General reactions of proteins and nitrogen balance By

General reactions of proteins and nitrogen balance By Dr. Mohammed Hosny Hassan, Lecturer of Medical Biochemistry, Faculty of Medicine, South Valley University

Nitrogen balance Definition: • It is the quantitative difference between nitrogen intake & output "loss" in gm per day. Nitrogen intake: • Nitrogen is taken in the form of dietary proteins. Nitrogen output "loss": • Derived from protein catabolism weather proteins of endogenous origin or of exogenous origin "dietary proteins". • Nitrogen is lost from the body through: 1. Urine: In the form of Urea, Uric acid, Ammonia, Amino acids, Creatinine, Creatine. 2. Stool "feces”. 3. Sweat. 4. Variable amounts are lost in milk & menstrual blood in females , skin "desquamated cells" , hair & nails By Dr. Mohammed Hosny Hassan, Lecturer of Medical Biochemistry, Faculty of Medicine, South Valley University

• There are three states of nitrogen balance: 1. Nitrogen equilibrium. 2. Positive nitrogen balance. 3. Negative nitrogen balance. By Dr. Mohammed Hosny Hassan, Lecturer of Medical Biochemistry, Faculty of Medicine, South Valley University

1. Nitrogen equilibrium: • Normally in an adult person under in which nitrogen intake equals nitrogen loss or output. 2. Positive nitrogen balance: – Exists when nitrogen intake is more than nitrogen output. – Occurs whenever new tissues are being built up e (protein anabolism). This occurs in: 1. 2. 3. 4. Children during their growth periods. Muscular training. Pregnancy & lactation. Recovery from wasting diseases e. g after surgery. By Dr. Mohammed Hosny Hassan, Lecturer of Medical Biochemistry, Faculty of Medicine, South Valley University

3. Negative nitrogen balance: – Exists when nitrogen output exceeds nitrogen intake. – It indicates excessive breakdown of tissue proteins (tissue catabolism). – It occurs in: • Chronic & debilitating diseases e. g. • D. M, hyperthyroidism, Cushing syndrome, infections & fevers. • Wasting diseases e. g. malignancies & T. B. • Inadequate intake of proteins e. g. • Starvation. • Malnutrition. • Gastro-intestinal diseases. • Loss of proteins e. g. • Chronic hemorrhage. • Burns. • Albuminuria or proteinuria. By Dr. Mohammed Hosny Hassan, Lecturer of Medical Biochemistry, Faculty of Medicine, South Valley University
General reaction of proteins • The most important reactions are: I). Transamination. II). Deamination. III) Decarboxylation. IV). Transmethylation. By Dr. Mohammed Hosny Hassan, Lecturer of Medical Biochemistry, Faculty of Medicine, South Valley University
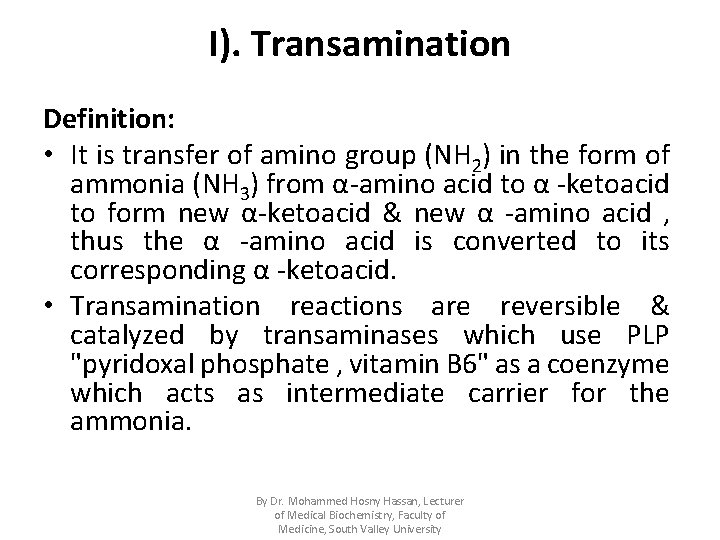
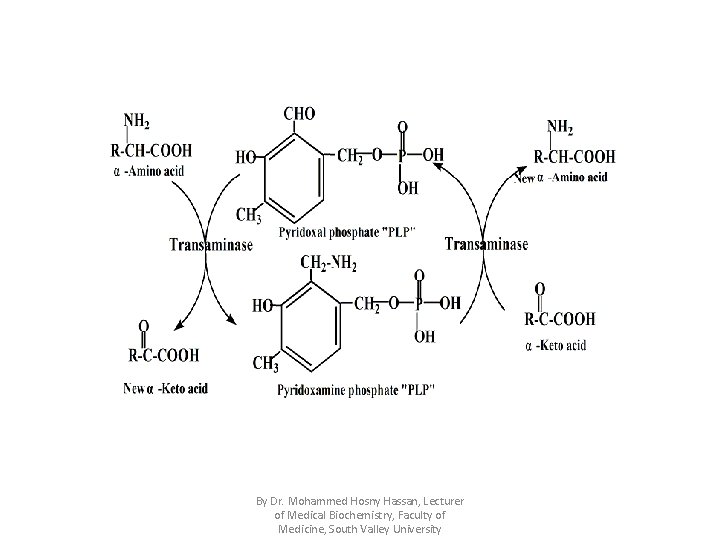
I). Transamination Definition: • It is transfer of amino group (NH 2) in the form of ammonia (NH 3) from α-amino acid to α -ketoacid to form new α-ketoacid & new α -amino acid , thus the α -amino acid is converted to its corresponding α -ketoacid. • Transamination reactions are reversible & catalyzed by transaminases which use PLP "pyridoxal phosphate , vitamin B 6" as a coenzyme which acts as intermediate carrier for the ammonia. By Dr. Mohammed Hosny Hassan, Lecturer of Medical Biochemistry, Faculty of Medicine, South Valley University
By Dr. Mohammed Hosny Hassan, Lecturer of Medical Biochemistry, Faculty of Medicine, South Valley University
Importance of transamination: 1. Production of non-essential amino acids through reaction of glutamate with α-keto acids. 2. Transamination can connect between carbohydrate & protein metabolism through kreb's cycle e. g. By Dr. Mohammed Hosny Hassan, Lecturer of Medical Biochemistry, Faculty of Medicine, South Valley University
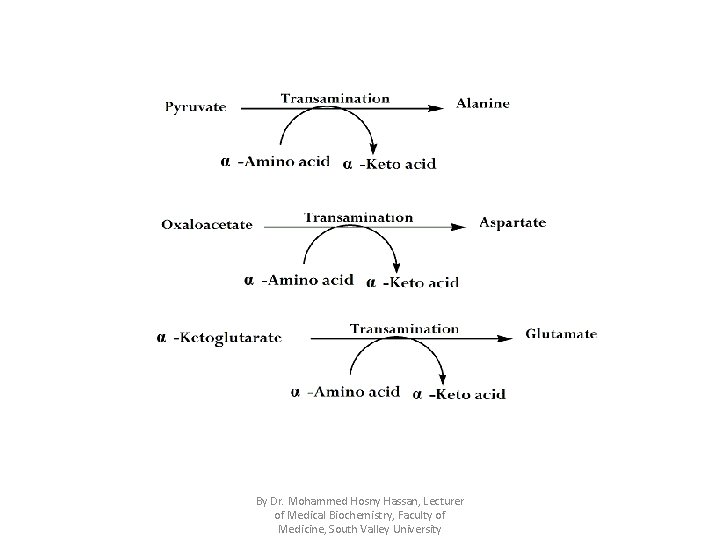
By Dr. Mohammed Hosny Hassan, Lecturer of Medical Biochemistry, Faculty of Medicine, South Valley University

• Among transaminases, three important transaminases which are ALT "G. P. T , Alanine transaminase" , AST "G. O. T , Aspartate transaminase" , glutamate transaminase. By Dr. Mohammed Hosny Hassan, Lecturer of Medical Biochemistry, Faculty of Medicine, South Valley University
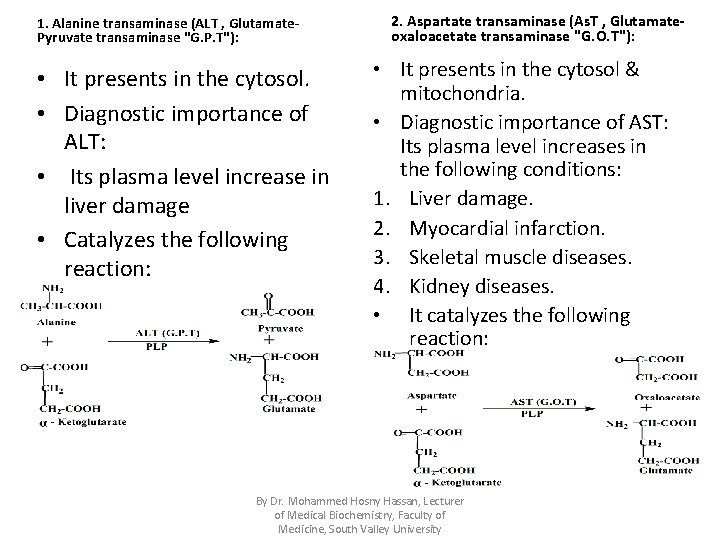
1. Alanine transaminase (ALT , Glutamate. Pyruvate transaminase "G. P. T"): • It presents in the cytosol. • Diagnostic importance of ALT: • Its plasma level increase in liver damage • Catalyzes the following reaction: 2. Aspartate transaminase (As. T , Glutamateoxaloacetate transaminase "G. O. T"): • It presents in the cytosol & mitochondria. • Diagnostic importance of AST: Its plasma level increases in the following conditions: 1. Liver damage. 2. Myocardial infarction. 3. Skeletal muscle diseases. 4. Kidney diseases. • It catalyzes the following reaction: By Dr. Mohammed Hosny Hassan, Lecturer of Medical Biochemistry, Faculty of Medicine, South Valley University
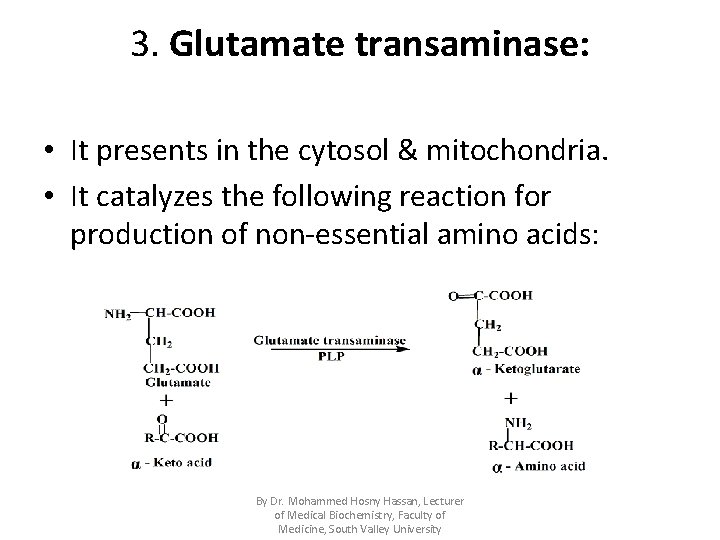
3. Glutamate transaminase: • It presents in the cytosol & mitochondria. • It catalyzes the following reaction for production of non-essential amino acids: By Dr. Mohammed Hosny Hassan, Lecturer of Medical Biochemistry, Faculty of Medicine, South Valley University

II). Deamination Definition: • It is removal of the amino group in the form of free ammonia from αamino acid converting it to its corresponding α -keto acid. Types: 1. Oxidative deamination: A. L-amino acid oxidase. B. D-amino acid oxidase. C. L-glutamate dehydrogenase. 2. Non-oxidative deamination: A. Dehydratases for hydroxy amino acids. B. Desulfhydrases for sulfur containing amino acids. 3. Specific deamination: Histidase. 4. Reductive deamination converting the amino acid into its corresponding fatty acid. 5. Hydrolytic deamination: A. For aspargine & glutamine. B. For nitrogenous bases (purines & pyrimidines). By Dr. Mohammed Hosny Hassan, Lecturer of Medical Biochemistry, Faculty of Medicine, South Valley University

1) Oxidative deamination Definition: • It is removal of amino group in the form of free ammonia secondary to dehydration (oxidation) of α-amino acid converting it to its corresponding αketo acid. • It is reversible reactions. • There are three oxidative deaminases: 1. L-amino acid oxidase. 2. D-amino acid oxidase. 3. L-glutamate dehydrogenase. By Dr. Mohammed Hosny Hassan, Lecturer of Medical Biochemistry, Faculty of Medicine, South Valley University
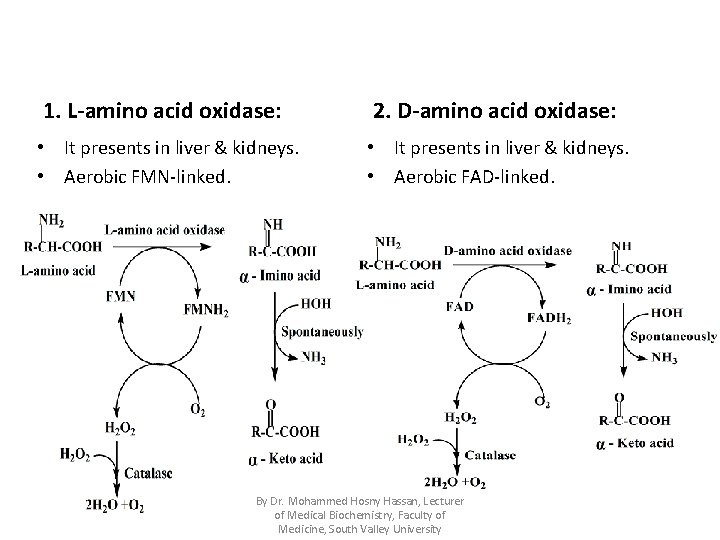
1. L-amino acid oxidase: 2. D-amino acid oxidase: • It presents in liver & kidneys. • Aerobic FMN-linked. • It presents in liver & kidneys. • Aerobic FAD-linked. By Dr. Mohammed Hosny Hassan, Lecturer of Medical Biochemistry, Faculty of Medicine, South Valley University
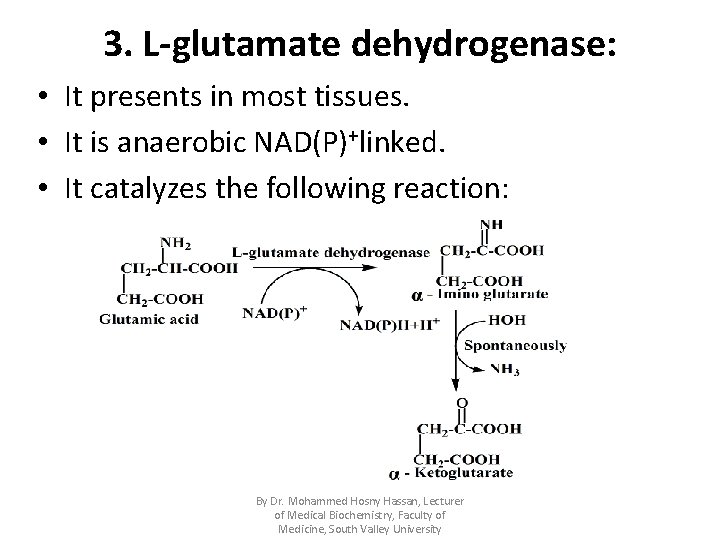
3. L-glutamate dehydrogenase: • It presents in most tissues. • It is anaerobic NAD(P)+linked. • It catalyzes the following reaction: By Dr. Mohammed Hosny Hassan, Lecturer of Medical Biochemistry, Faculty of Medicine, South Valley University
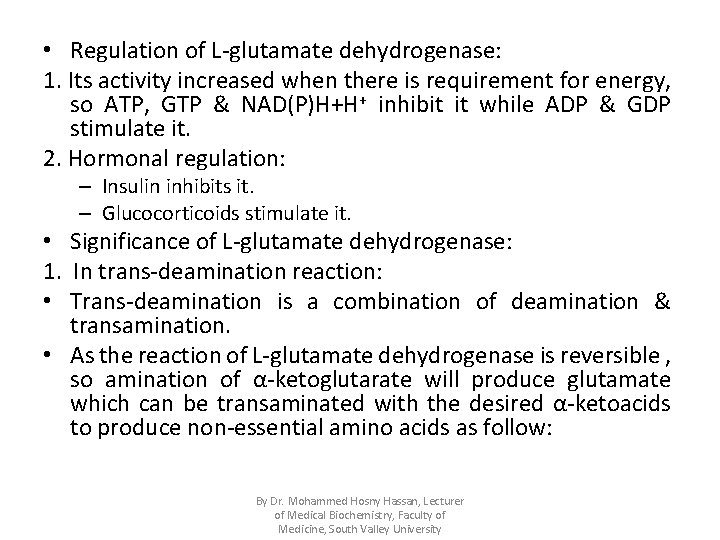
• Regulation of L-glutamate dehydrogenase: 1. Its activity increased when there is requirement for energy, so ATP, GTP & NAD(P)H+H+ inhibit it while ADP & GDP stimulate it. 2. Hormonal regulation: – Insulin inhibits it. – Glucocorticoids stimulate it. • Significance of L-glutamate dehydrogenase: 1. In trans-deamination reaction: • Trans-deamination is a combination of deamination & transamination. • As the reaction of L-glutamate dehydrogenase is reversible , so amination of α-ketoglutarate will produce glutamate which can be transaminated with the desired α-ketoacids to produce non-essential amino acids as follow: By Dr. Mohammed Hosny Hassan, Lecturer of Medical Biochemistry, Faculty of Medicine, South Valley University
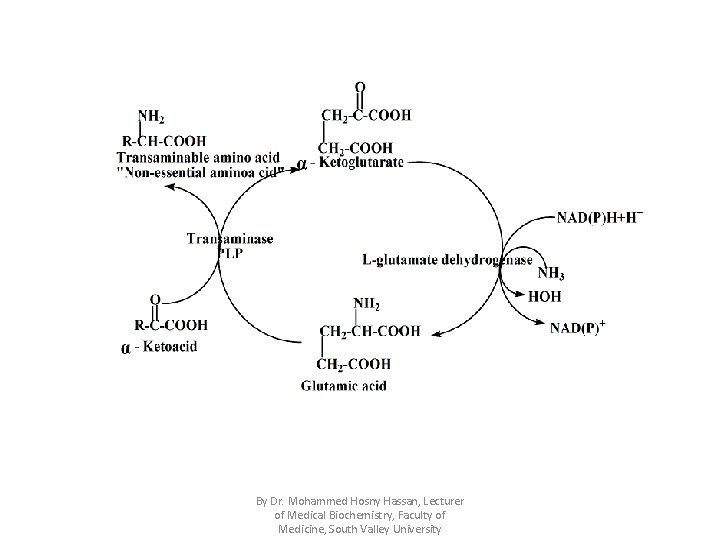
By Dr. Mohammed Hosny Hassan, Lecturer of Medical Biochemistry, Faculty of Medicine, South Valley University
2. Pathological conditions: in which there is accumulation of ammonia as in patients with liver diseases e. g. liver cirrhosis , ammonia will reacts with α-ketoglutarate in the presence of L-glutamate dehydrogenase producing glutamate & as there is excess ammonia , it will depletes α -ketoglutarate from kreb's cycle & inhibits it in the brain , so no ATP production in the brain resulting in encephalopathy. By Dr. Mohammed Hosny Hassan, Lecturer of Medical Biochemistry, Faculty of Medicine, South Valley University

2) Non-oxidative deamination Definition: • It is removal of amino group in the form of free ammonia from α-amino acid without oxidation converting it to its corresponding αketo acid , using PLP as a coenzyme. • It occurs to certain amino acids. By Dr. Mohammed Hosny Hassan, Lecturer of Medical Biochemistry, Faculty of Medicine, South Valley University
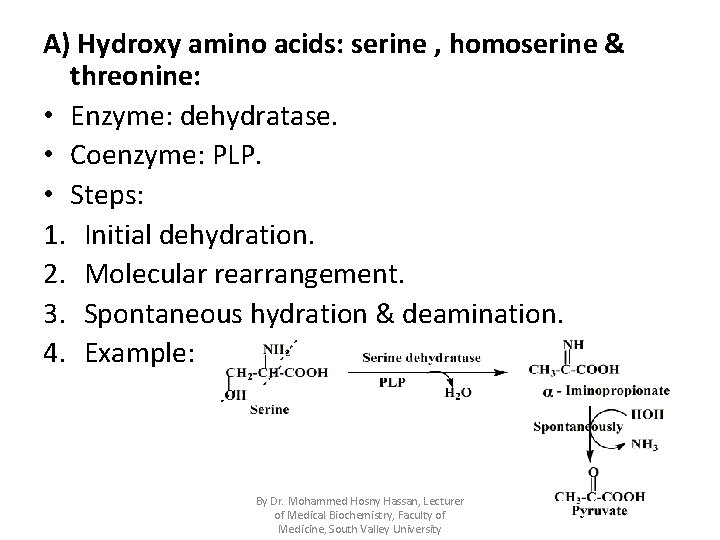
A) Hydroxy amino acids: serine , homoserine & threonine: • Enzyme: dehydratase. • Coenzyme: PLP. • Steps: 1. Initial dehydration. 2. Molecular rearrangement. 3. Spontaneous hydration & deamination. 4. Example: By Dr. Mohammed Hosny Hassan, Lecturer of Medical Biochemistry, Faculty of Medicine, South Valley University
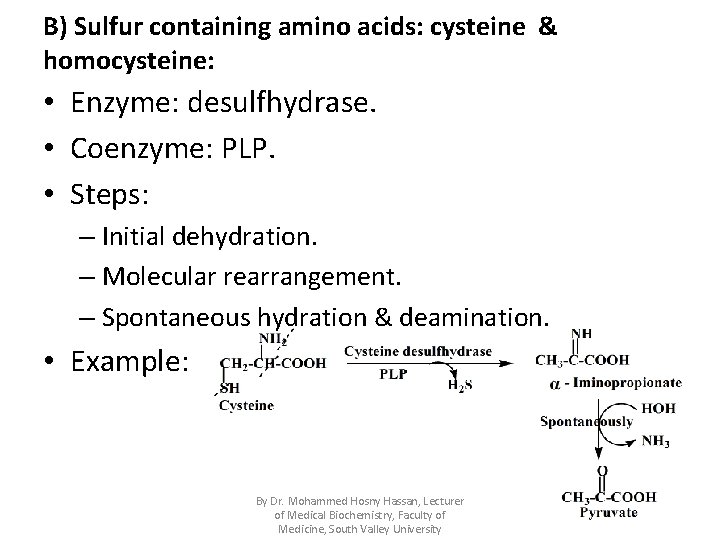
B) Sulfur containing amino acids: cysteine & homocysteine: • Enzyme: desulfhydrase. • Coenzyme: PLP. • Steps: – Initial dehydration. – Molecular rearrangement. – Spontaneous hydration & deamination. • Example: By Dr. Mohammed Hosny Hassan, Lecturer of Medical Biochemistry, Faculty of Medicine, South Valley University
III). Decarboxylation reactions "Biogenic amines" Definition of biogenic amines: • These are products of simple decarboxylation reactions ( removal of carbon dioxide) of the amino acids or their derivatives & some of which are biologically active. • Catalyzed by decarboxylase which uses PLP as a coenzyme. • Examples: By Dr. Mohammed Hosny Hassan, Lecturer of Medical Biochemistry, Faculty of Medicine, South Valley University

1. Dopamine from dihydroxy phenylalanine "DOPA": Functions of dopamine: 1. It is hypothalamic neurotransmitter. 2. It is a precursor for noradrenaline in adrenergic nerve endings & adrenal medulla and adrenaline in the adrenal medulla. By Dr. Mohammed Hosny Hassan, Lecturer of Medical Biochemistry, Faculty of Medicine, South Valley University

2. Histamine from histidine • Site of synthesis of histamine: 1. Basophils & mast cells. 2. Histaminergic receptors in the brain. 3. Gastric mucosa. • Actions of histamine: 1. Vasodilataion, so it is inflammatory agents in allergy. 2. Hypothalamic neurotransmitter. 3. Increases the HCL secretion, so blockage of its receptors e. g. cimetidine will prevent HCL secretion, so used in the treatment of gastric hyperacidity & peptic ulcer. By Dr. Mohammed Hosny Hassan, Lecturer of Medical Biochemistry, Faculty of Medicine, South Valley University
3. Serotonin from tryptophan Site of synthesis of serotonin: 1. Intestinal mucosal cells "argentaffin cells" , so in carcinoid syndrome , in which primary liver tumor sends metastasis to argentaffin cells causing argentaffinoma ( tumor of argentaffin cells) → excessive production of serotonin with lack of tryptophan conversion into niacin →pellagra. 2. Platelets "stored in it", brain, gastric mucosa, basophils & mast cells. Functions of serotonin: 1. Vasoconstriction & Smooth muscle contraction e. g. bronchoconstriction & diarrhea. 2. Excitatory neurotransmitter in the brain. By Dr. Mohammed Hosny Hassan, Lecturer of Medical Biochemistry, Faculty of Medicine, South Valley University

4. γ-Amino butyric acid "GABA" from glutamic acid: Functions of GABA: • Inhibitory neurotransmitter. • Decreased PLP "vit. B 6" causes decrease in GABA concentration in the brain leading to convulsions especially in infants. By Dr. Mohammed Hosny Hassan, Lecturer of Medical Biochemistry, Faculty of Medicine, South Valley University
IV). Transmethylation Definition: • It is transfer of methyl group from methyl donor to methyl acceptor producing a biologically active or an extractable product. • Catalyzed by methyl transferases. • Site: in many tissues mainly liver. • Methyl donors: Ø The major donor of methyl group in the body is the active form of methionine i. e. S-adenosyl methionine "SAM". By Dr. Mohammed Hosny Hassan, Lecturer of Medical Biochemistry, Faculty of Medicine, South Valley University

• Methyl acceptors: 1. Noradrenaline to adrenaline. 2. Guanidoacetic acid to guanidoacetic acid "creatine“. 3. Uracil to thymine. By Dr. Mohammed Hosny Hassan, Lecturer of Medical Biochemistry, Faculty of Medicine, South Valley University N-methyl

Questions Q 1: Mention various states of nitrogen balance. Q 2: Compare between each of the following: A) ALT & AST. B) L-amino acid oxidase & D- amino acid oxidase. Q 3: Discuss non-oxidative deamination. Q 4: Define transmethyaltion and give examples on methyl acceptors. Q 5: Define biogenic amines and mention two examples. By Dr. Mohammed Hosny Hassan, Lecturer of Medical Biochemistry, Faculty of Medicine, South Valley University
- Slides: 31