General Reactions Dr Ron Rusay General Chemical Reactions





























- Slides: 60

General Reactions Dr. Ron Rusay

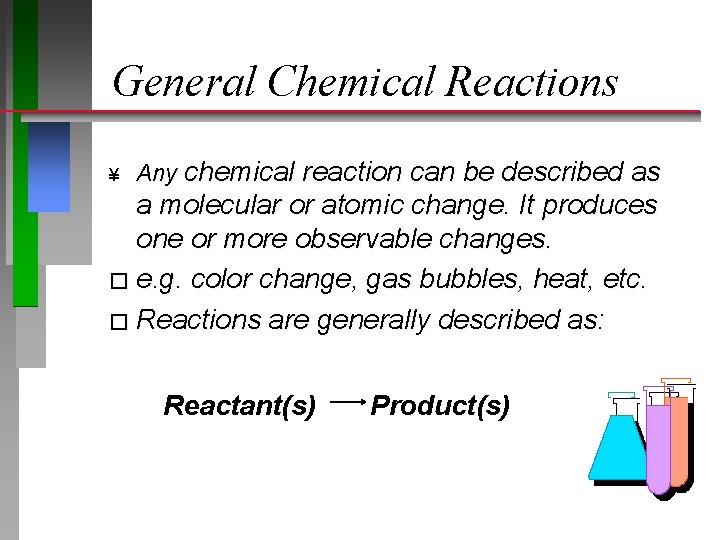
General Chemical Reactions ¥ Any chemical reaction can be described as a molecular or atomic change. It produces one or more observable changes. � e. g. color change, gas bubbles, heat, etc. � Reactions are generally described as: Reactant(s) Product(s)

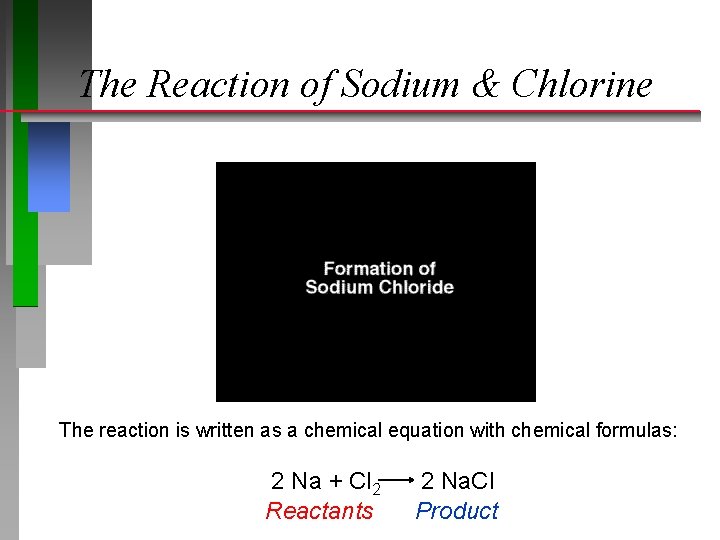
The Reaction of Sodium & Chlorine The reaction is written as a chemical equation with chemical formulas: 2 Na + Cl 2 Reactants 2 Na. Cl Product

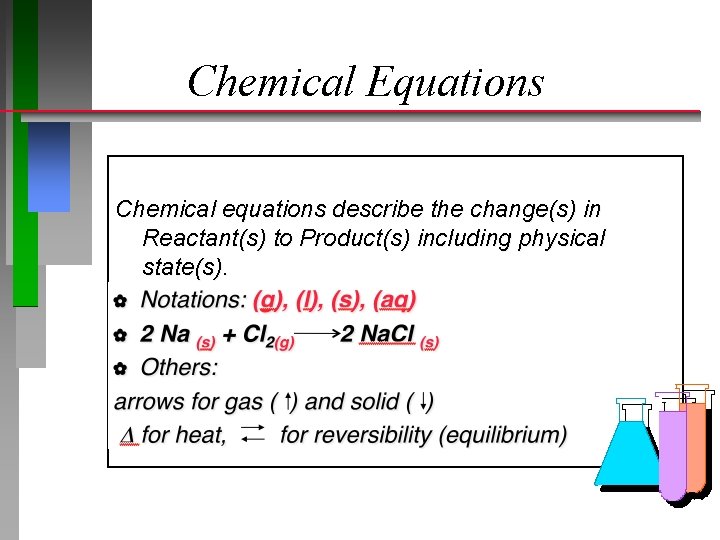
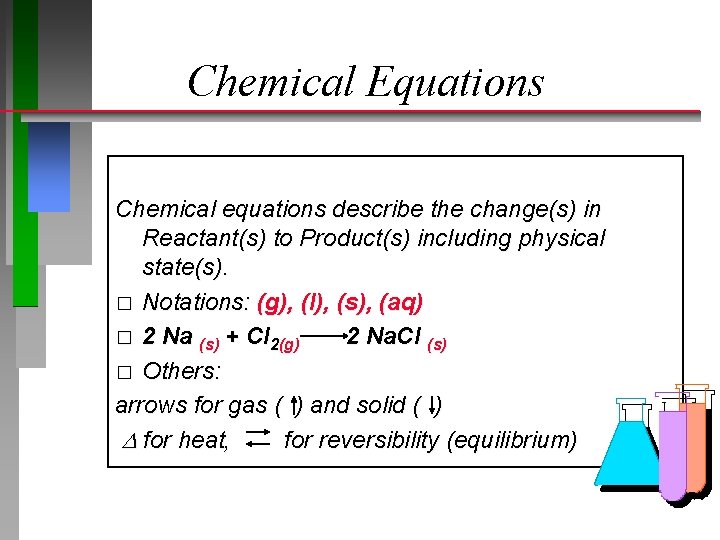
Chemical Equations Chemical equations describe the change(s) in Reactant(s) to Product(s) including physical state(s). � Notations: (g), (l), (s), (aq)

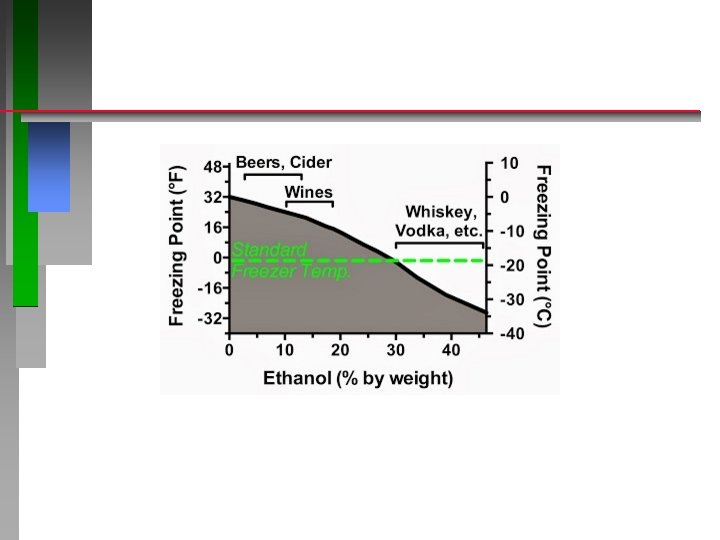
Types of Chemical Reactions � Combination (Synthesis) � Decomposition � Single Displacement � Double Displacement � Combustion: Oxidation-Reduction _________ � Biological Reactions: Enzyme Catalysts Example: Fermentation http: //www. piney. com/Bab. Ninkasi. html)

General Chemical Reactions http: //chemconnections. org/general/movies/rxn-types. mov

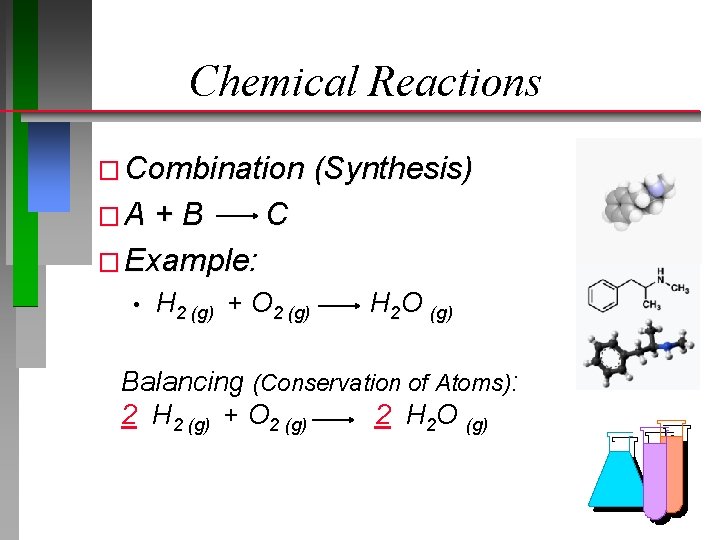
Chemical Reactions � Combination (Synthesis) �A +B C � Example: • H 2 (g) + O 2 (g) H 2 O (g) Balancing (Conservation of Atoms): 2 H 2 (g) + O 2 (g) 2 H 2 O (g)

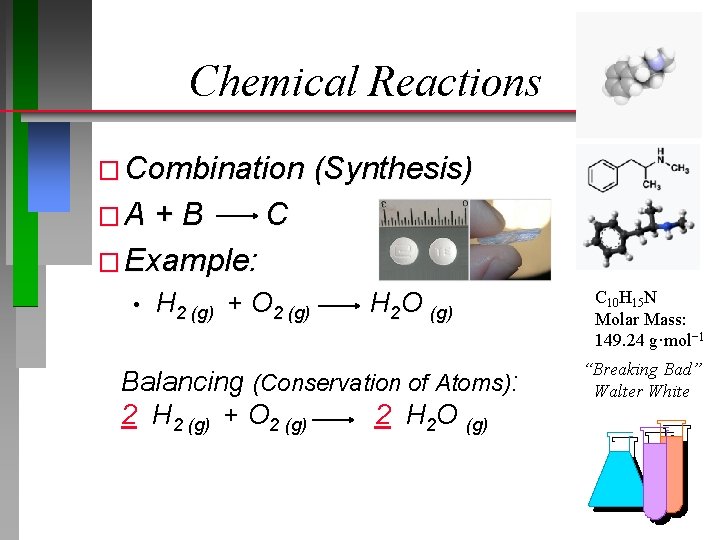
Chemical Reactions � Combination (Synthesis) �A +B C � Example: • H 2 (g) + O 2 (g) H 2 O (g) Balancing (Conservation of Atoms): 2 H 2 (g) + O 2 (g) 2 H 2 O (g) C 10 H 15 N Molar Mass: 149. 24 g·mol− 1 “Breaking Bad” Walter White

Synthesis of Water http: //chemconnections. org/general/movies/H 2 O-form. MOV Three Balloons: https: //www. youtube. com/watch? v=a 6 q. GIMq. DKw. A&index=4&list=PLA 3383 CE 72437 FC 43

An Unwanted Synthesis of Water Combustion & the Hindenburg 1937

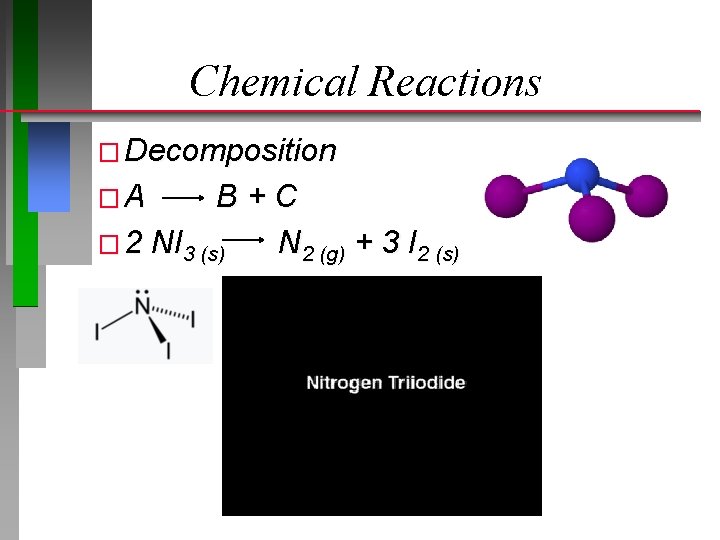
Chemical Reactions � Decomposition �A B+C � 2 NI 3 (s) N 2 (g) + 3 I 2 (s)

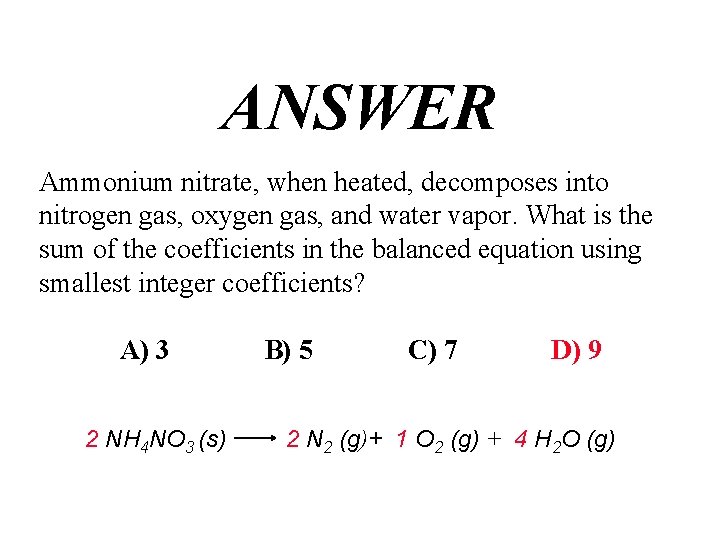
QUESTION Ammonium nitrate, when heated, decomposes into nitrogen gas, oxygen gas, and water vapor. What is the sum of the coefficients in the balanced equation using smallest integer coefficients? A) 3 B) 5 C) 7 D) 9

ANSWER Ammonium nitrate, when heated, decomposes into nitrogen gas, oxygen gas, and water vapor. What is the sum of the coefficients in the balanced equation using smallest integer coefficients? A) 3 2 NH 4 NO 3 (s) B) 5 C) 7 D) 9 2 N 2 (g)+ 1 O 2 (g) + 4 H 2 O (g)

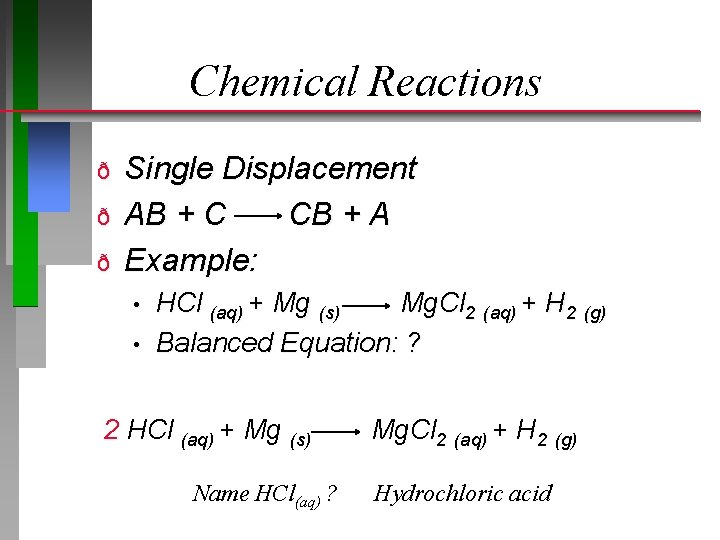
Chemical Reactions ð ð ð Single Displacement AB + C CB + A Example: • • HCl (aq) + Mg (s) Mg. Cl 2 (aq) + H 2 (g) Balanced Equation: ? 2 HCl (aq) + Mg (s) Name HCl(aq) ? Mg. Cl 2 (aq) + H 2 (g) Hydrochloric acid

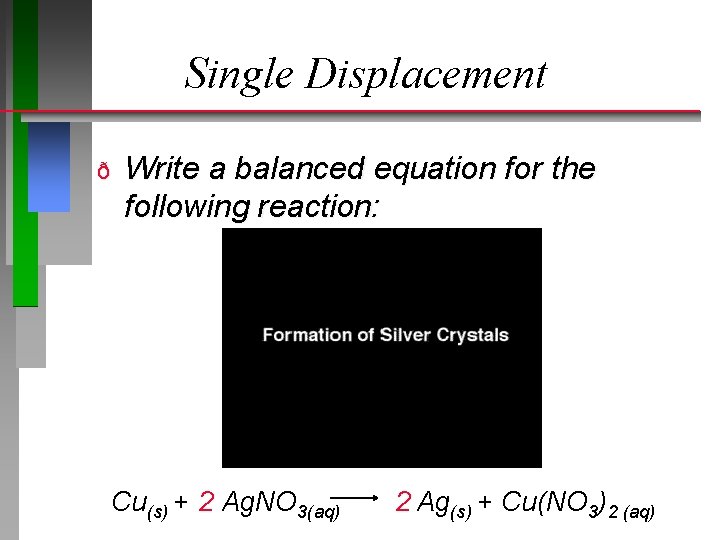
Single Displacement ð Write a balanced equation for the following reaction: Cu(s) + 2 Ag. NO 3(aq) 2 Ag(s) + Cu(NO 3)2 (aq)

Molecular Modeling (Individual or Collaborative) Report Form (Replacement pages for Molecular Model Lab pp. 97 -103) http: //chemconnections. org/general/chem 108/Chemistry%20108%20 Molecular%20 Modeling%20 Form%20 F all%202017. pdf Turn-in individually or one per group Due TODAY

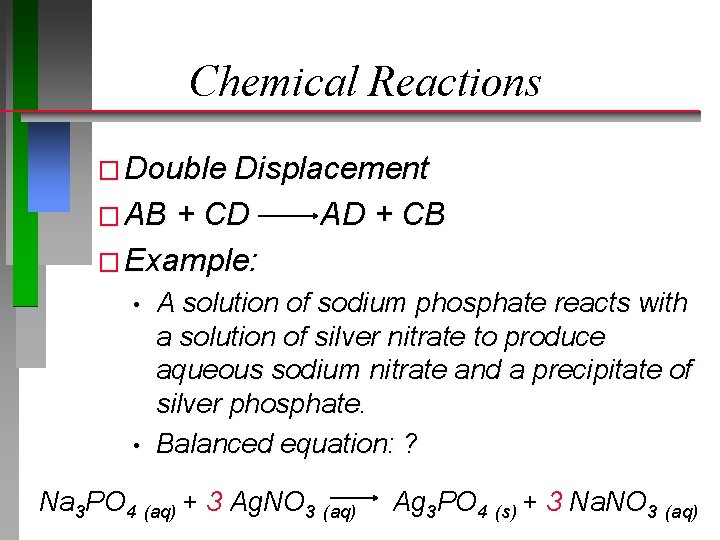
Chemical Reactions � Double Displacement � AB + CD AD + CB � Example: • • A solution of sodium phosphate reacts with a solution of silver nitrate to produce aqueous sodium nitrate and a precipitate of silver phosphate. Balanced equation: ? Na 3 PO 4 (aq) + 3 Ag. NO 3 (aq) Ag 3 PO 4 (s) + 3 Na. NO 3 (aq)

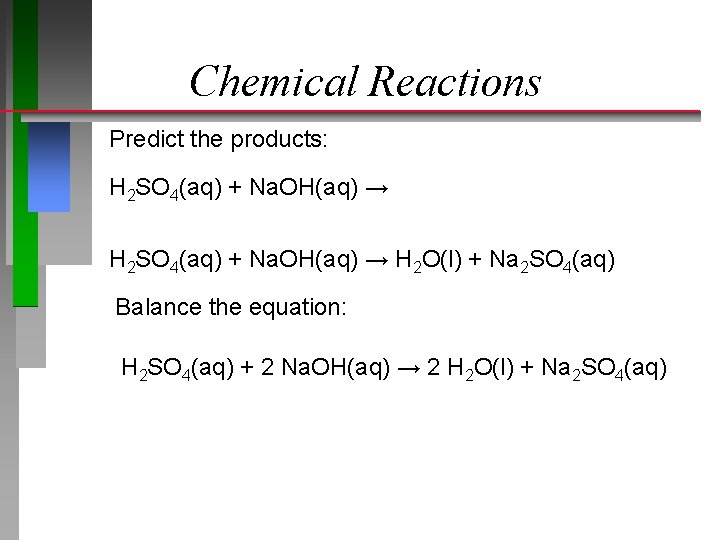
Chemical Reactions Predict the products: H 2 SO 4(aq) + Na. OH(aq) → H 2 O(l) + Na 2 SO 4(aq) Balance the equation: H 2 SO 4(aq) + 2 Na. OH(aq) → 2 H 2 O(l) + Na 2 SO 4(aq)

Post lab https: //phet. colorado. edu/sims/html/balancing-chemical-equations/latest/balancing-chemicalequations_en. html

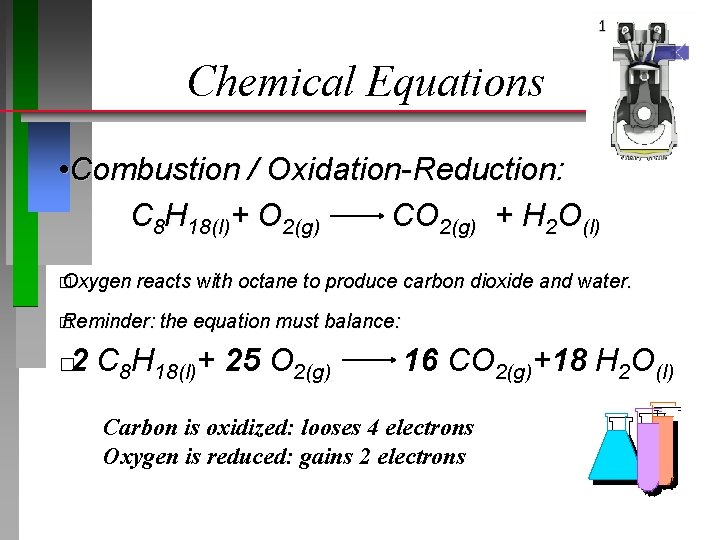
Chemical Equations • Combustion / Oxidation-Reduction: C 8 H 18(l)+ O 2(g) CO 2(g) + H 2 O(l) � Oxygen reacts with octane to produce carbon dioxide and water. � Reminder: the equation must balance: 2 C 8 H 18(l)+ 25 O 2(g) � 16 CO 2(g)+18 H 2 O(l) Carbon is oxidized: looses 4 electrons Oxygen is reduced: gains 2 electrons

QUESTION

ANSWER D) 13 2 C 8 H 18 (l)+ 25 O 2(g) 16 CO 2(g)+18 H 2 O(l) 2 C 4 H 10 (l)+ 13 O 2(g) 8 CO 2(g)+ 10 H 2 O(l) O 2 should be balanced last since it contains only one type of element and balancing it will not cause an imbalance in another element.

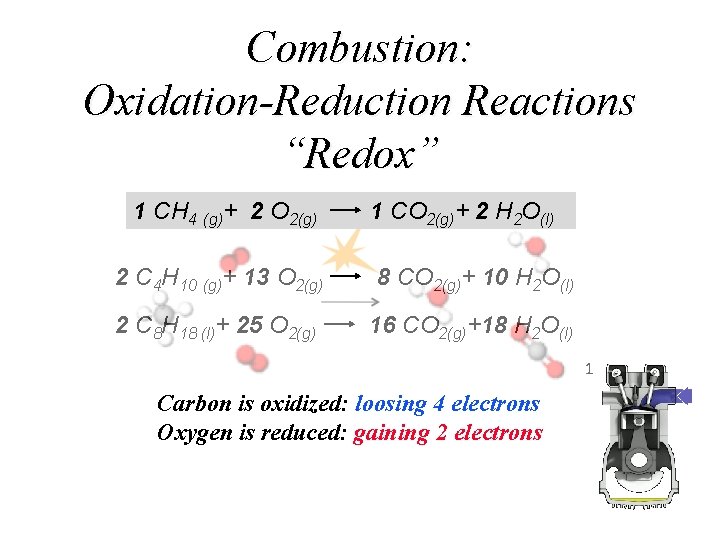
Combustion: Oxidation-Reduction Reactions “Redox” 1 CH 4 (g)+ 2 O 2(g) 1 CO 2(g)+ 2 H 2 O(l) 2 C 4 H 10 (g)+ 13 O 2(g) 8 CO 2(g)+ 10 H 2 O(l) 2 C 8 H 18 (l)+ 25 O 2(g) 16 CO 2(g)+18 H 2 O(l) Carbon is oxidized: loosing 4 electrons Oxygen is reduced: gaining 2 electrons

Combustion Products Formulas & Multiple Proportions http: //chemconnections. org/general/movies/multiple-proportions. MOV Carbon can also be oxidized by loosing 2 electrons Oxygen remains reduced by gaining 2 electrons 2 CH 4 (g)+ 3 O 2(g) 1 CH 4 (g)+ 2 O 2(g) 2 COg)+ 4 H 2 O(l) 1 CO 2(g)+ 2 H 2 O(l)

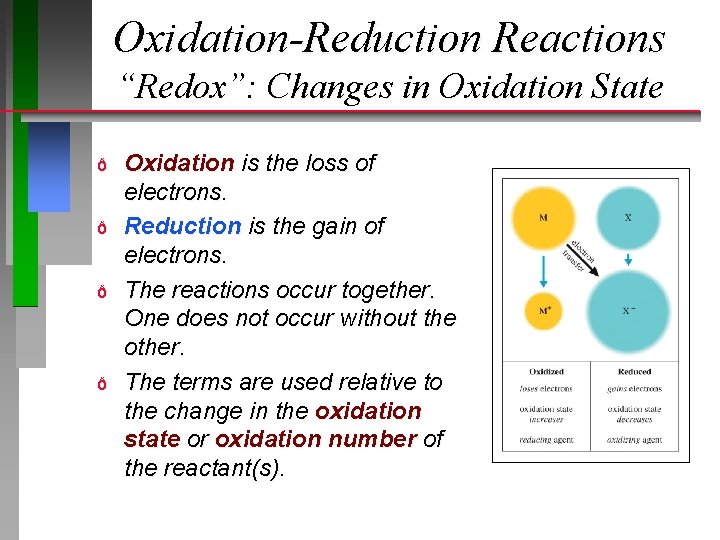
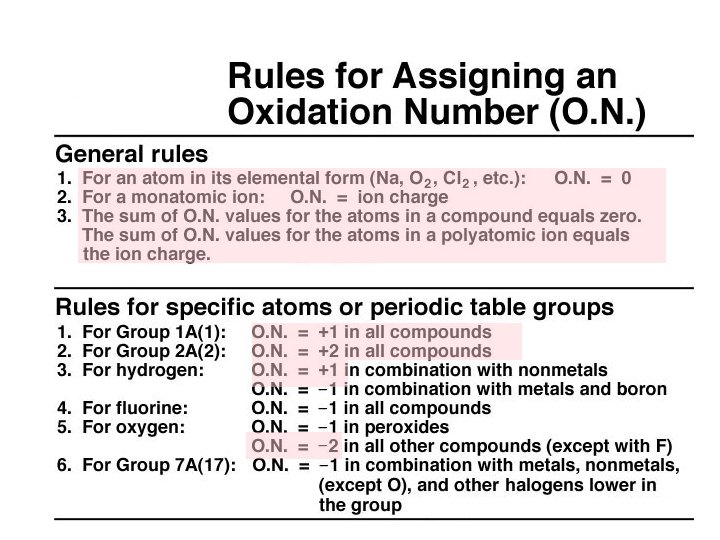
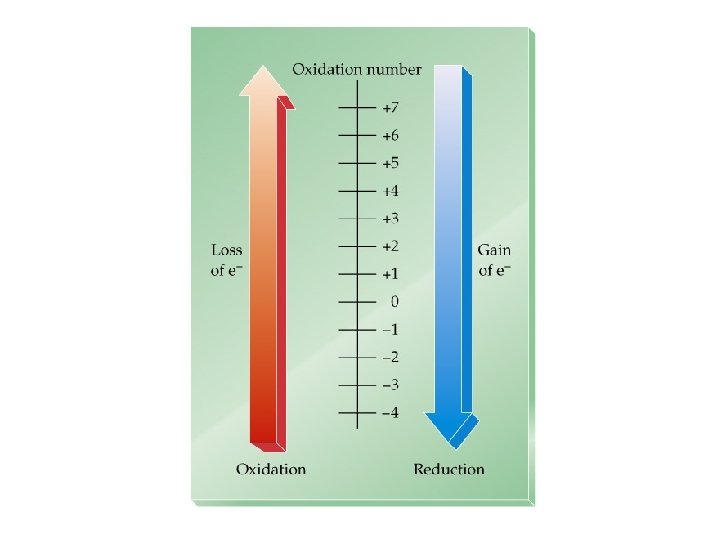
Oxidation-Reduction Reactions “Redox”: Changes in Oxidation State ð ð Oxidation is the loss of electrons. Reduction is the gain of electrons. The reactions occur together. One does not occur without the other. The terms are used relative to the change in the oxidation state or oxidation number of the reactant(s).

Oxidation Number (State) http: //chemconnections. org/general/movies/Oxid%20 States. MOV


Tutorial https: //www. youtube. com/watch? v=w 0 Rf. MRDy 34 w

Tutorial https: //www. youtube. com/watch? v=w 0 Rf. MRDy 34 w

QUESTION

ANSWER B) NO 2 Oxygen has an oxidation state of – 2 when part of a compound.

QUESTION (More Challenging) What is the oxidation number of chromium in ammonium dichromate? A) +3 Formula: ? B) +4 C) +5 (NH 4)2 Cr 2 O 7 D) +6

ANSWER What is the oxidation number of chromium in ammonium dichromate? A) +3 B) +4 C) +5 2 x + 7(-2) = -2 Cr 2 O 722 x = +14 -2 x = 12/2 D) +6

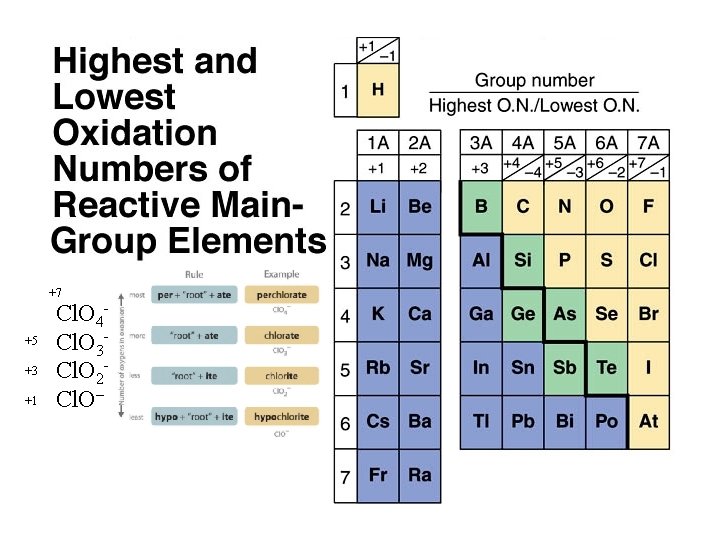
+7 +5 +3 +1 Cl. O 4– Cl. O 3– Cl. O 2– Cl. O–

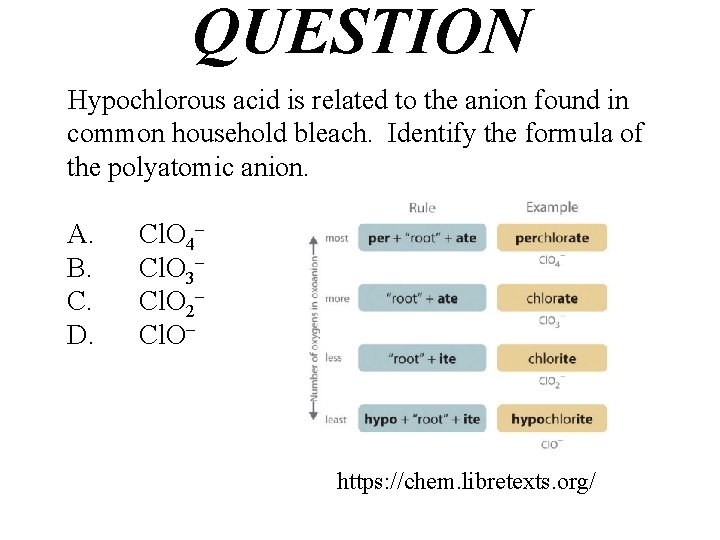
QUESTION Hypochlorous acid is related to the anion found in common household bleach. Identify the formula of the polyatomic anion. A. B. C. D. Cl. O 4– Cl. O 3– Cl. O 2– Cl. O– https: //chem. libretexts. org/

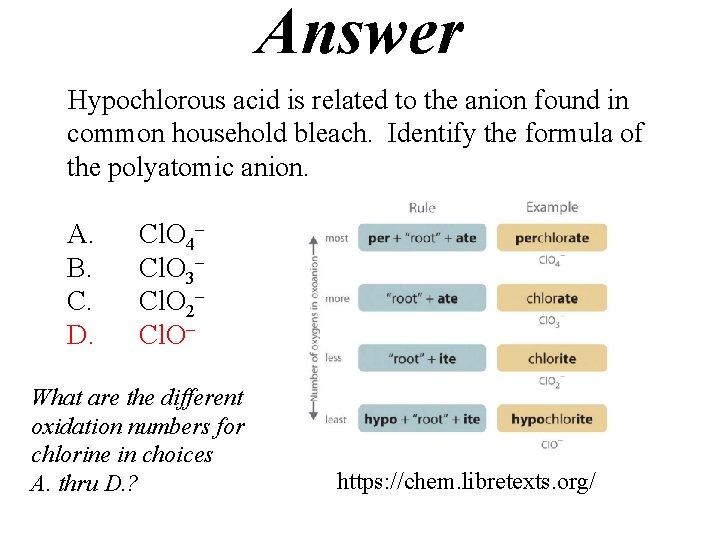
Answer Hypochlorous acid is related to the anion found in common household bleach. Identify the formula of the polyatomic anion. A. B. C. D. Cl. O 4– Cl. O 3– Cl. O 2– Cl. O– What are the different oxidation numbers for chlorine in choices A. thru D. ? https: //chem. libretexts. org/


QUESTION In a redox reaction, oxidation and reduction must both occur. Which statement is an accurate statement? A. The substance (atom) that is oxidized has a lower oxidation number in the product. B. The substance that is oxidized gains electrons. C. The substance that is oxidized must have a higher oxidation number afterwards. D. The substance that is oxidized must combine with oxygen.

ANSWER In a redox reaction, oxidation and reduction must both occur. Which statement is an accurate statement? A. The substance (atom) that is oxidized has a lower oxidation number in the product. B. The substance that is oxidized gains electrons. C. The substance that is oxidized must have a higher oxidation number afterwards. D. The substance that is oxidized must combine with oxygen.

Activity increases going down the row https: //www. youtube. com/watch? v=UO 0 CKJ 0 ubw. M Cesium: https: //www. youtube. com/watch? v=D 4 p. Qz 3 TC 0 Jo Rubidium: https: //www. youtube. com/watch? v=i. P 6 CRZd. Du 6 o Activity increases going up the row Which would be most reactive? A) Li + I 2 B) Na + Cl 2 C) Cs + F 2 Write a balanced equation for the rubidium reacting.

QUESTION

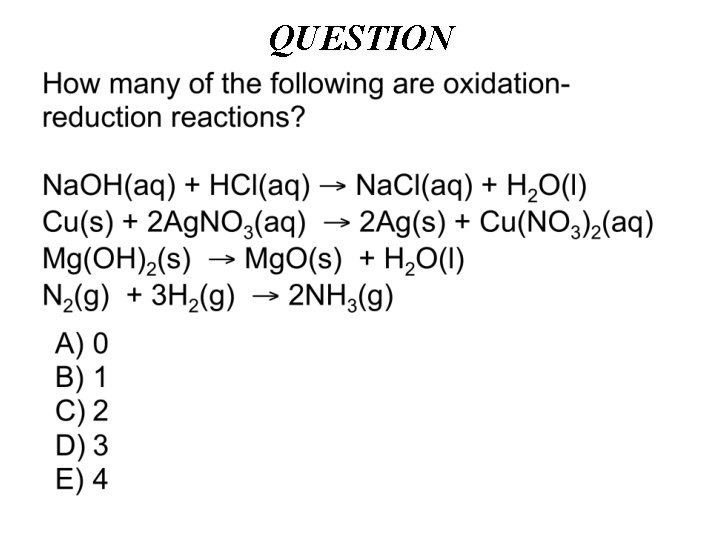
ANSWER C) 2 0 0 Cu(s) + 2 Ag. NO 3(aq) 2 Ag(s) + Cu(NO 3)2(aq) 0 0 N 2(g) + 3 H 2(g) 2 NH 3(g) If an element is a reactant or product, it is a redox reaction. The oxidation number = 0.

QUESTION Cu(s) + 2 Ag. NO 3(aq) 2 Ag(s) + Cu(NO 3)2(aq) TRUE (A) / FALSE (B) Copper is oxidized in the reaction and silver is reduced.

0 +1 Answer 0 +2 Cu(s) + 2 Ag. NO 3(aq) 2 Ag(s) + Cu(NO 3)2(aq) TRUE (A) / FALSE (B) Copper is oxidized in the reaction and silver is reduced.

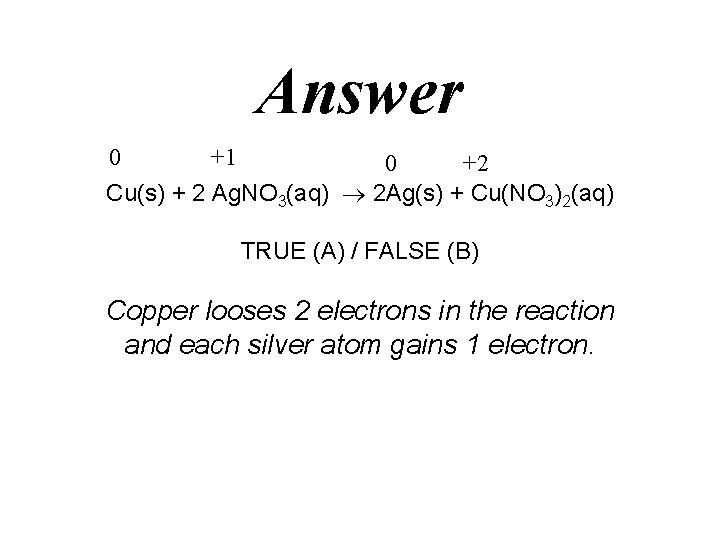
Question 0 +1 0 +2 Cu(s) + 2 Ag. NO 3(aq) 2 Ag(s) + Cu(NO 3)2(aq) TRUE (A) / FALSE (B) Copper looses 2 electrons in the reaction and each silver atom gains 1 electron.

Answer 0 +1 0 +2 Cu(s) + 2 Ag. NO 3(aq) 2 Ag(s) + Cu(NO 3)2(aq) TRUE (A) / FALSE (B) Copper looses 2 electrons in the reaction and each silver atom gains 1 electron.

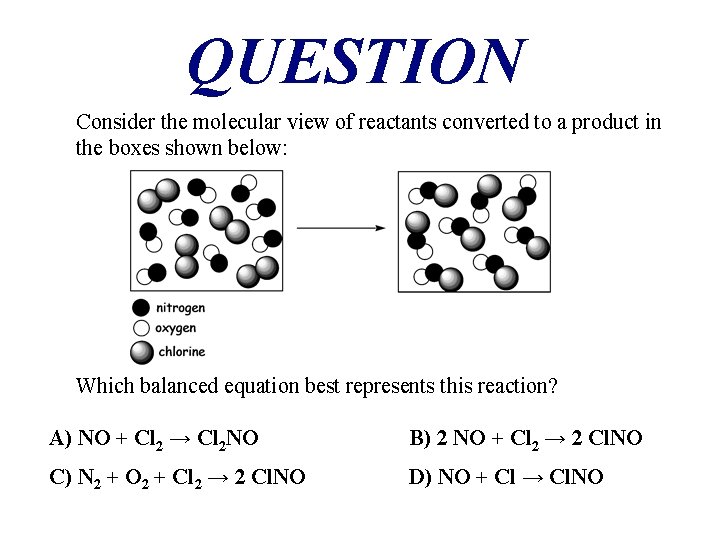
QUESTION Consider the molecular view of reactants converted to a product in the boxes shown below: Which balanced equation best represents this reaction? A) NO + Cl 2 → Cl 2 NO B) 2 NO + Cl 2 → 2 Cl. NO C) N 2 + O 2 + Cl 2 → 2 Cl. NO D) NO + Cl → Cl. NO

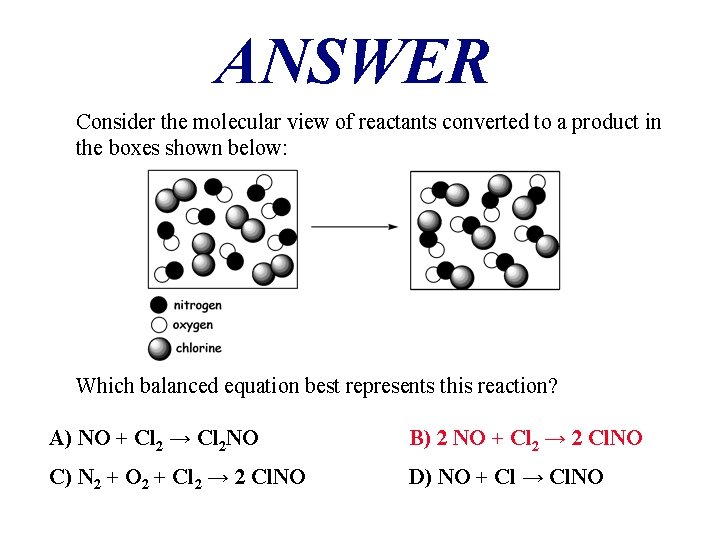
ANSWER Consider the molecular view of reactants converted to a product in the boxes shown below: Which balanced equation best represents this reaction? A) NO + Cl 2 → Cl 2 NO B) 2 NO + Cl 2 → 2 Cl. NO C) N 2 + O 2 + Cl 2 → 2 Cl. NO D) NO + Cl → Cl. NO


Molecular Modeling (Individual or Collaborative) Report Form (Replacement pages for Molecular Model Lab pp. 97 -103) http: //chemconnections. org/general/chem 108/Chemistry%20108%20 Molecular%20 Modeling%20 Form%20 F all%202017. pdf Computers & Internet available in PS 110, if needed http: //molview. org Turn-in individually or one per group Due Today

Moles / Molar Mass and Molecular Formulas i-clicker QUIZ QUESTIONS http: //chemconnections. org/general/chem 108/Moles-Molar Mass Quiz. html Submit individually on-line Refer to Calendar & Resources pages for links

Molecular Shapes Quiz) http: //chemconnections. org/general/chem 108/Molecular%20 Shapes%20 Quiz. htm l Submit individually on-line DUE Friday

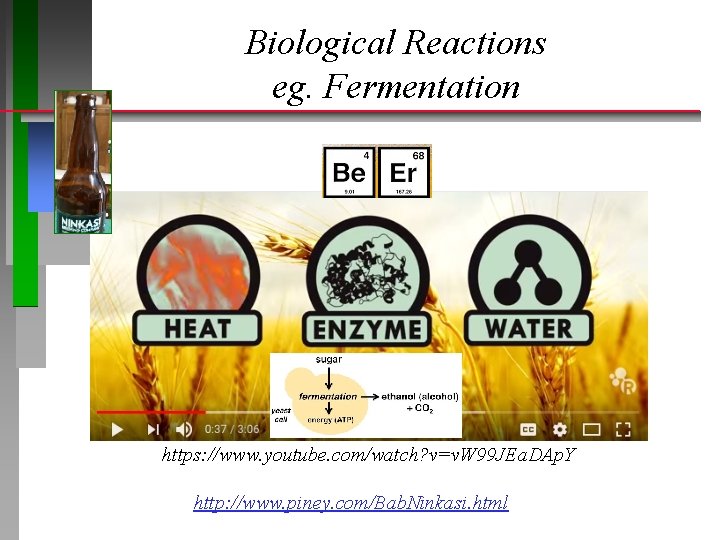
Biological Reactions eg. Fermentation https: //www. youtube. com/watch? v=v. W 99 JEa. DAp. Y http: //www. piney. com/Bab. Ninkasi. html

https: //www. youtube. com/watch? v=OKRJ 73 Ro 5 jw https: //www. youtube. com/watch? v=r. L 14 u. Lb. SLLc

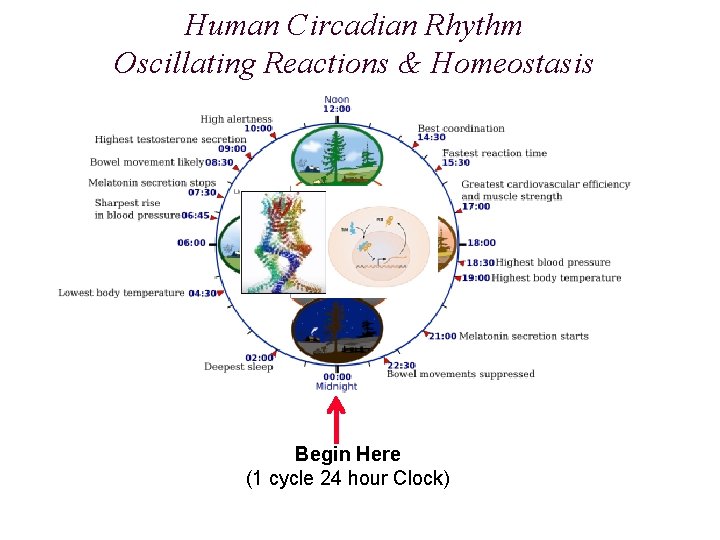
Human Circadian Rhythm Oscillating Reactions & Homeostasis Begin Here (1 cycle 24 hour Clock)


General Chemical Reactions ¥ � � � Any chemical reaction can be described as a molecular or atomic change. It produces one or more observable changes. e. g. color change, gas bubbles, heat, etc. Reactions are generally described as Reactant(s) Product(s) The reaction is written as a chemical equation with chemical formulas: 2 Na + Cl 2 2 Na. Cl

The Reaction of Sodium & Chlorine

Chemical Equations Chemical equations describe the change(s) in Reactant(s) to Product(s) including physical state(s). � Notations: (g), (l), (s), (aq) � 2 Na (s) + Cl 2(g) 2 Na. Cl (s) � Others: arrows for gas ( ) and solid ( ) for heat, for reversibility (equilibrium)

QUESTION
 Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Rusay
Rusay Types of reactions
Types of reactions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Are kc and kp equal
Are kc and kp equal Five chemical changes
Five chemical changes 5 general types of chemical reactions
5 general types of chemical reactions What are the five general types of chemical reactions
What are the five general types of chemical reactions 10 examples of redox reaction
10 examples of redox reaction Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Stoichiometry mole island diagram
Stoichiometry mole island diagram Examples of chemical change
Examples of chemical change Types of redox reactions
Types of redox reactions Types of reactions
Types of reactions Types of reactions chemistry
Types of reactions chemistry Types of reaction
Types of reaction Predicting products of chemical reactions
Predicting products of chemical reactions 4 types of chemical reactions
4 types of chemical reactions Non examples of chemical reactions
Non examples of chemical reactions Chapter 10 chemical reactions answer key
Chapter 10 chemical reactions answer key The calculations of quantities in chemical reactions
The calculations of quantities in chemical reactions Principles of immuno chemical reactions
Principles of immuno chemical reactions Predicting products of chemical reactions
Predicting products of chemical reactions Predicting products synthesis
Predicting products synthesis Combination reaction example
Combination reaction example Unit 11 chemical reactions
Unit 11 chemical reactions Toxic reactions chemical equations lesson 68 answers
Toxic reactions chemical equations lesson 68 answers Four types of chemical reactions
Four types of chemical reactions Www.biology-roots.com
Www.biology-roots.com Describing chemical reactions
Describing chemical reactions Chemical reactions classification
Chemical reactions classification Chemical reactions in everyday life
Chemical reactions in everyday life Chemical reactions reactants and products
Chemical reactions reactants and products Chemical reactions study guide
Chemical reactions study guide Chapter 9 chemical reactions
Chapter 9 chemical reactions Rate constant and equilibrium constant
Rate constant and equilibrium constant Chapter 9 chemical reactions
Chapter 9 chemical reactions What are the 4 types of chemical reactions
What are the 4 types of chemical reactions Chapter 8 review chemical equations and reactions
Chapter 8 review chemical equations and reactions Four types of chemical reactions
Four types of chemical reactions Chapter 9 study guide chemical reactions
Chapter 9 study guide chemical reactions Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Balancing equations chapter 8
Balancing equations chapter 8 5 chemical reactions
5 chemical reactions Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Examples of double replacement reactions
Examples of double replacement reactions Chapter 11 chemical reactions answer key
Chapter 11 chemical reactions answer key Chapter 11 chemical reactions practice problems
Chapter 11 chemical reactions practice problems Physical properties of esters
Physical properties of esters Summary of chemical reactions
Summary of chemical reactions Yeast chemical reaction
Yeast chemical reaction Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Solvent in chemical reactions
Solvent in chemical reactions Three types of chemical reactions
Three types of chemical reactions Mass relationships in chemical reactions
Mass relationships in chemical reactions Indications of chemical reaction
Indications of chemical reaction Describing chemical reactions
Describing chemical reactions Chemical reaction rules
Chemical reaction rules In a chemical reaction... atoms aren't rearranged
In a chemical reaction... atoms aren't rearranged Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions