General Organic and Biological Chemistry Fourth Edition Karen

General, Organic, and Biological Chemistry Fourth Edition Karen Timberlake Chapter 5 Compounds and Their Bonds 5. 5 Covalent Compounds: Sharing Electrons © 2013 Pearson Education, Inc. Lectures
Covalent Bonds Covalent bonds form when atoms of nonmetals share electrons to complete octets. Valence electrons are not transferred, but shared to achieve stability. © 2013 Pearson Education, Inc. Chapter 5, Section 5 2
Formation of H 2 In the simplest covalent molecule, H 2 , the H atoms § increase attraction as they move closer. § share electrons to achieve a stable configuration. § form a covalent bond. © 2013 Pearson Education, Inc. Chapter 5, Section 5 3
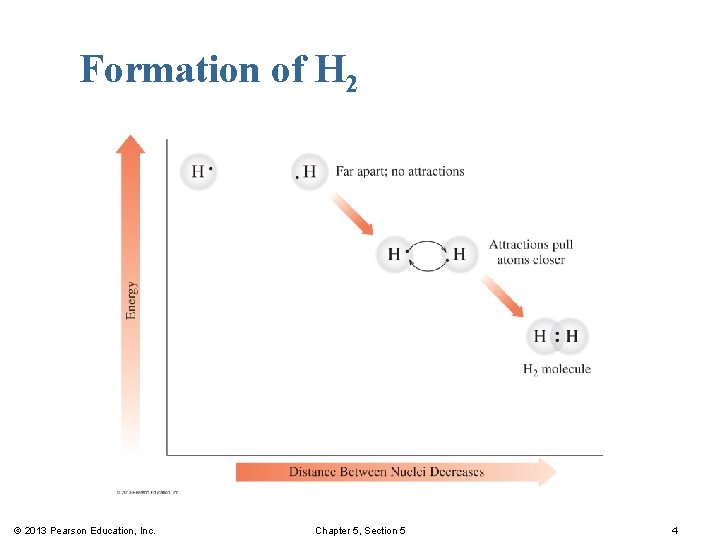
Formation of H 2 © 2013 Pearson Education, Inc. Chapter 5, Section 5 4
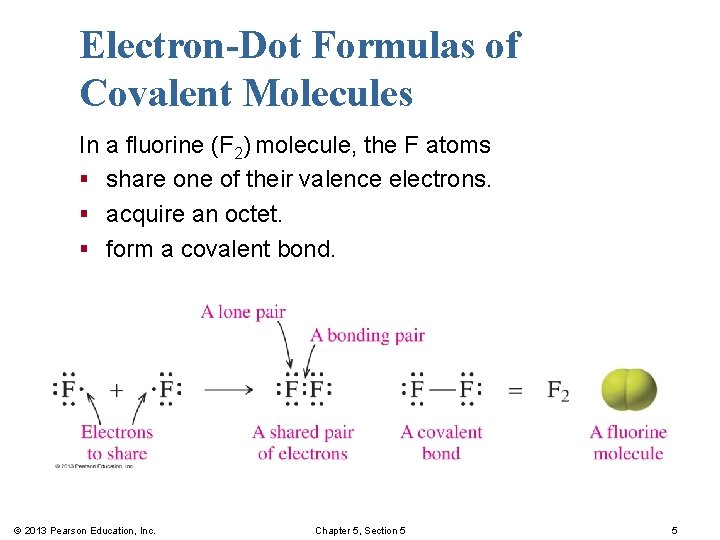
Electron-Dot Formulas of Covalent Molecules In a fluorine (F 2) molecule, the F atoms § share one of their valence electrons. § acquire an octet. § form a covalent bond. © 2013 Pearson Education, Inc. Chapter 5, Section 5 5
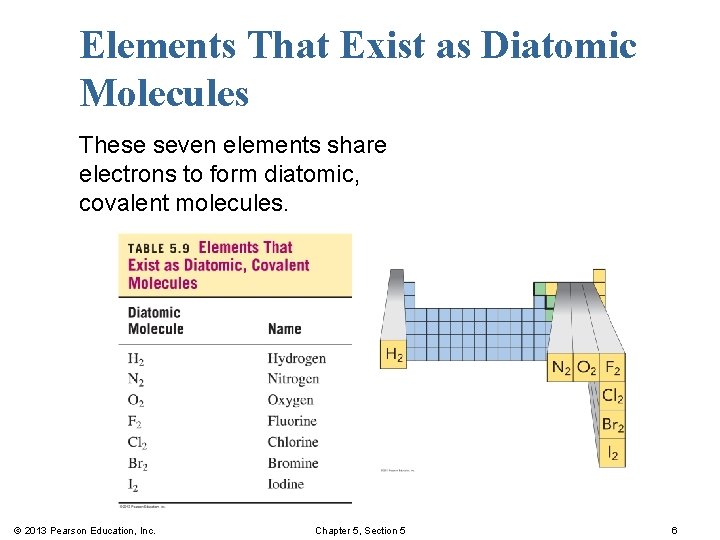
Elements That Exist as Diatomic Molecules These seven elements share electrons to form diatomic, covalent molecules. © 2013 Pearson Education, Inc. Chapter 5, Section 5 6
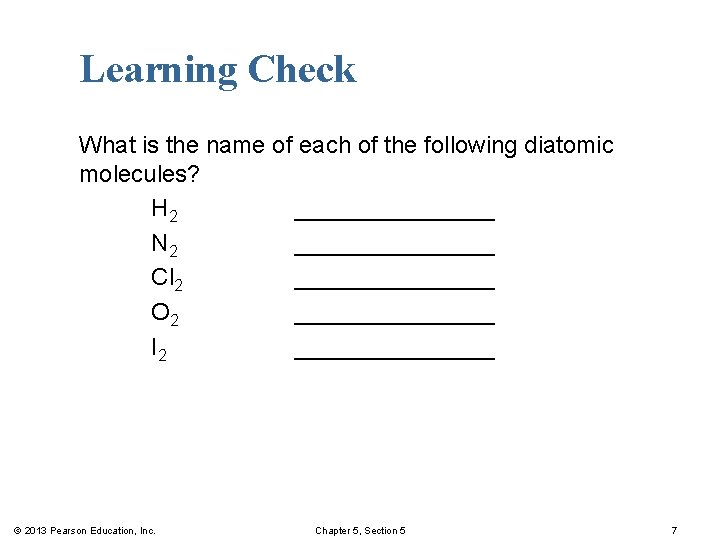
Learning Check What is the name of each of the following diatomic molecules? H 2 ________ N 2 ________ Cl 2 ________ O 2 ________ I 2 ________ © 2013 Pearson Education, Inc. Chapter 5, Section 5 7
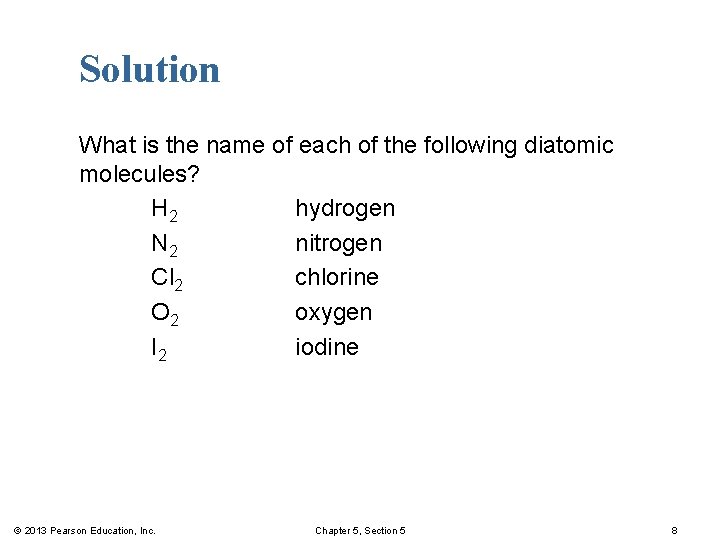
Solution What is the name of each of the following diatomic molecules? H 2 hydrogen N 2 nitrogen Cl 2 chlorine O 2 oxygen I 2 iodine © 2013 Pearson Education, Inc. Chapter 5, Section 5 8
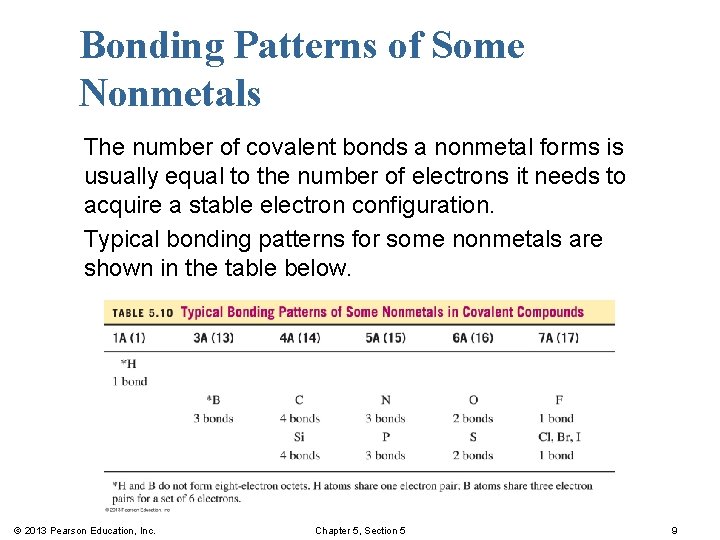
Bonding Patterns of Some Nonmetals The number of covalent bonds a nonmetal forms is usually equal to the number of electrons it needs to acquire a stable electron configuration. Typical bonding patterns for some nonmetals are shown in the table below. © 2013 Pearson Education, Inc. Chapter 5, Section 5 9
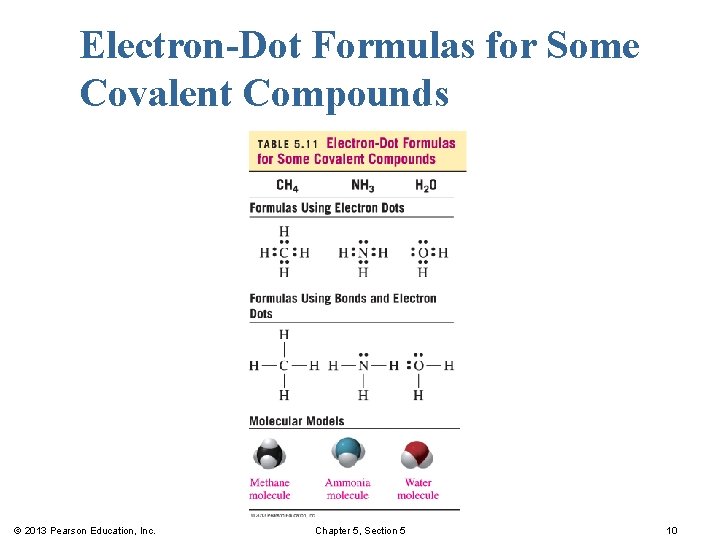
Electron-Dot Formulas for Some Covalent Compounds © 2013 Pearson Education, Inc. Chapter 5, Section 5 10
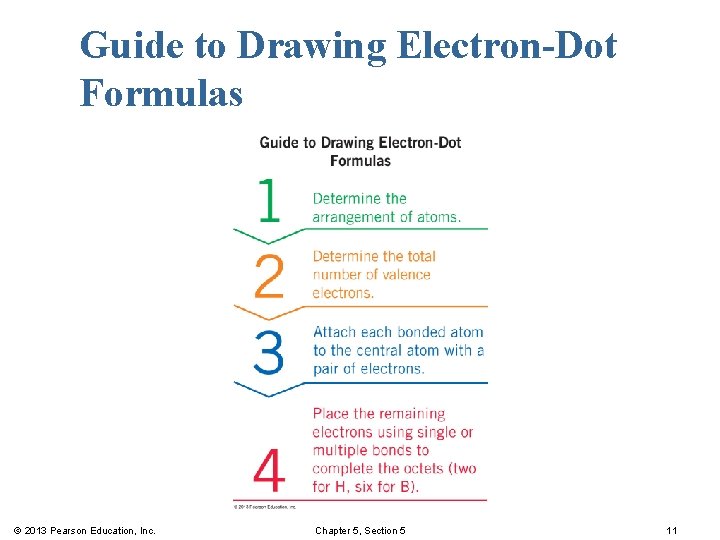
Guide to Drawing Electron-Dot Formulas © 2013 Pearson Education, Inc. Chapter 5, Section 5 11
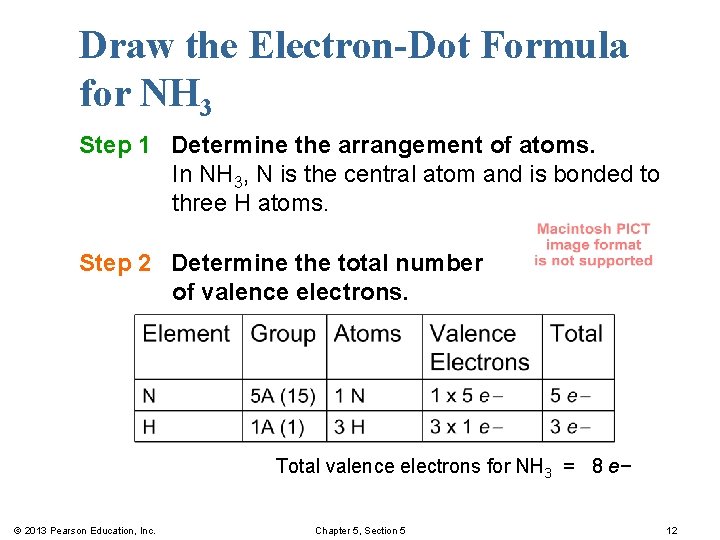
Draw the Electron-Dot Formula for NH 3 Step 1 Determine the arrangement of atoms. In NH 3, N is the central atom and is bonded to three H atoms. Step 2 Determine the total number of valence electrons. Total valence electrons for NH 3 = 8 e− © 2013 Pearson Education, Inc. Chapter 5, Section 5 12
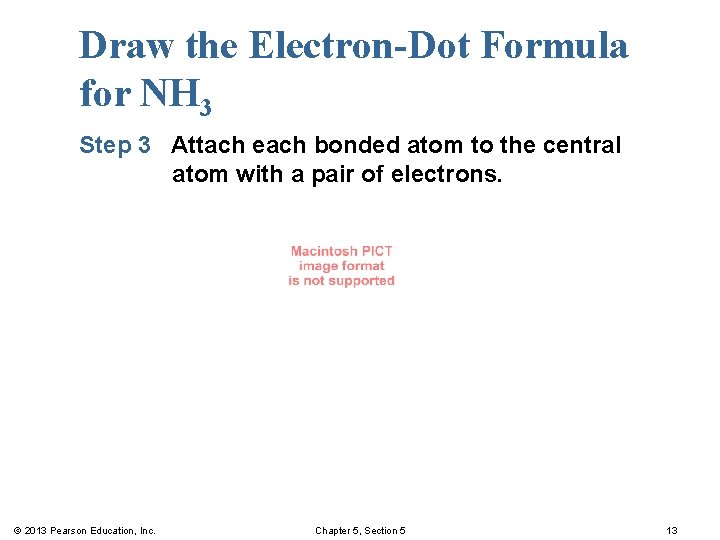
Draw the Electron-Dot Formula for NH 3 Step 3 Attach each bonded atom to the central atom with a pair of electrons. © 2013 Pearson Education, Inc. Chapter 5, Section 5 13
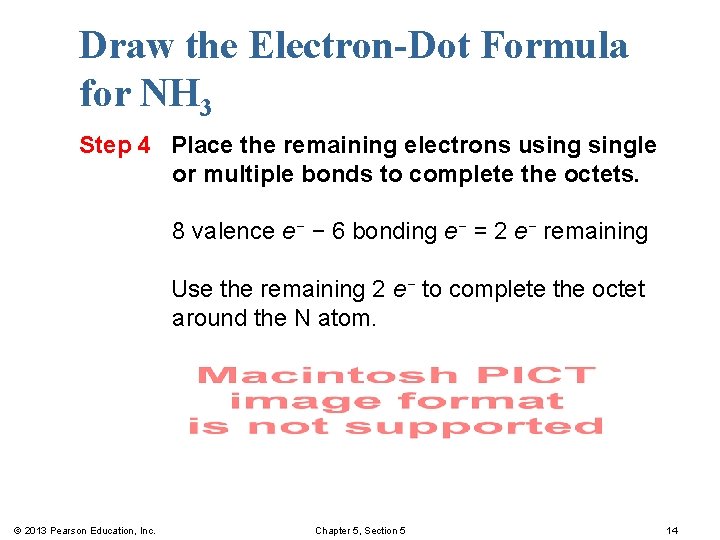
Draw the Electron-Dot Formula for NH 3 Step 4 Place the remaining electrons usingle or multiple bonds to complete the octets. 8 valence e− − 6 bonding e− = 2 e− remaining Use the remaining 2 e− to complete the octet around the N atom. © 2013 Pearson Education, Inc. Chapter 5, Section 5 14
Learning Check Draw the electron-dot formula for CCl 4. © 2013 Pearson Education, Inc. Chapter 5, Section 5 15
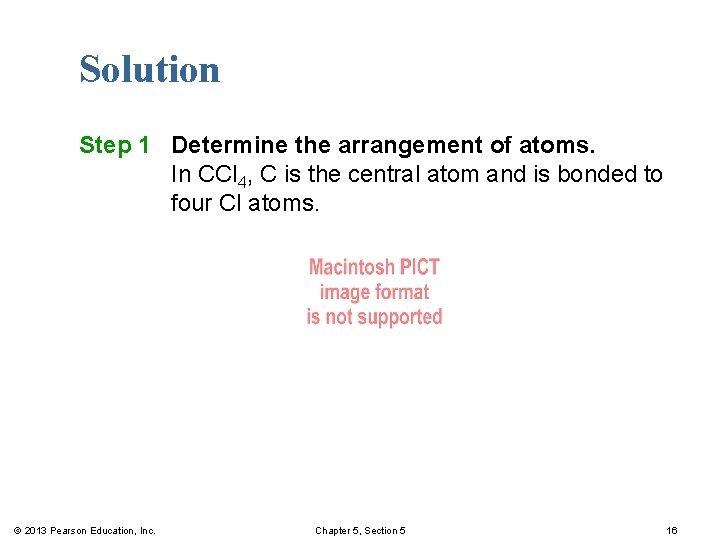
Solution Step 1 Determine the arrangement of atoms. In CCl 4, C is the central atom and is bonded to four Cl atoms. © 2013 Pearson Education, Inc. Chapter 5, Section 5 16
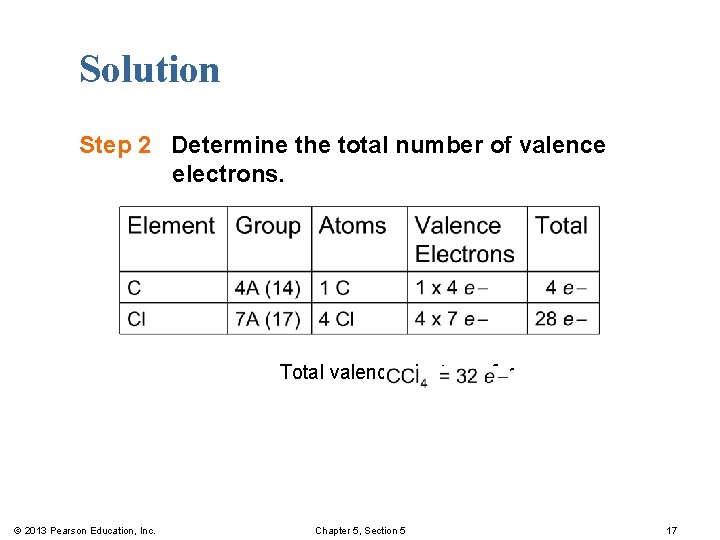
Solution Step 2 Determine the total number of valence electrons. Total valence electrons for © 2013 Pearson Education, Inc. Chapter 5, Section 5 17
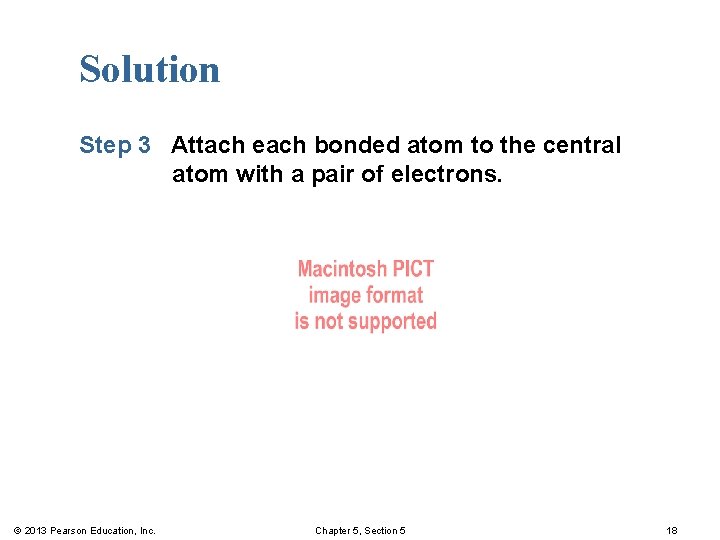
Solution Step 3 Attach each bonded atom to the central atom with a pair of electrons. © 2013 Pearson Education, Inc. Chapter 5, Section 5 18
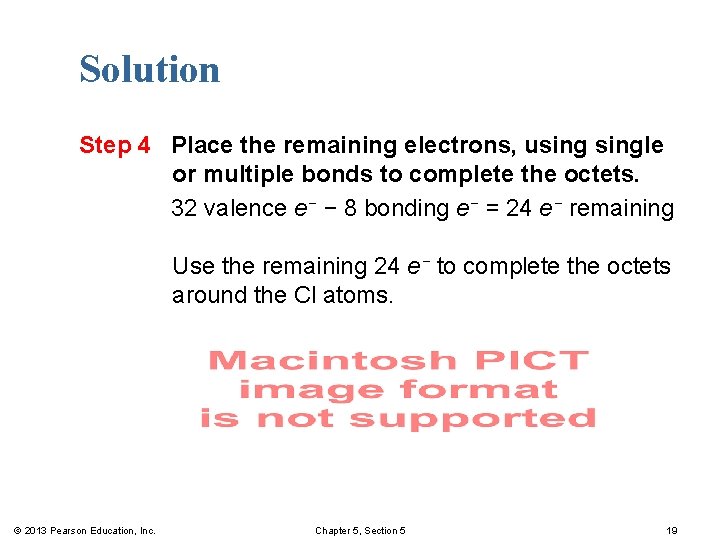
Solution Step 4 Place the remaining electrons, usingle or multiple bonds to complete the octets. 32 valence e− − 8 bonding e− = 24 e− remaining Use the remaining 24 e− to complete the octets around the Cl atoms. © 2013 Pearson Education, Inc. Chapter 5, Section 5 19
Exceptions to the Octet Rule Not all atoms have octets. § Some can have less than an octet, such as H, which requires only 2 electrons, B, which requires only 3 electrons, and Be, which requires only 4 electrons. § Some can have expanded octets, such as P, which can have 10 electrons, S, which can have 12 electrons, and Cl, Br and I, which can have 14 electrons © 2013 Pearson Education, Inc. Chapter 5, Section 5 20
Single and Multiple Bonds In many covalent compounds, atoms share two or three pairs of electrons to complete their octets. § In a single bond, one pair of electrons is shared. § In a double bond, two pairs of electrons are shared. § In a triple bond, three pairs of electrons are shared. © 2013 Pearson Education, Inc. Chapter 5, Section 5 21
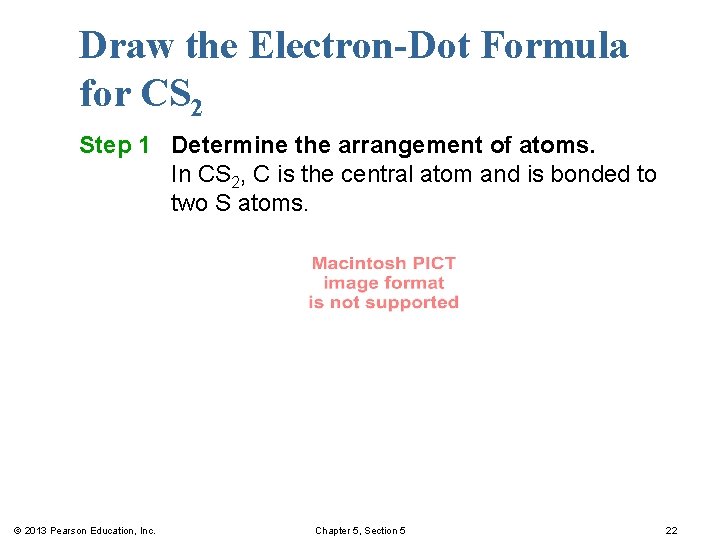
Draw the Electron-Dot Formula for CS 2 Step 1 Determine the arrangement of atoms. In CS 2, C is the central atom and is bonded to two S atoms. © 2013 Pearson Education, Inc. Chapter 5, Section 5 22
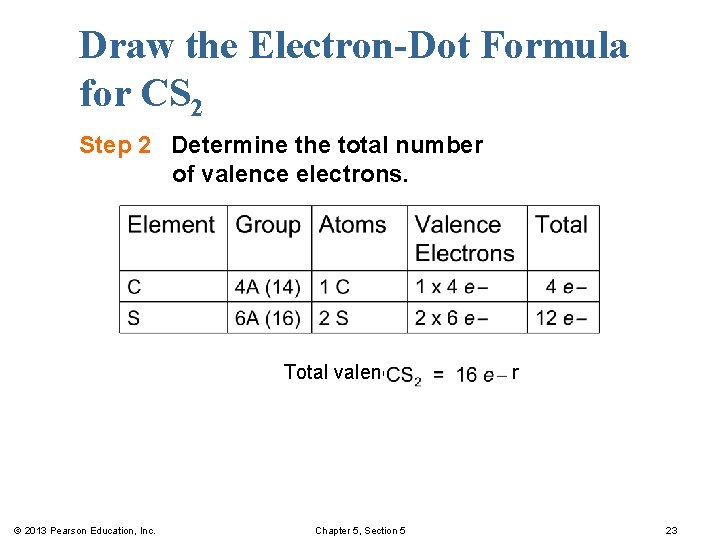
Draw the Electron-Dot Formula for CS 2 Step 2 Determine the total number of valence electrons. Total valence electrons for © 2013 Pearson Education, Inc. Chapter 5, Section 5 23
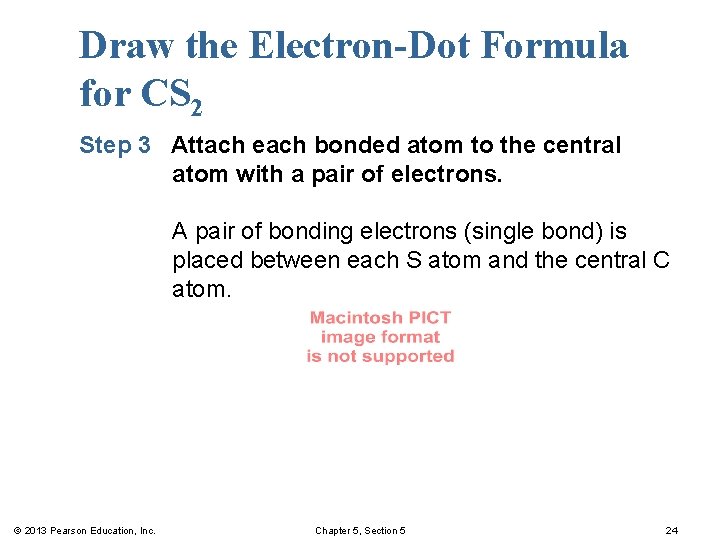
Draw the Electron-Dot Formula for CS 2 Step 3 Attach each bonded atom to the central atom with a pair of electrons. A pair of bonding electrons (single bond) is placed between each S atom and the central C atom. © 2013 Pearson Education, Inc. Chapter 5, Section 5 24
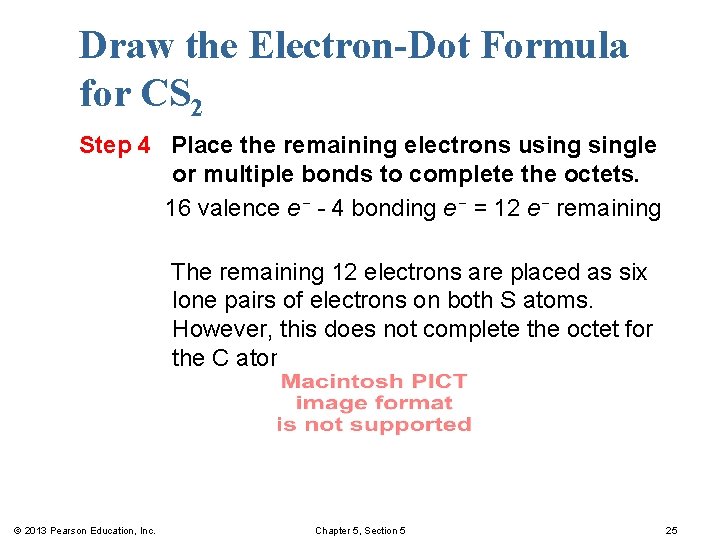
Draw the Electron-Dot Formula for CS 2 Step 4 Place the remaining electrons usingle or multiple bonds to complete the octets. 16 valence e− - 4 bonding e− = 12 e− remaining The remaining 12 electrons are placed as six lone pairs of electrons on both S atoms. However, this does not complete the octet for the C atom. © 2013 Pearson Education, Inc. Chapter 5, Section 5 25
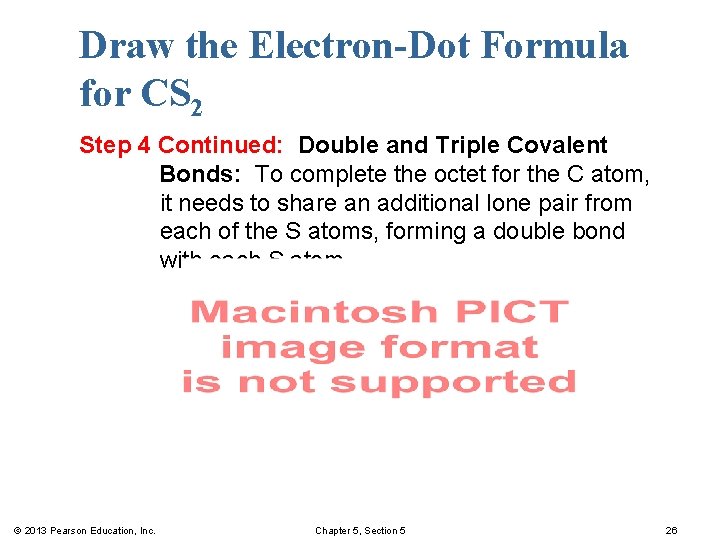
Draw the Electron-Dot Formula for CS 2 Step 4 Continued: Double and Triple Covalent Bonds: To complete the octet for the C atom, it needs to share an additional lone pair from each of the S atoms, forming a double bond with each S atom. © 2013 Pearson Education, Inc. Chapter 5, Section 5 26
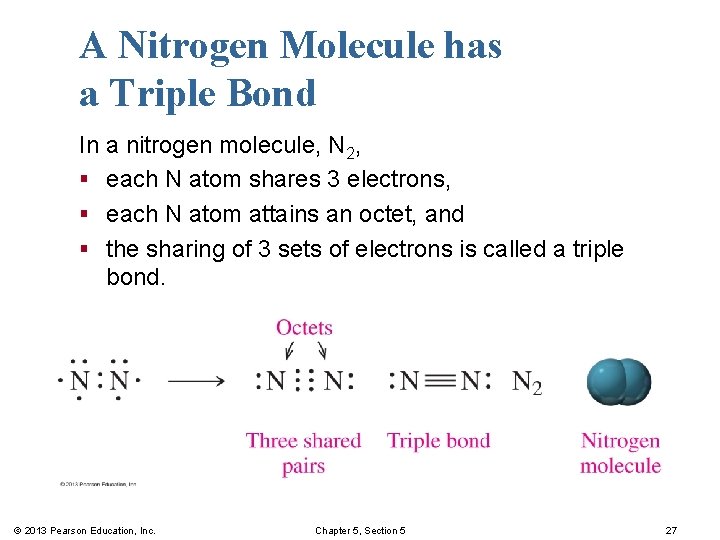
A Nitrogen Molecule has a Triple Bond In a nitrogen molecule, N 2, § each N atom shares 3 electrons, § each N atom attains an octet, and § the sharing of 3 sets of electrons is called a triple bond. © 2013 Pearson Education, Inc. Chapter 5, Section 5 27
Resonance Structures Resonance structures are § two or more electron-dot formulas for the same arrangement of atoms. § related by a double-headed arrow ( ). § written by changing the location of a double bond between the central atom and a different attached atom. © 2013 Pearson Education, Inc. Chapter 5, Section 5 28
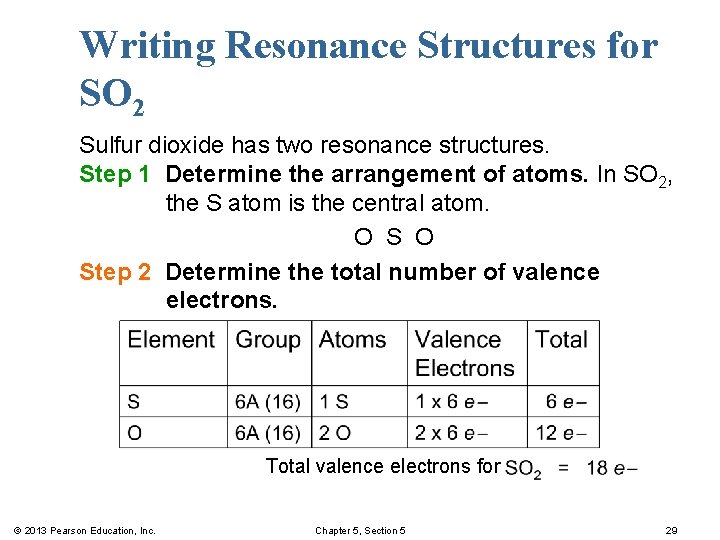
Writing Resonance Structures for SO 2 Sulfur dioxide has two resonance structures. Step 1 Determine the arrangement of atoms. In SO 2, the S atom is the central atom. O Step 2 Determine the total number of valence electrons. Total valence electrons for © 2013 Pearson Education, Inc. Chapter 5, Section 5 29
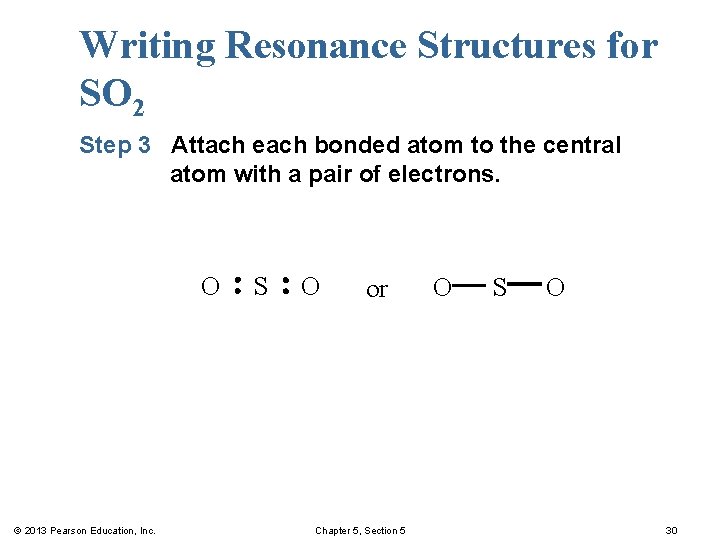
Writing Resonance Structures for SO 2 Step 3 Attach each bonded atom to the central atom with a pair of electrons. O © 2013 Pearson Education, Inc. S O or Chapter 5, Section 5 O S O 30
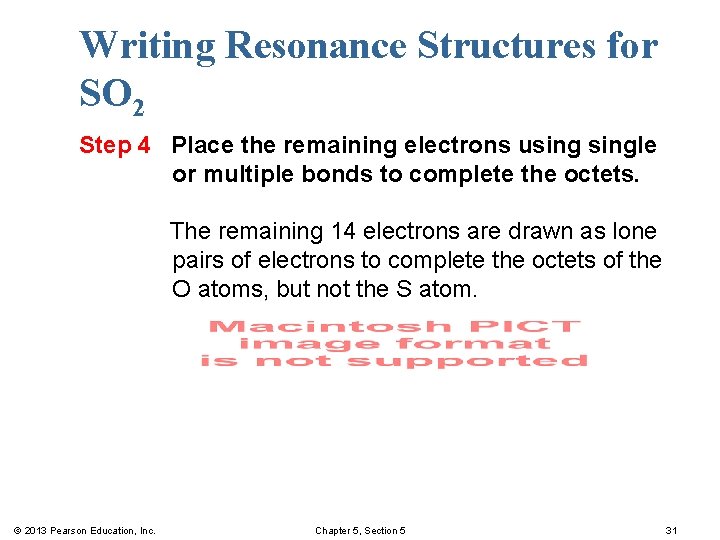
Writing Resonance Structures for SO 2 Step 4 Place the remaining electrons usingle or multiple bonds to complete the octets. The remaining 14 electrons are drawn as lone pairs of electrons to complete the octets of the O atoms, but not the S atom. © 2013 Pearson Education, Inc. Chapter 5, Section 5 31
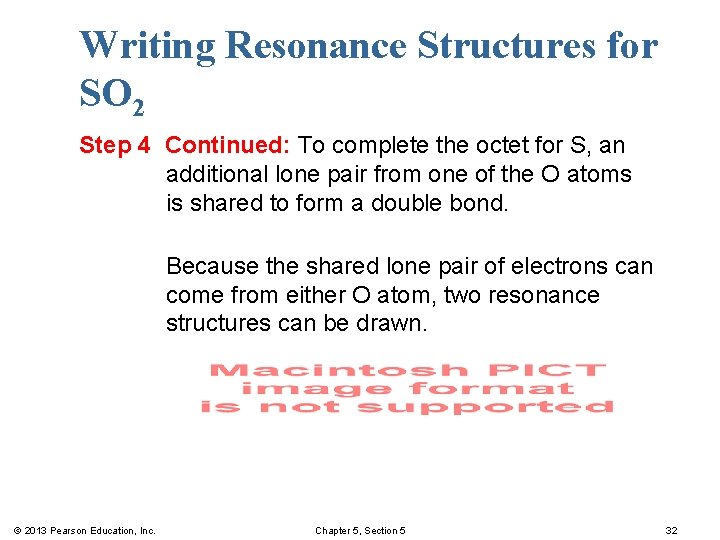
Writing Resonance Structures for SO 2 Step 4 Continued: To complete the octet for S, an additional lone pair from one of the O atoms is shared to form a double bond. Because the shared lone pair of electrons can come from either O atom, two resonance structures can be drawn. © 2013 Pearson Education, Inc. Chapter 5, Section 5 32
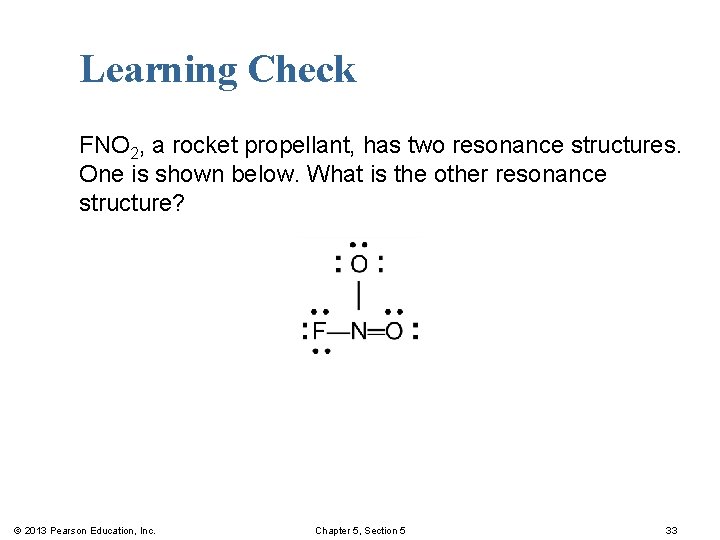
Learning Check FNO 2, a rocket propellant, has two resonance structures. One is shown below. What is the other resonance structure? © 2013 Pearson Education, Inc. Chapter 5, Section 5 33
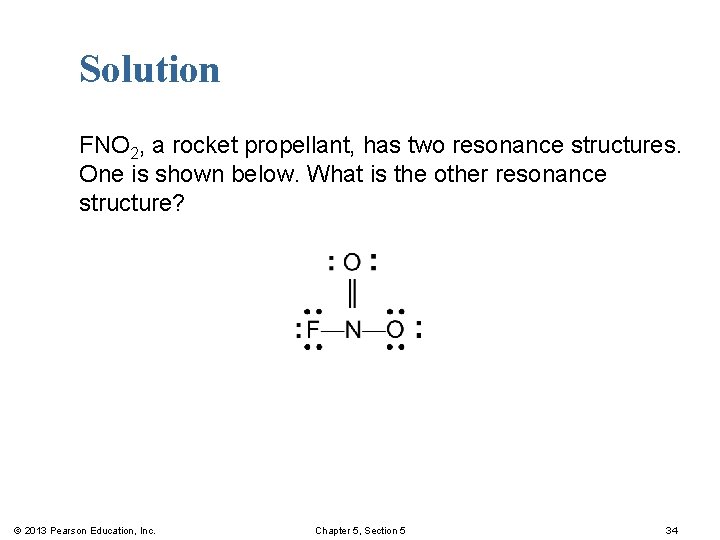
Solution FNO 2, a rocket propellant, has two resonance structures. One is shown below. What is the other resonance structure? © 2013 Pearson Education, Inc. Chapter 5, Section 5 34
- Slides: 34