General Phase Diagram Sections Arthur D Pelton Centre


- Slides: 38

General Phase Diagram Sections Arthur D. Pelton Centre de Recherche en Calcul Thermochimique École Polytechnique de Montréal, Québec, CANADA General rules of construction of all true phase diagram sections; Proper choice of variables and constants to give a “true” phase diagram section (with a unique equilibrium state at each point); A general algorithm for calculating all true phase diagram sections. www. factsage. com

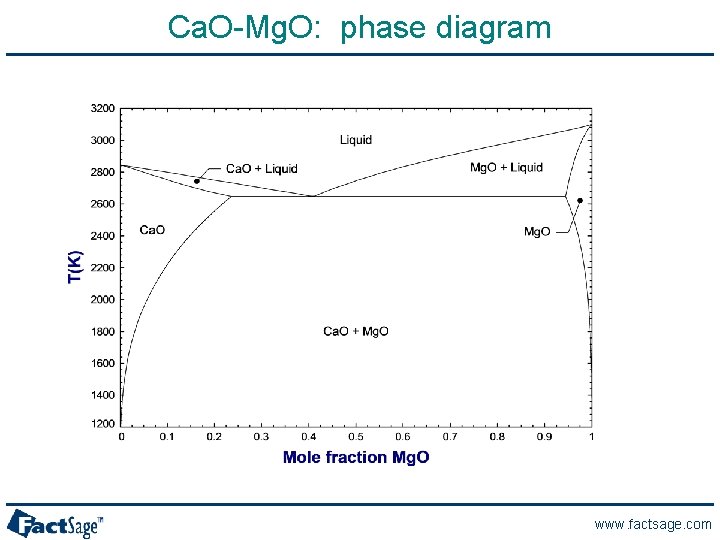
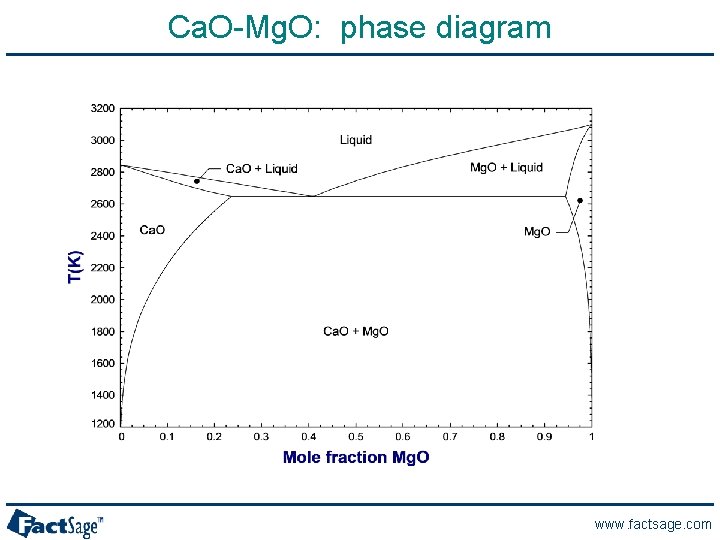
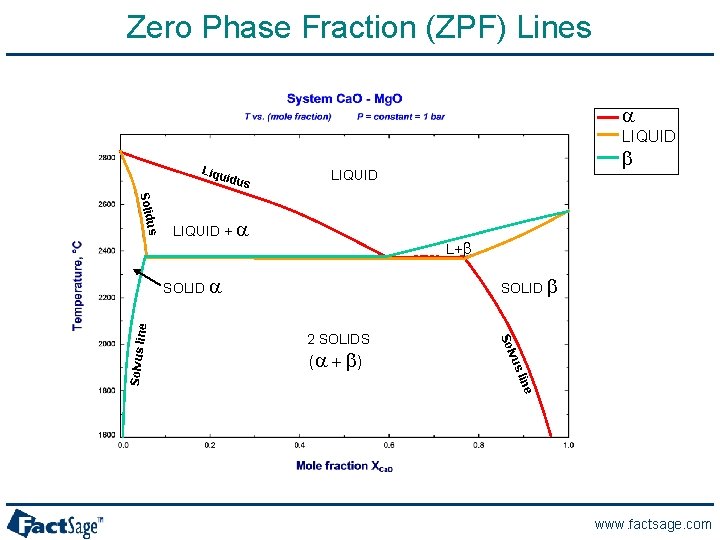
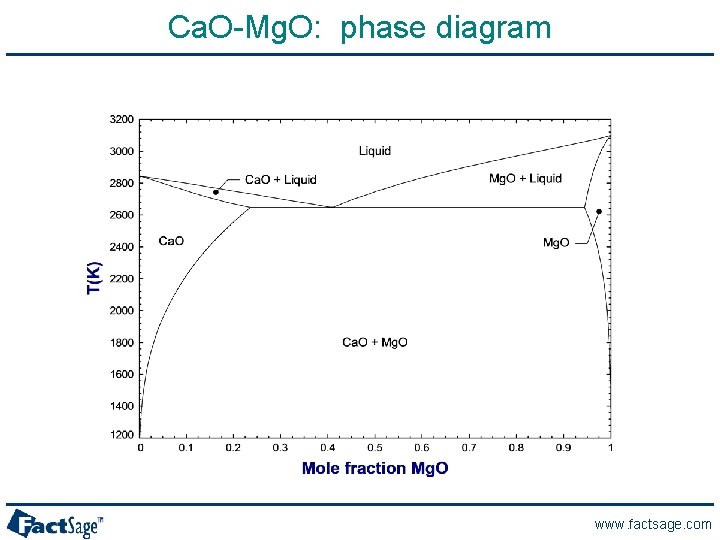
Ca. O-Mg. O: phase diagram www. factsage. com

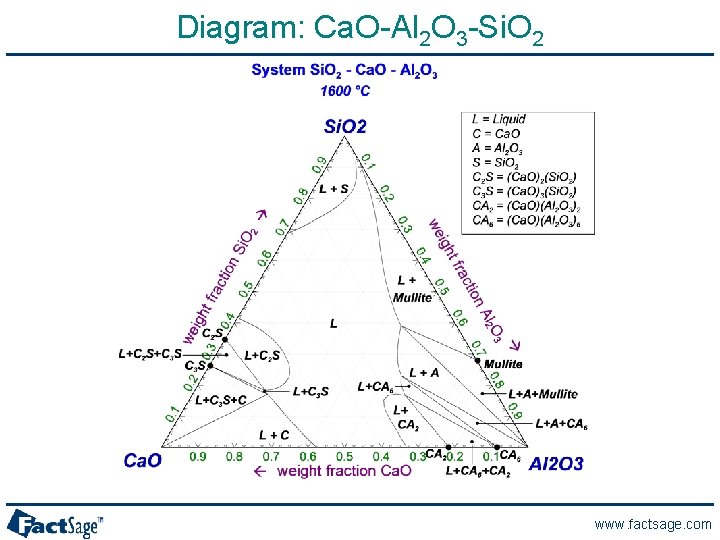
Diagram: Ca. O-Al 2 O 3 -Si. O 2 www. factsage. com

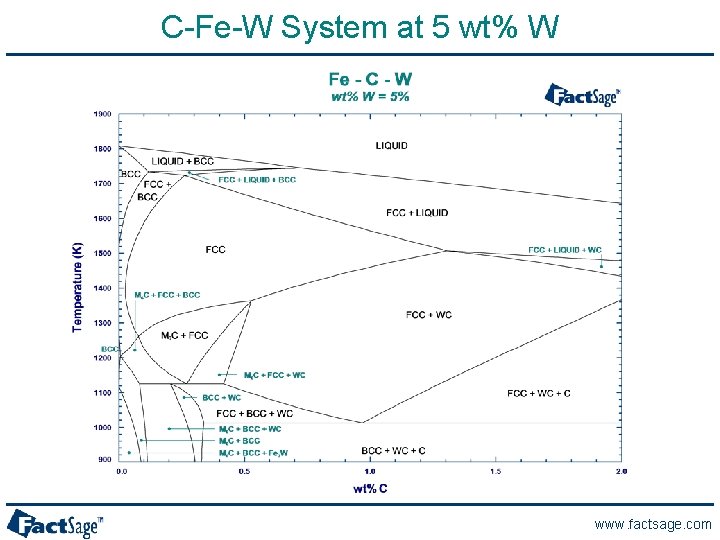
C-Fe-W System at 5 wt% W www. factsage. com

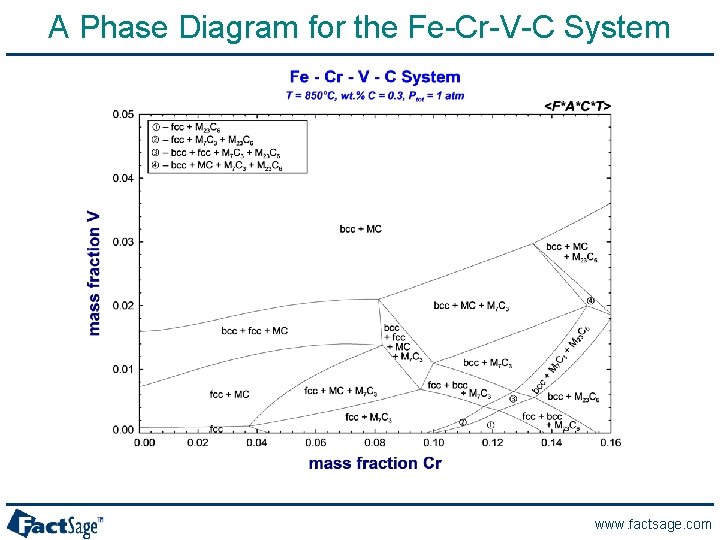
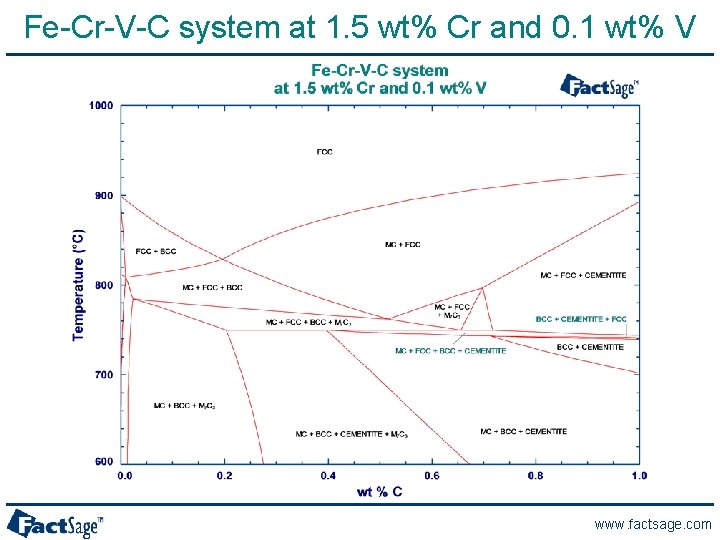
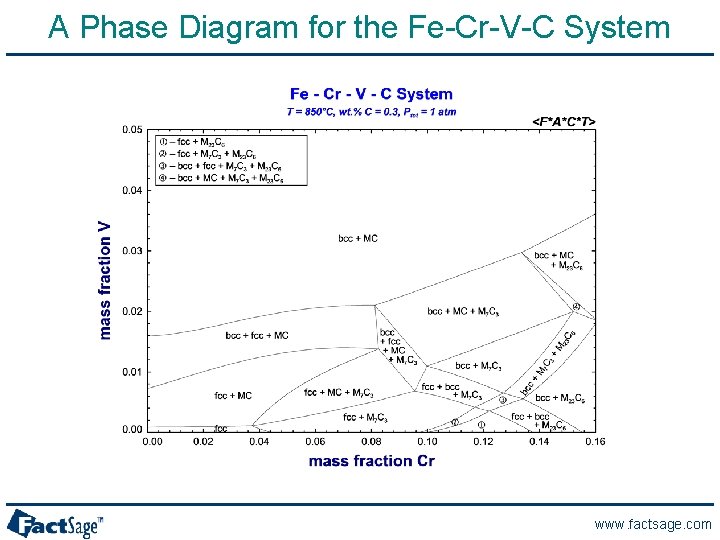
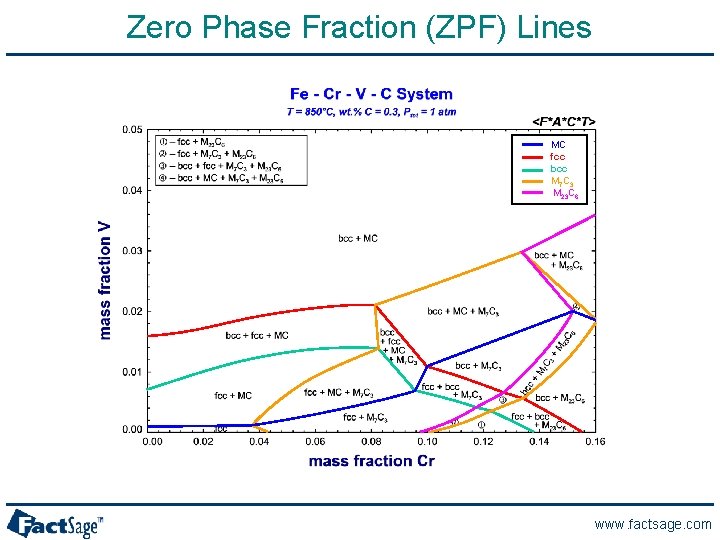
A Phase Diagram for the Fe-Cr-V-C System www. factsage. com

Fe-Cr-V-C system at 1. 5 wt% Cr and 0. 1 wt% V www. factsage. com

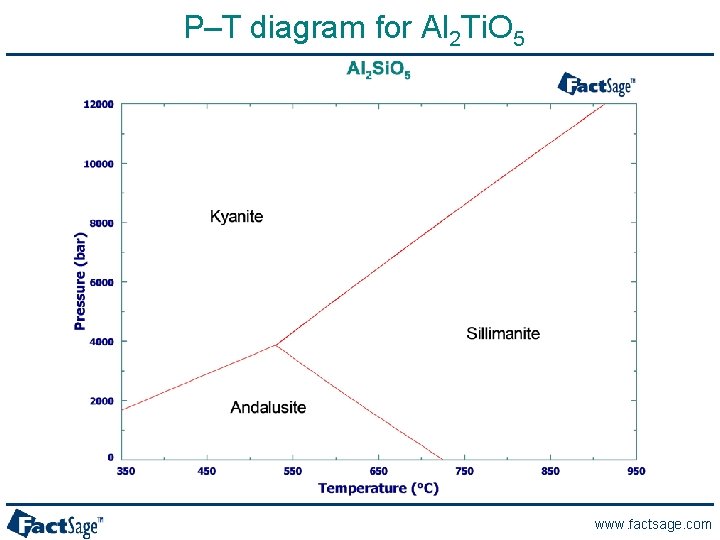
P–T diagram for Al 2 Ti. O 5 www. factsage. com

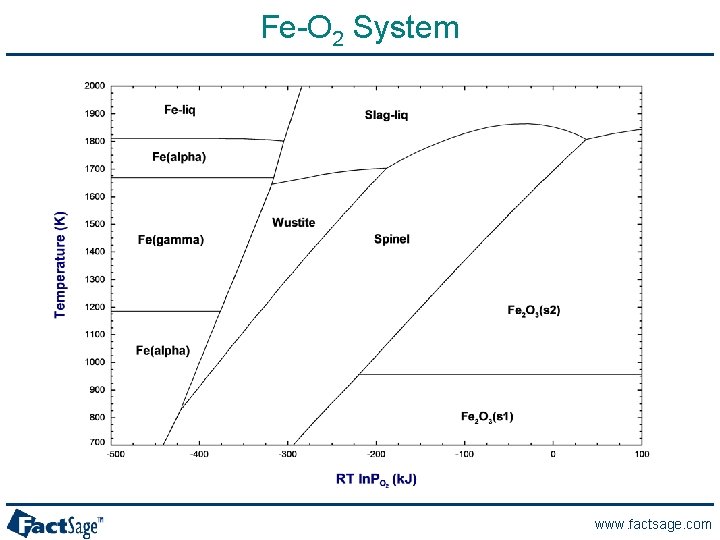
Fe-O 2 System www. factsage. com

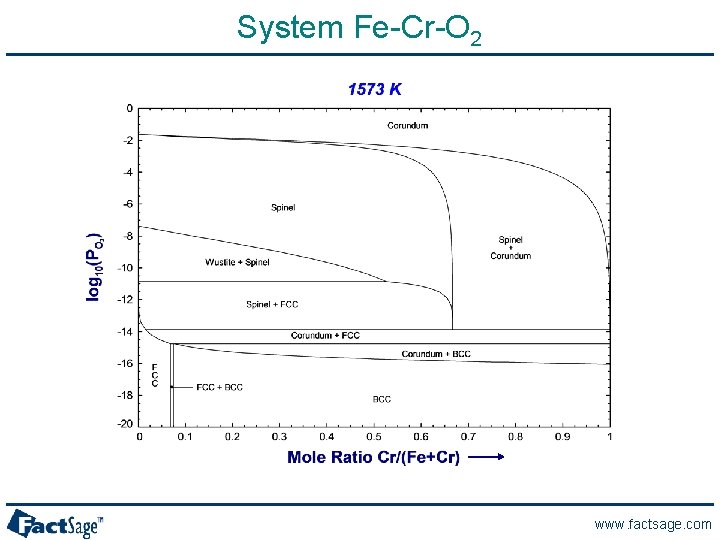
System Fe-Cr-O 2 www. factsage. com

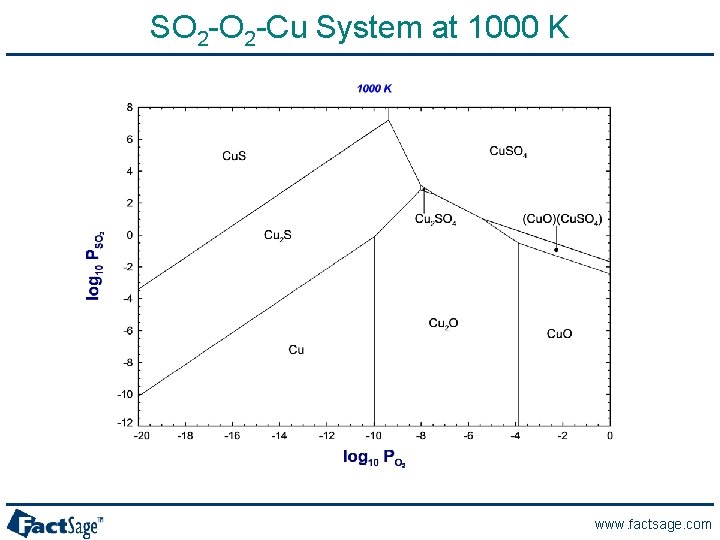
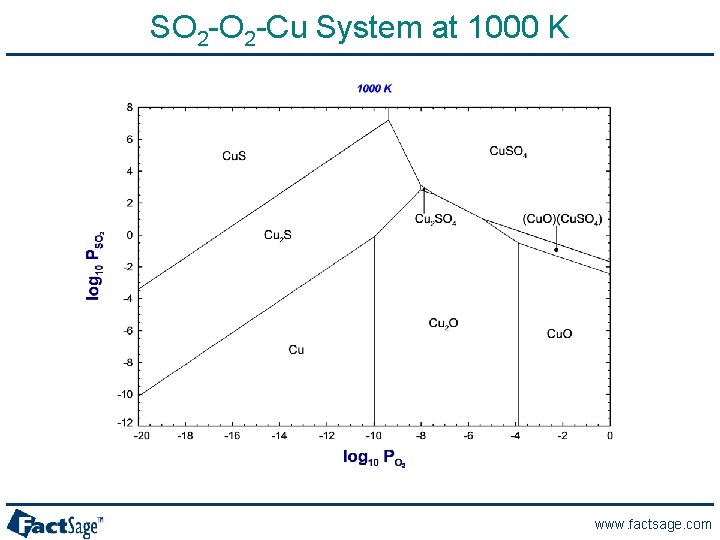
SO 2 -Cu System at 1000 K www. factsage. com

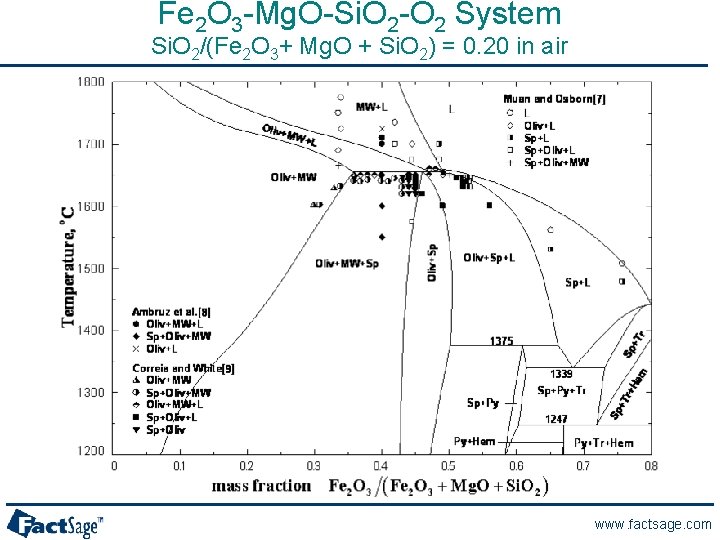
Fe 2 O 3 -Mg. O-Si. O 2 -O 2 System Si. O 2/(Fe 2 O 3+ Mg. O + Si. O 2) = 0. 20 in air www. factsage. com

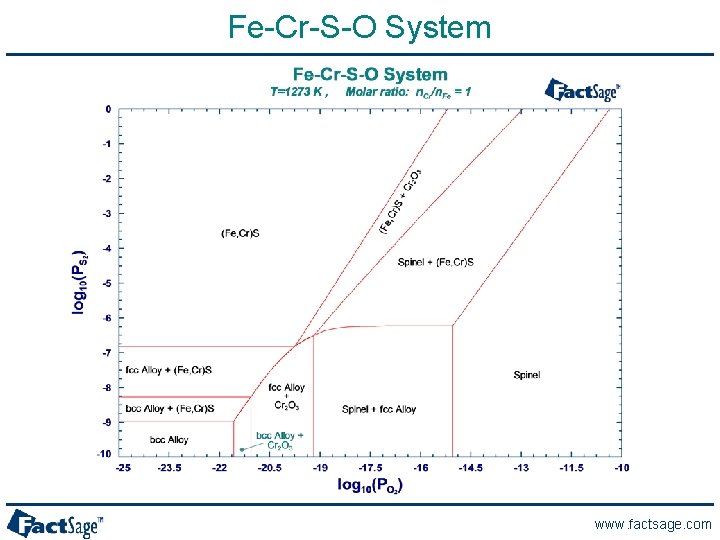
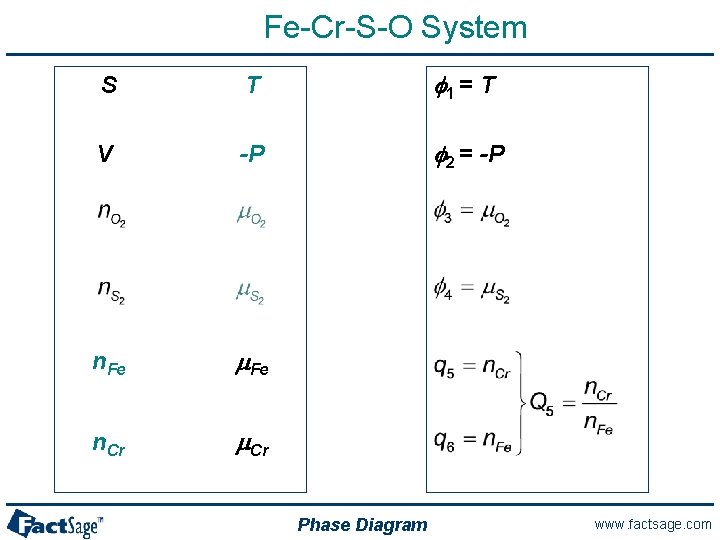
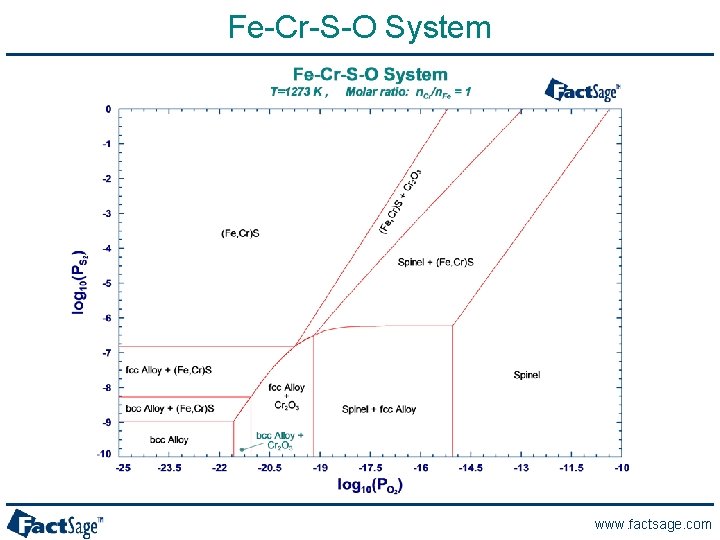
Fe-Cr-S-O System www. factsage. com

A Phase Diagram for the Fe-Cr-V-C System www. factsage. com

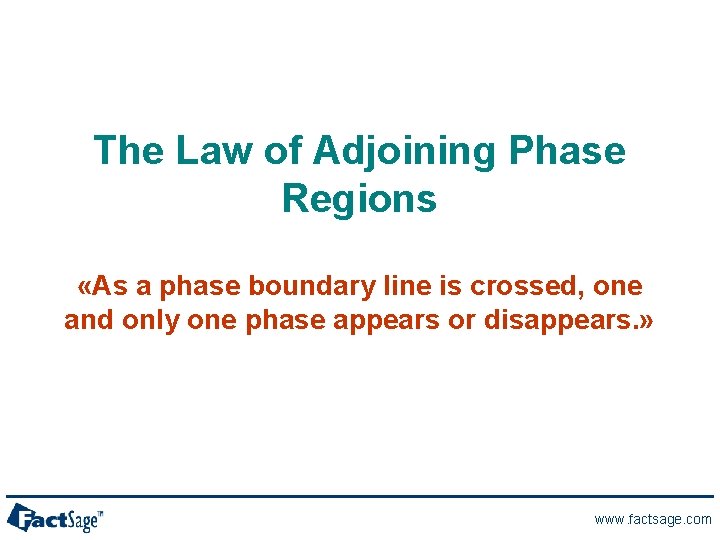
The Law of Adjoining Phase Regions «As a phase boundary line is crossed, one and only one phase appears or disappears. » www. factsage. com

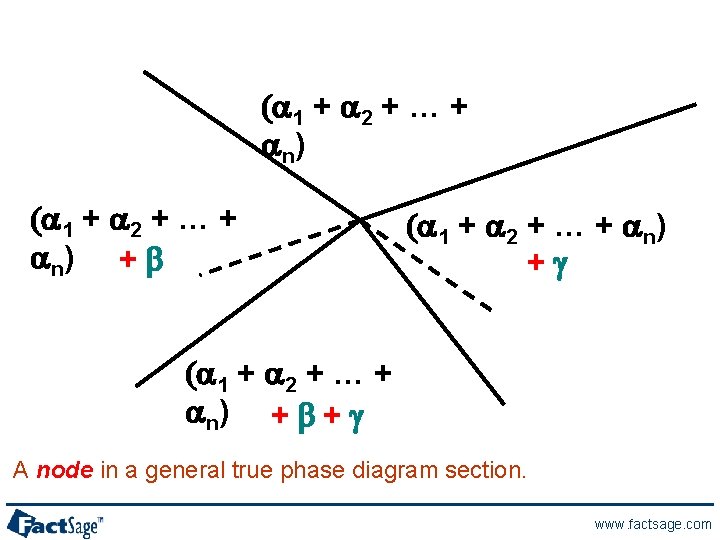
(a 1 + a 2 + … + a n) + b (a 1 + a 2 + … + an) +g (a 1 + a 2 + … + a n) + b + g A node in a general true phase diagram section. www. factsage. com

Ca. O-Mg. O: phase diagram www. factsage. com

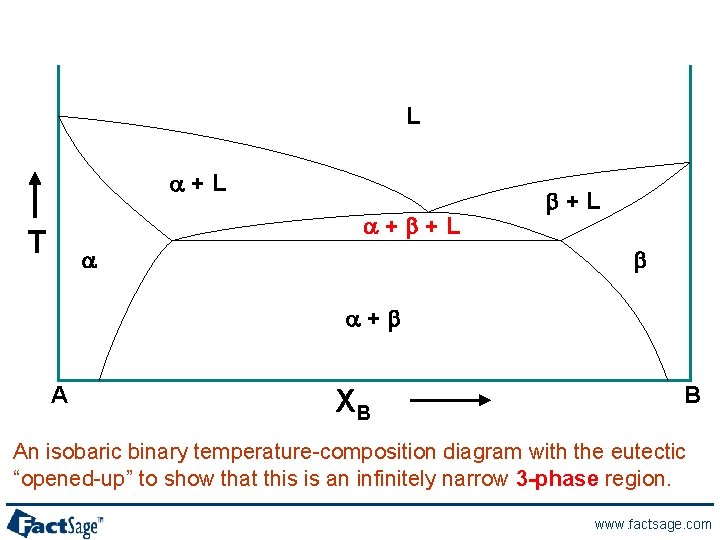
L a+b+L T a b+L b a+b A XB B An isobaric binary temperature-composition diagram with the eutectic “opened-up” to show that this is an infinitely narrow 3 -phase region. www. factsage. com

SO 2 -Cu System at 1000 K www. factsage. com

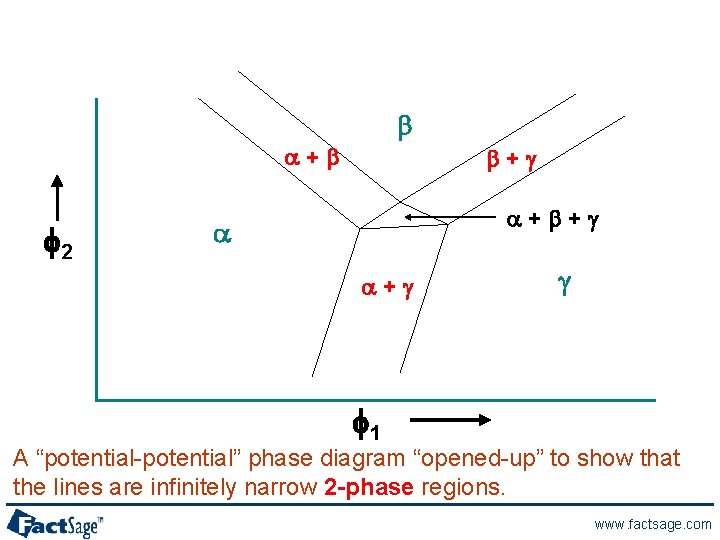
b a+b f 2 b+g a+b+g a a+g g f 1 A “potential-potential” phase diagram “opened-up” to show that the lines are infinitely narrow 2 -phase regions. www. factsage. com

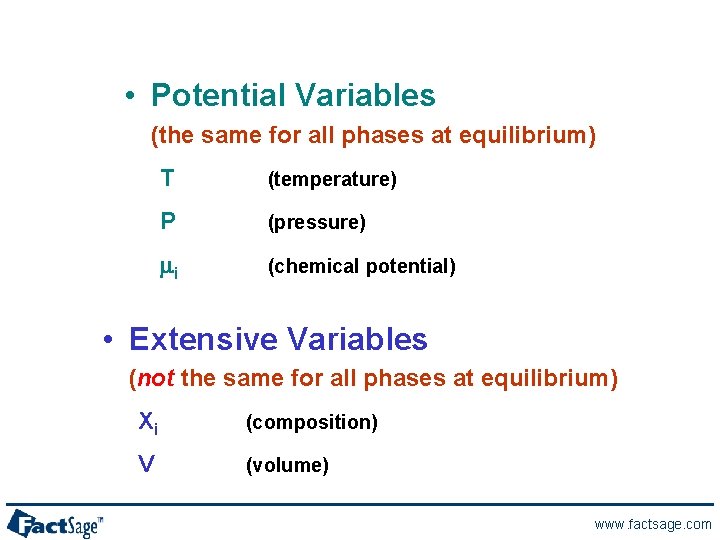
• Potential Variables (the same for all phases at equilibrium) T (temperature) P (pressure) mi (chemical potential) • Extensive Variables (not the same for all phases at equilibrium) Xi (composition) V (volume) www. factsage. com

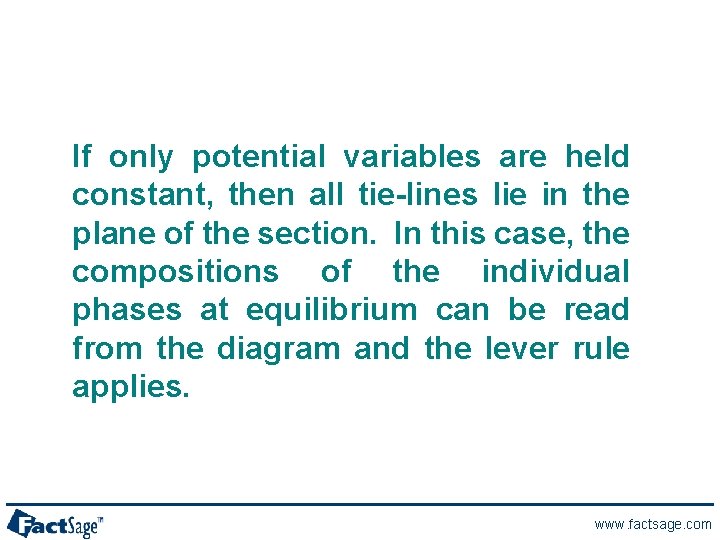
If only potential variables are held constant, then all tie-lines lie in the plane of the section. In this case, the compositions of the individual phases at equilibrium can be read from the diagram and the lever rule applies. www. factsage. com

Zero Phase Fraction (ZPF) Lines MC fcc bcc M 7 C 3 M 23 C 6 www. factsage. com

Zero Phase Fraction (ZPF) Lines a LIQUID Liqu idus us Solid LIQUID + a L+b SOLID b 2 SOLIDS vus (a + b ) Sol ne SOLID a line Solvus li b LIQUID www. factsage. com

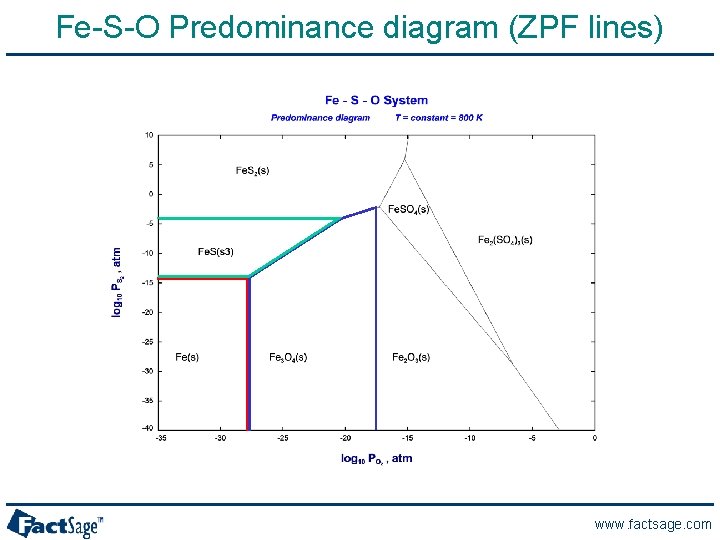
Fe-S-O Predominance diagram (ZPF lines) www. factsage. com

Choice of variables to always give a true phase diagram (single-valued) everywhere www. factsage. com

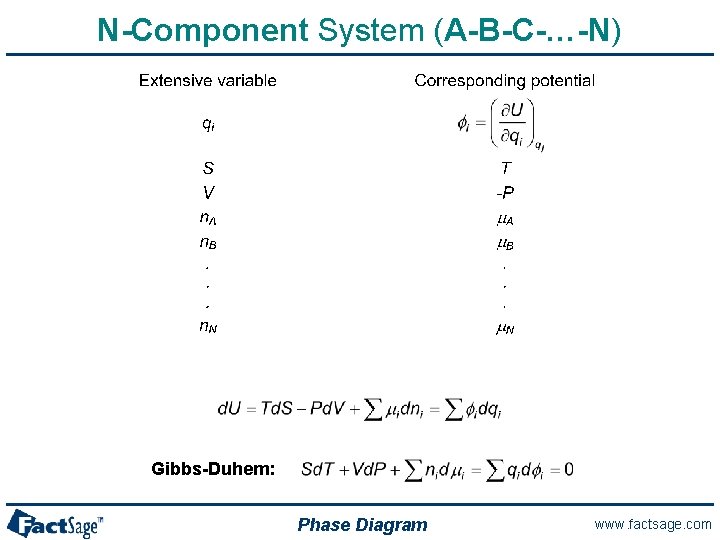
N-Component System (A-B-C-…-N) Gibbs-Duhem: Phase Diagram www. factsage. com

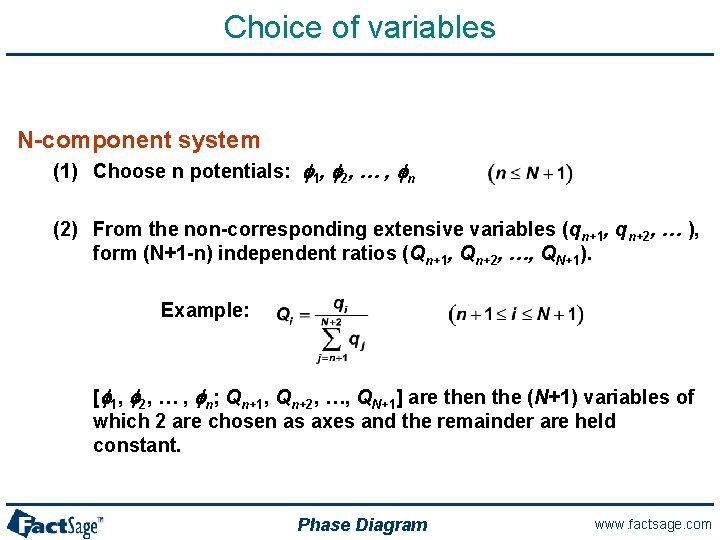
Choice of variables N-component system (1) Choose n potentials: f 1, f 2, … , fn (2) From the non-corresponding extensive variables (qn+1, qn+2, … ), form (N+1 -n) independent ratios (Qn+1, Qn+2, …, QN+1). Example: [f 1, f 2, … , fn; Qn+1, Qn+2, …, QN+1] are then the (N+1) variables of which 2 are chosen as axes and the remainder are held constant. Phase Diagram www. factsage. com

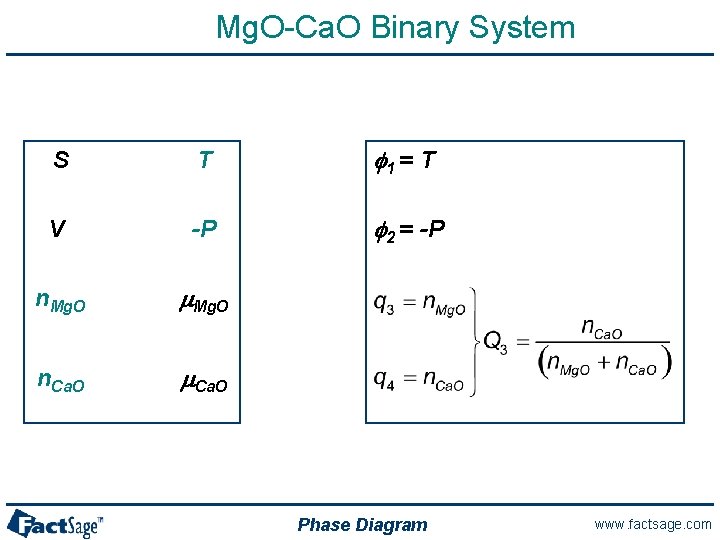
Mg. O-Ca. O Binary System S T f 1 = T V -P f 2 = -P n. Mg. O m. Mg. O n. Ca. O m. Ca. O Phase Diagram www. factsage. com

Ca. O-Mg. O: phase diagram www. factsage. com

Fe-Cr-S-O System S T f 1 = T V -P f 2 = -P n. Fe m. Fe n. Cr m. Cr Phase Diagram www. factsage. com

Fe-Cr-S-O System www. factsage. com

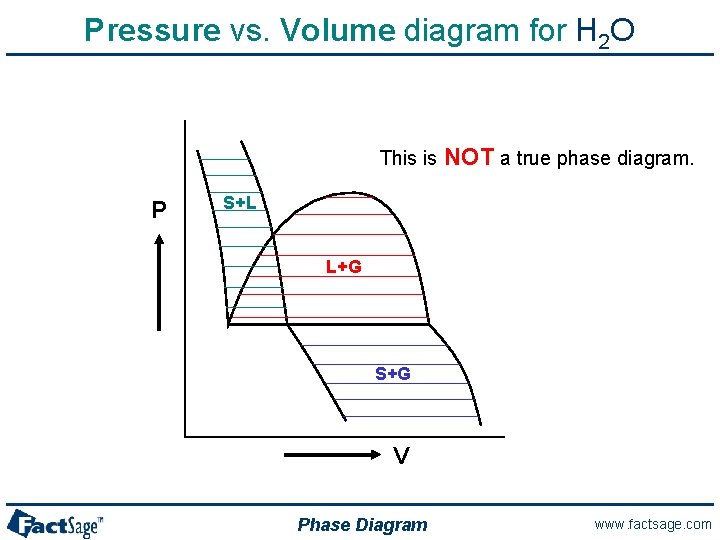
Pressure vs. Volume diagram for H 2 O This is P NOT a true phase diagram. S+L L+G S+G V Phase Diagram www. factsage. com

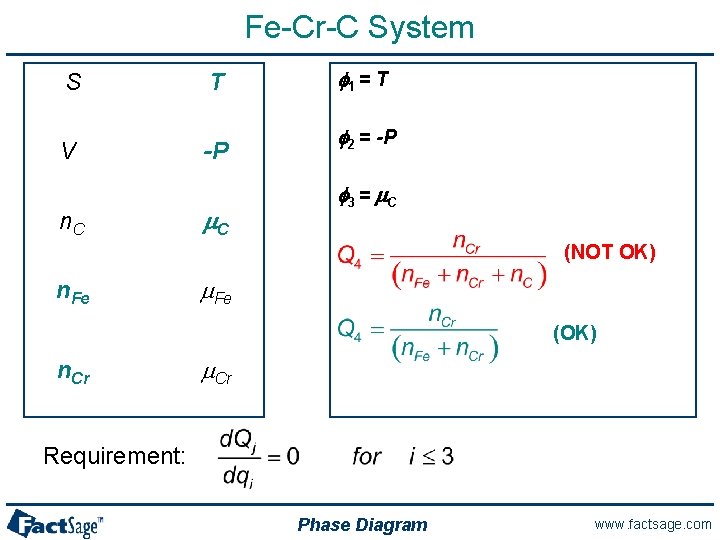
Fe-Cr-C System S T V -P n. C m. C f 1 = T f 2 = -P f 3 = m. C (NOT OK) n. Fe m. Fe (OK) n. Cr m. Cr Requirement: Phase Diagram www. factsage. com

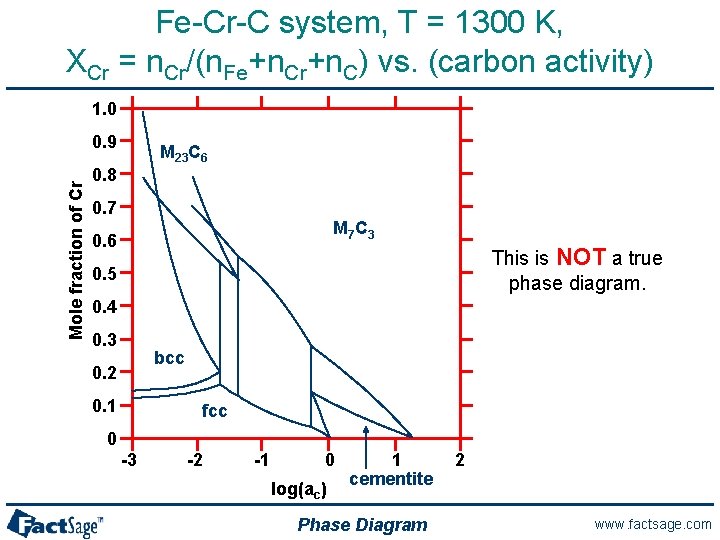
Fe-Cr-C system, T = 1300 K, XCr = n. Cr/(n. Fe+n. Cr+n. C) vs. (carbon activity) 1. 0 Mole fraction of Cr 0. 9 M 23 C 6 0. 8 0. 7 M 7 C 3 0. 6 This is NOT a true phase diagram. 0. 5 0. 4 0. 3 bcc 0. 2 0. 1 fcc 0 -3 -2 -1 0 log(ac) 1 cementite Phase Diagram 2 www. factsage. com

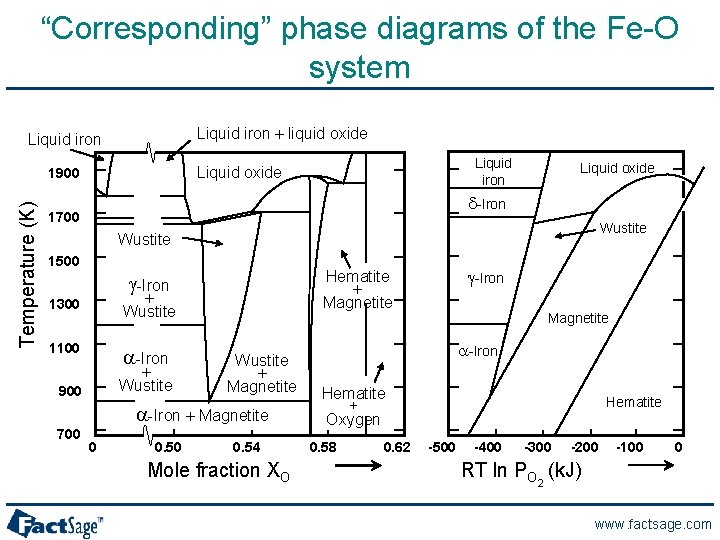
“Corresponding” phase diagrams of the Fe-O system Liquid iron + liquid oxide Liquid iron Temperature (K) Liquid iron Liquid oxide 1900 Liquid oxide d-Iron 1700 Wustite 1500 + Wustite 1300 1100 a-Iron + Wustite 900 Wustite + Magnetite a-Iron + Magnetite 700 0 0. 50 g-Iron Hematite + Magnetite g-Iron 0. 54 Mole fraction XO Magnetite a-Iron Hematite + Oxygen 0. 58 0. 62 Hematite -500 -400 -300 -200 -100 0 RT ln PO 2 (k. J) www. factsage. com

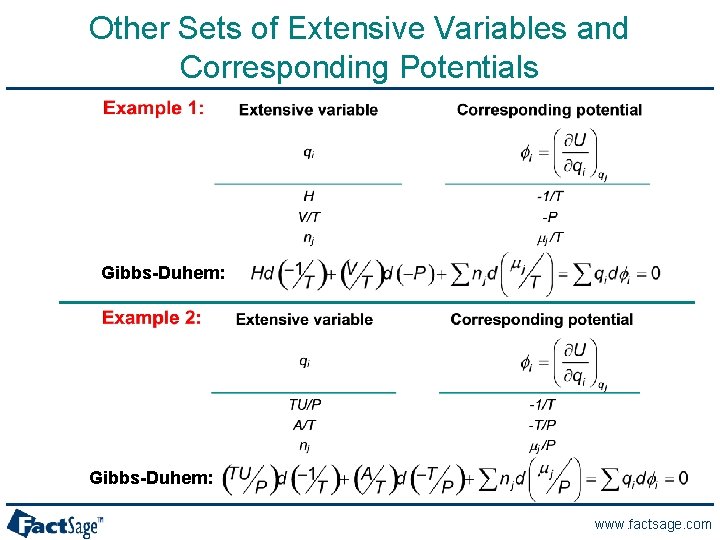
Other Sets of Extensive Variables and Corresponding Potentials Gibbs-Duhem: www. factsage. com

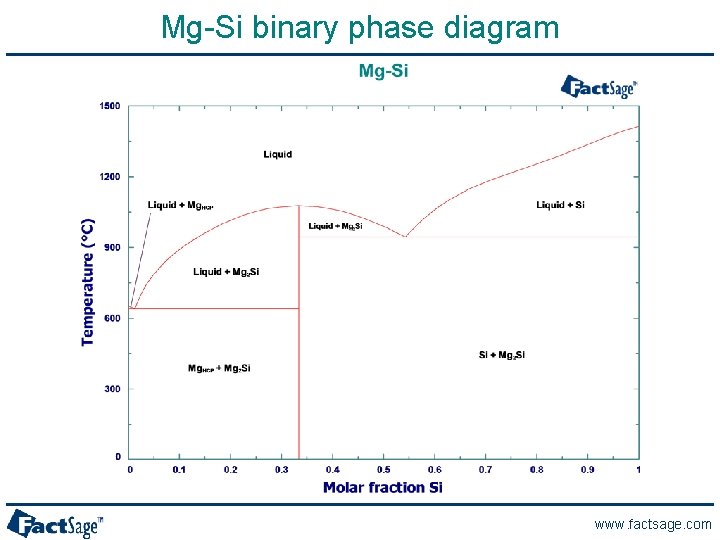
Mg-Si binary phase diagram www. factsage. com

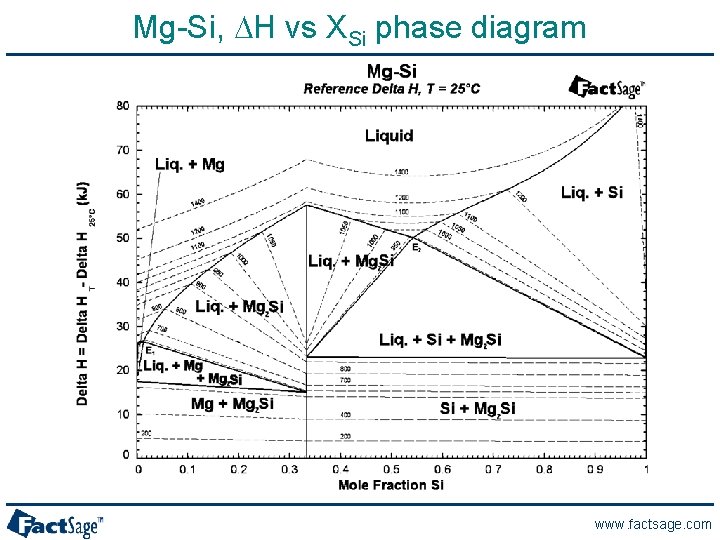
Mg-Si, DH vs XSi phase diagram www. factsage. com