GENERAL PHARMACOLOGY PHARMACOKINETICS BIOAVAILABILITY BIOAVAILABILITY Definition Rate and

GENERAL PHARMACOLOGY

PHARMACOKINETICS
BIOAVAILABILITY
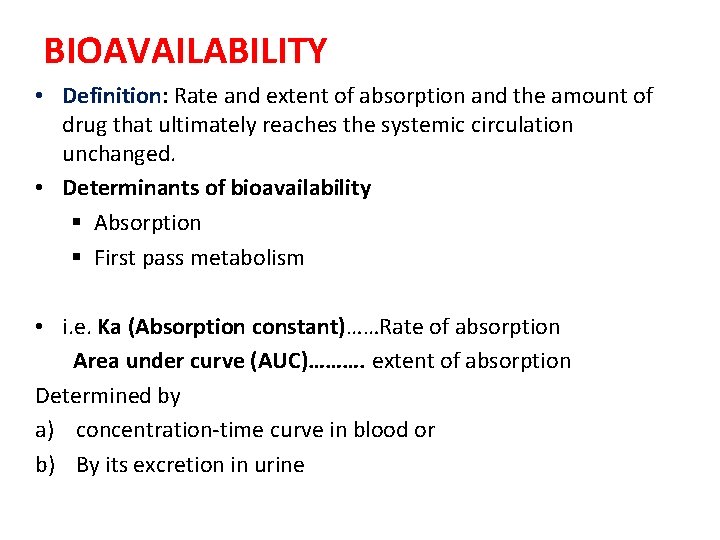
BIOAVAILABILITY • Definition: Rate and extent of absorption and the amount of drug that ultimately reaches the systemic circulation unchanged. • Determinants of bioavailability § Absorption § First pass metabolism • i. e. Ka (Absorption constant)……Rate of absorption Area under curve (AUC)………. extent of absorption Determined by a) concentration-time curve in blood or b) By its excretion in urine
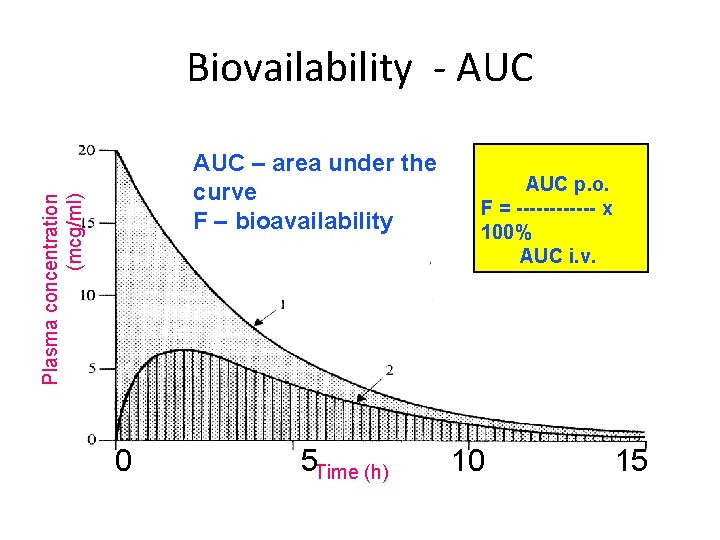
Biovailability - AUC Plasma concentration (mcg/ml) AUC – area under the curve F – bioavailability 0 5 Time (h) AUC p. o. F = ------ x 100% AUC i. v. 10 15
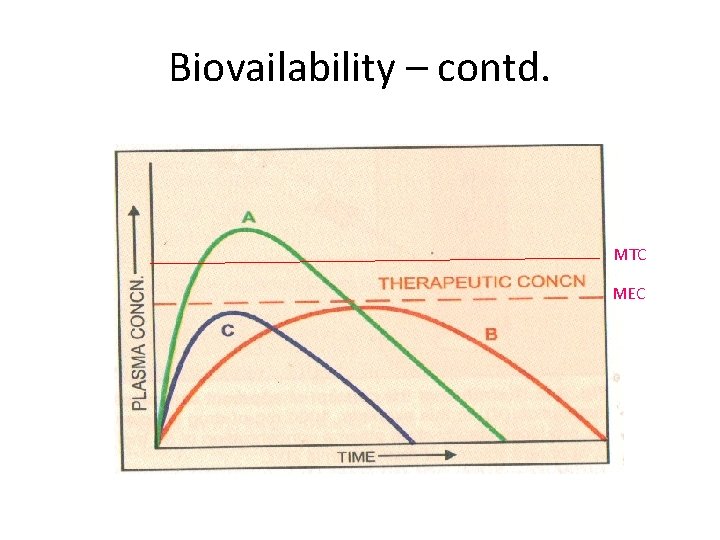
Biovailability – contd. MTC MEC
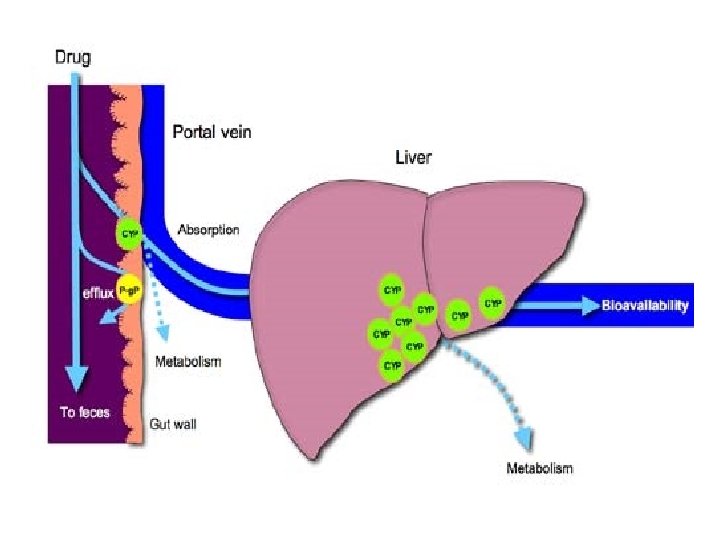
• Bioavailability of drug injected i. v. is 100%, but is frequently lower after oral ingestion because(a) the drug may be incompletely absorbed. (b) the absorbed drug may undergo first pass metabolism in the intestinal wall/liver or be excreted in bile. • Incomplete bioavailability after SC or IM Injection is less common, but may occur due to local binding of the drug.
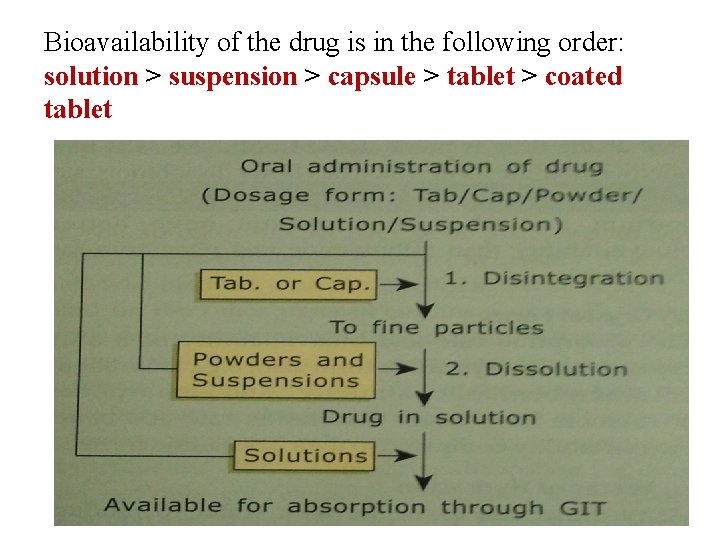
Bioavailability of the drug is in the following order: solution > suspension > capsule > tablet > coated tablet
FACTORS AFFECTING BIOAVAILABILITY: PHARMACEUTICAL FACTORS • Particle size • Salt form • Crystal form • Water of hydration • Nature of excipients and Adjuvants • Degree of ionisation PHARMACOLOGICAL FACTORS • Gastric emptying • GI disease • Food and other substances • First pass effect • Drug drug interactions • Pharmacogenetic factors

METHODS TO FACILITATE ABSORPTION • Increasing diffusion through the tissue by adding hyaluronidase , an enzyme which breaks down the intracellular matrix • Increasing local blood flow by hot fomentation or doing massage
BIOEQUIVALENCE • Two preparations of a drug are considered bioequivalent when the rate and extent of bioavailability of the drug from them is not significantly different under suitable test conditions.
DRUG DISTRIBUTION

• It is the passage of drug from the circulation to the tissue and site of its action. This is determined by parameter, volume of distribution(Vd). • Conc gradient being plasma tissues Distribution controls drug actions, efficacy and side effect.
DRUG DISTRIBUTION STEPS OF DRUG DISTRIBUTION 1. Dilution in blood- The drug has to reach equilibrium with itself in the blood. 2. Movement into extracellular fluid - Drugs need to be able to get out from vascular system. 3. Uptake into cells- The drug must be able to cross the cell membrane.
EXTENT OF DRUG DISTRIBUTION DEPENDS ON • • Lipid solubility Ionization at physiological p. H Extent of binding to plasma and tissue protiens Presence of tissue specific transporters • Difference in regional blood flow • Fat : lean body mass ratio • Diseases like CHF, uremia , cirrhosis

• Movement of drug proceeds until an equilibrium is established between unbound drug in plasma and tissue fluids. Subsequently, there is a parallel decline in both due to elimination.
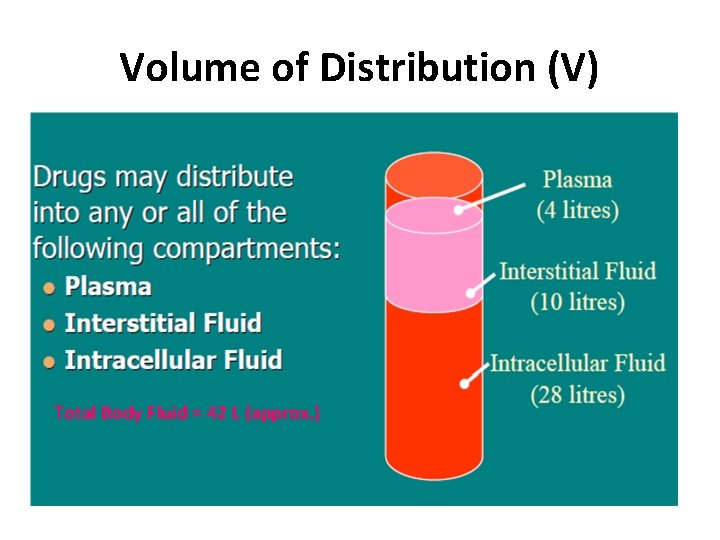
Volume of Distribution (V) Total Body Fluid = 42 L (approx. )
Volume of Distribution (V) • Chloroquin – 13000 liters, Digoxin – 420 L, Morphine – 250 L and Propranolol – 280 L • Streptomycin and Gentamicin – 18 L (WHY ? ) `Vd` is an imaginary Volume of Fluid which will accommodate the entire quantity of the drug in the body, if the concentration throughout this imaginary volume were same as that in plasma
VOLUME OF DISTRIBUTION • Gives information on HOW extensively the drug is distributed to the rest of the body compared to plasma. • Used to calculate a loading dose
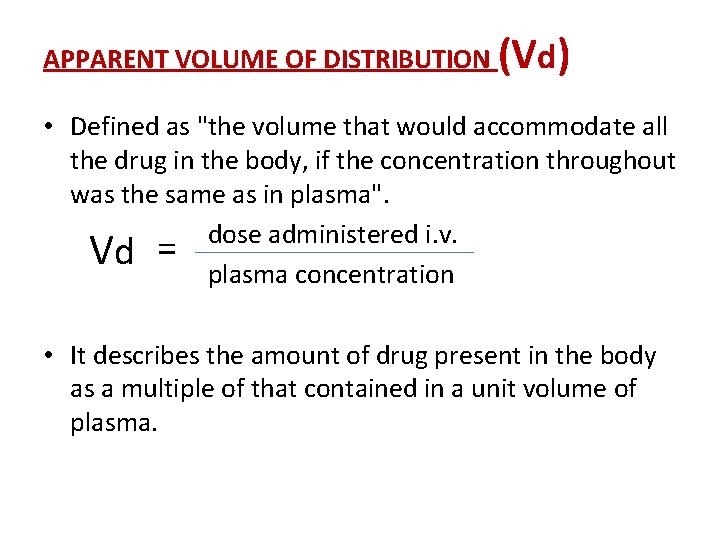
APPARENT VOLUME OF DISTRIBUTION (Vd) • Defined as "the volume that would accommodate all the drug in the body, if the concentration throughout was the same as in plasma". dose administered i. v. Vd = plasma concentration • It describes the amount of drug present in the body as a multiple of that contained in a unit volume of plasma.
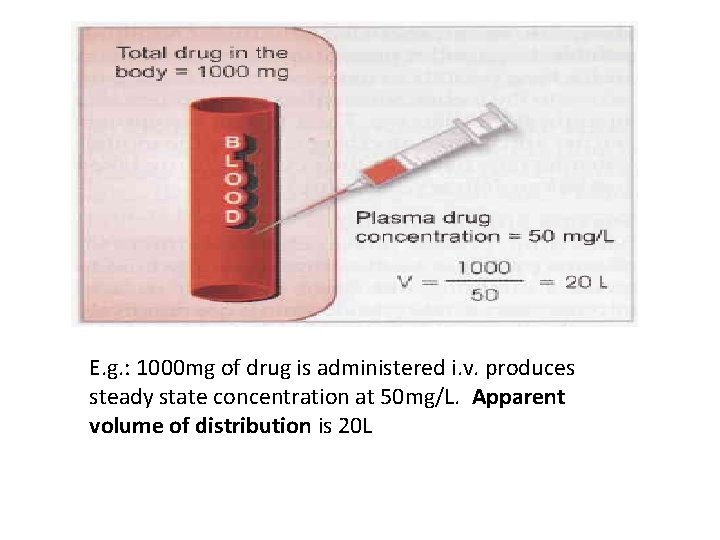
E. g. : 1000 mg of drug is administered i. v. produces steady state concentration at 50 mg/L. Apparent volume of distribution is 20 L
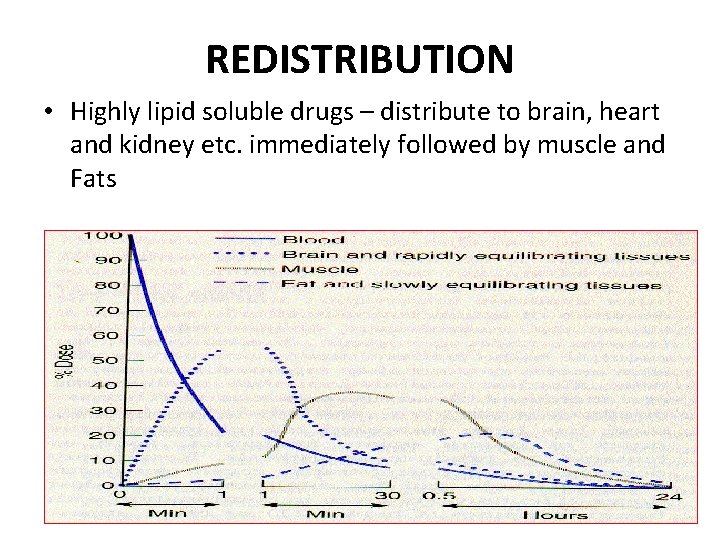
REDISTRIBUTION • Highly lipid soluble drugs – distribute to brain, heart and kidney etc. immediately followed by muscle and Fats
REDISTRIBUTION • Highly lipid-soluble drugs get initially distributed to organs with high blood flow, i. e. brain, heart, kidney, etc. Later, less vascular but more bulky tissues (muscle, fat) take up the drugplasma concentration falls and the drug is withdrawn from these sites. If the site of action of the drug was in one of the highly perfused organs, redistribution results in termination of drug action. • Greater the lipid solubility of the drug, faster is its redistribution. • E. g. : – Thiopentone sod. – Oral diazepam – Nitrazepam
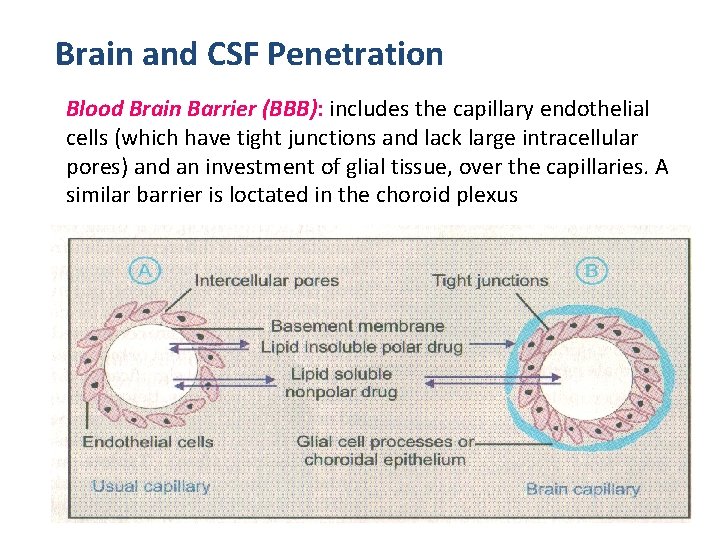
Brain and CSF Penetration Blood Brain Barrier (BBB): includes the capillary endothelial cells (which have tight junctions and lack large intracellular pores) and an investment of glial tissue, over the capillaries. A similar barrier is loctated in the choroid plexus
Brain and CSF Penetration – contd. • BBB is lipoidal and limits the entry of non-lipid soluble drugs (amikacin, gentamicin, neostigmine etc. ). (Only lipid soluble unionized drugs penetrate and have action on the CNS) • Efflux carriers like P-gp (glycoprotein) present in brain capillary endothelial cell (also in intestinal mucosal, renal tubular, hepatic canicular, placental and testicular cells) extrude drugs that enter brain by other processes. (Inflammation of meanings of brain increases permeability of BBB) • Dopamine (DA) does not enter brain, but its precursor levodopa does. This is used in parkinsonism.
Placental Transfer • Only lipid soluble Drugs can penetrate – limitation of hydrophillic drugs • Placental P-gp serves as limiting factor • But, REMEMBER, its an incomplete barrier – some influx transporters operate • Thalidomide
PLASMA PROTEIN BINDING Acidic drugs generally bind to plasma albumin and basic drugs to α 1 acid glycoprotein. IMPORTANCE: (i) Highly plasma protein bound drugs are largely restricted to the vascular compartment (ii) The bound fraction is not available for action (iii) High degree of protein binding generally makes the drug long acting (iv) Generally expressed plasma concentrations of the drug refer to bound as well as free drug. (v) Drug interaction

TISSUE STORAGE Drugs may also accumulate in specific organs or get bound to specific tissue constituents, e. g. : • Heart and skeletal muscles – digoxin (to muscle proteins) • Liver – chloroquine, tetracyclines, digoxin • Kidney – digoxin, chloroquine • Thyroid gland – iodine • Brain – chlorpromazine, isoniazid, acetazolamide • Retina – chloroquine (to nucleoproteins) • Iris – ephedrine, atropine (to melanin) • Bones and teeth – tetracyclines, heavy metals (to mucopolysaccharide of connective tissue) • Adipose tissues – thiopental, ether, minocycline, DDT

- Slides: 29