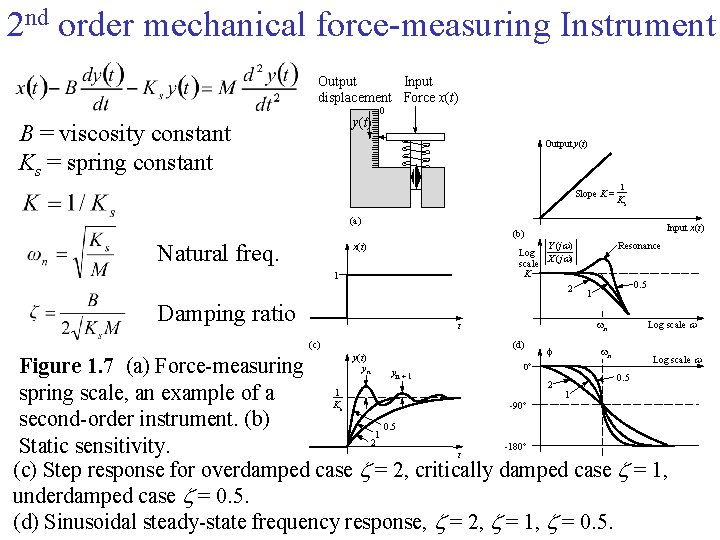
2 nd order mechanical force-measuring Instrument Output Input displacement Force x(t) y(t) B = viscosity constant Ks = spring constant 0 Output y(t) Slope K = 1 Ks (a) Input x(t) (b) Natural freq. Y (j ) Log scale X (j ) K 2 x(t) 1 Damping ratio (d) y(t) yn 0. 5 1 wn t (c) Resonance f wn Log scale 0° Figure 1. 7 (a) Force-measuring y 0. 5 2 1 1 spring scale, an example of a K -90° second-order instrument. (b) 0. 5 1 2 -180° Static sensitivity. t (c) Step response for overdamped case = 2, critically damped case = 1, underdamped case = 0. 5. (d) Sinusoidal steady-state frequency response, = 2, = 1, = 0. 5. s n+1

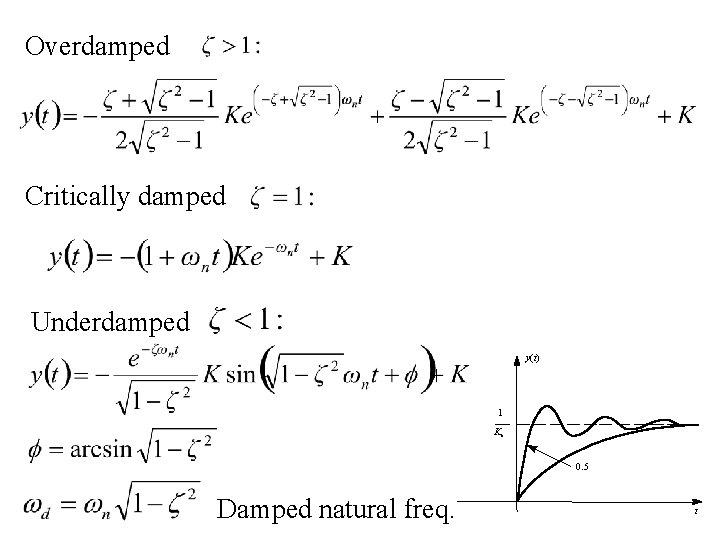
Overdamped Critically damped Underdamped y(t) 1 Ks 0. 5 Damped natural freq. t

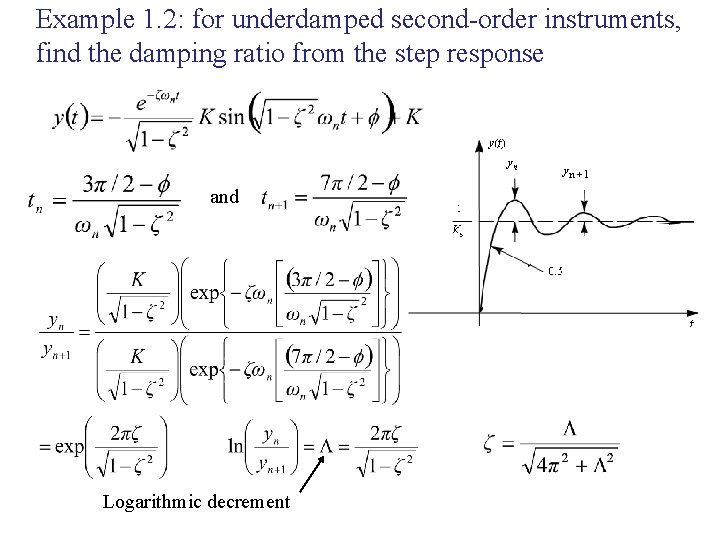
Example 1. 2: for underdamped second-order instruments, find the damping ratio from the step response and Logarithmic decrement

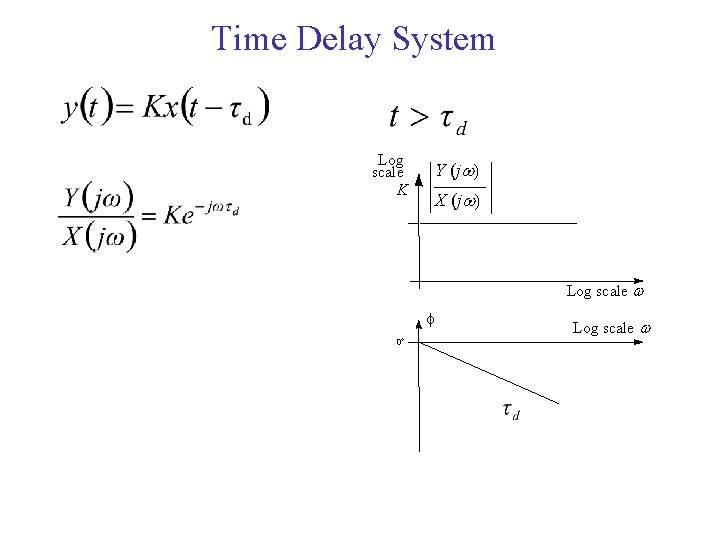
Time Delay System Log scale K Y (j ) X (j ) Log scale f 0° Log scale

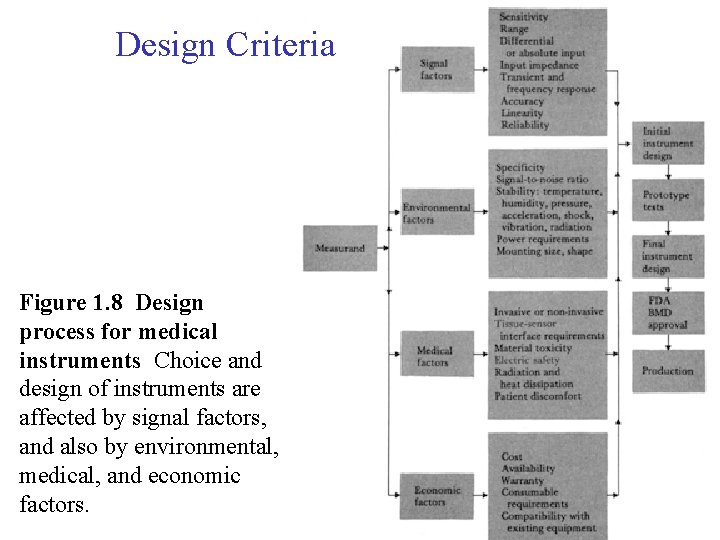
Design Criteria Figure 1. 8 Design process for medical instruments Choice and design of instruments are affected by signal factors, and also by environmental, medical, and economic factors.

Commercial Medical Instrumentation Development Process • Ideas: come from people working in the health care • Detailed evaluation and signed disclosure • Feasibility analysis and product description • Medical need • Technical feasibility • Brief business plan (financial, sales, patents, standards, competition) • Product Specification (interface, size, weight, color) “What” is required but nothing about “how” • Design and development (software and hardware)

Commercial Medical Instrumentation Development Process • Prototype development • Testing on animals or human subjects • Final design review (test results for, specifications, subject feedback, cost) • Production (packaging, manual and documents) • Technical support

Regulation of Medical Devices Medical devices is “any item promoted for a medical purpose that does not rely on chemical action to achieve its intended effect” 2 Ways for Medical Devices Classification First Way: (based on potential hazards) Class I: general controls Class II: performance standards Class III: premarketing approval Second Method: (see Table 1. 2 in textbook) preamendment, postamendment, substantially equivalent, implant, custom, investigational, transitional

Regulation of Medical Devices Second Way of classifications: (see Table 1. 2 in textbook) Preamendment: Devices on the market before 5/28/1976 Postamendment: Devices on the market after 5/28/1976 Substantially equivalent: Equivalent to preamendment devices Implant: devices inserted in human body and intended to remain there for >30 days. Custom: Devices not available to other licensed and not in finished form Investigational: Unapproved devices undergoing clinical investigation Transitional: devices that were regulated as drugs and now defined as medical devices