GENERAL CHEMISTRY 101 Matter Mixture Homogeneous Mixture Physical





- Slides: 26

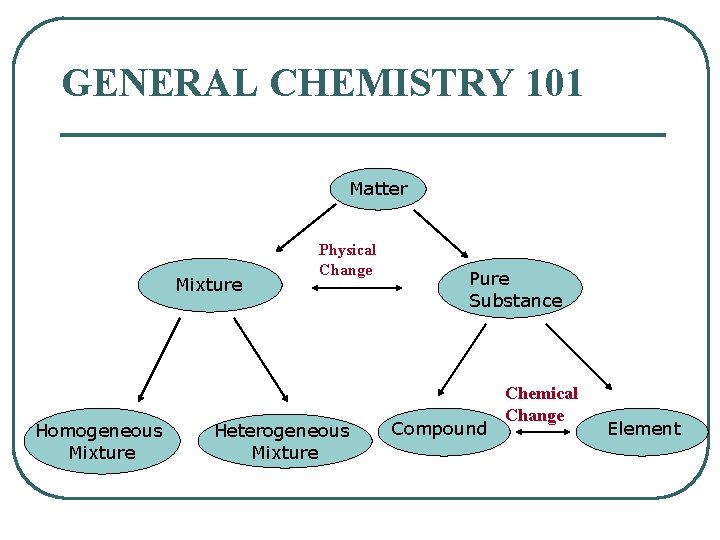
GENERAL CHEMISTRY 101 Matter Mixture Homogeneous Mixture Physical Change Heterogeneous Mixture Pure Substance Compound Chemical Change Element

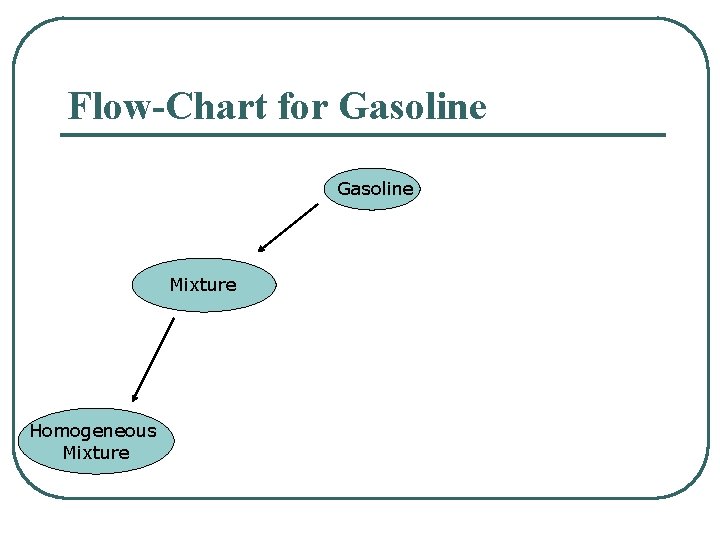
Flow-Chart for Gasoline Mixture Homogeneous Mixture

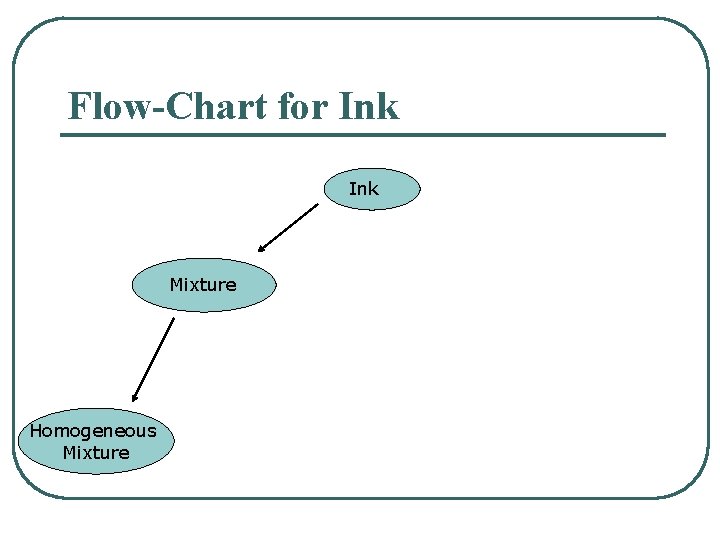
Flow-Chart for Ink Mixture Homogeneous Mixture

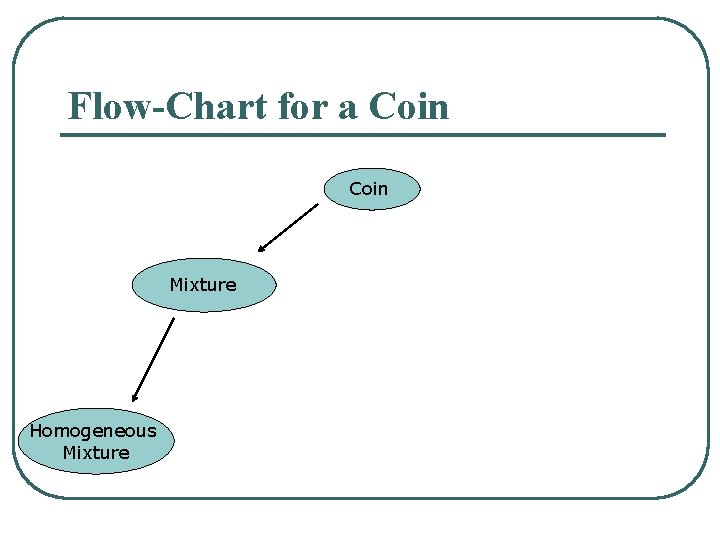
Flow-Chart for a Coin Mixture Homogeneous Mixture

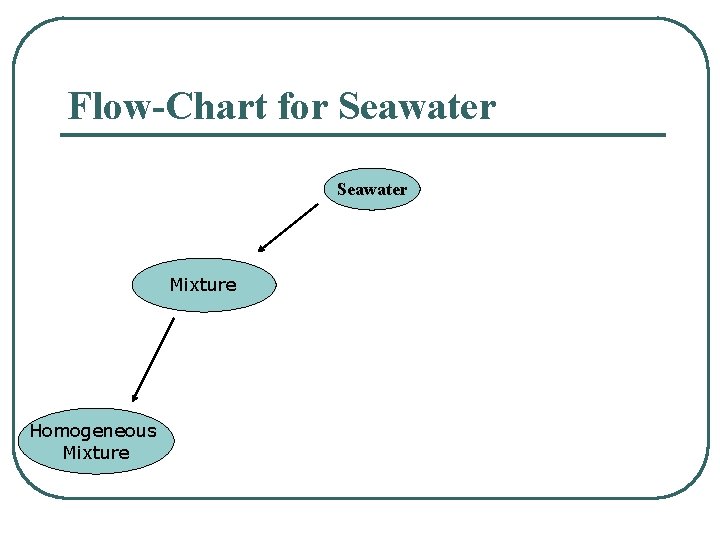
Flow-Chart for Seawater Mixture Homogeneous Mixture

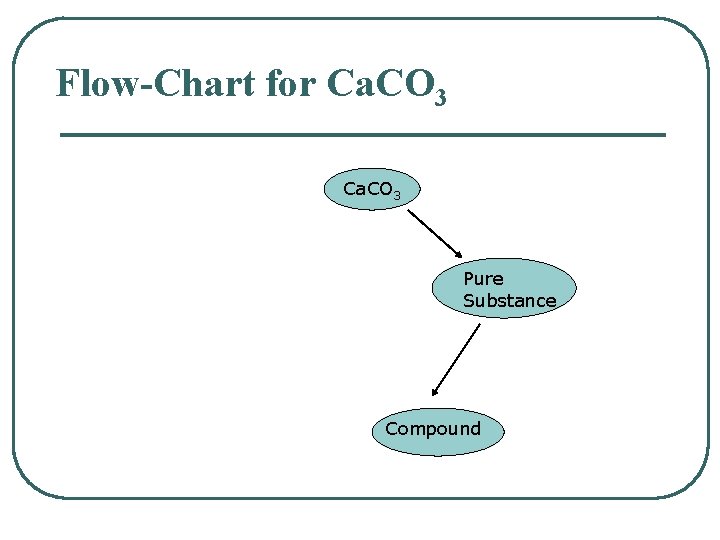
Flow-Chart for Ca. CO 3 Pure Substance Compound

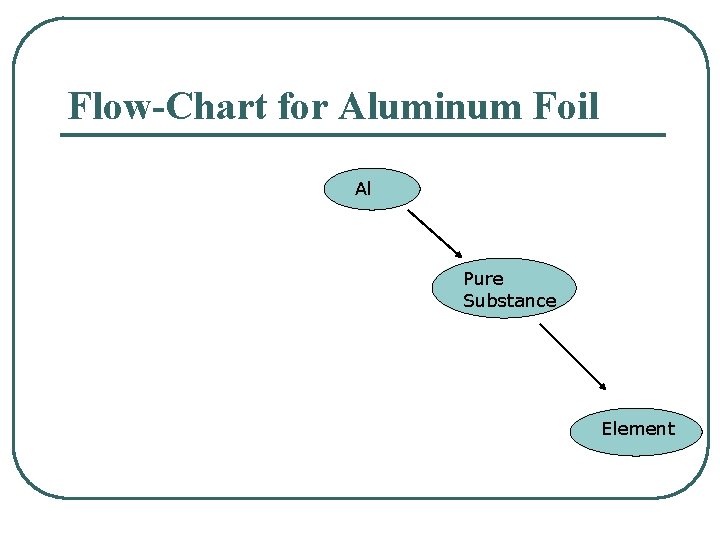
Flow-Chart for Aluminum Foil Al Pure Substance Element

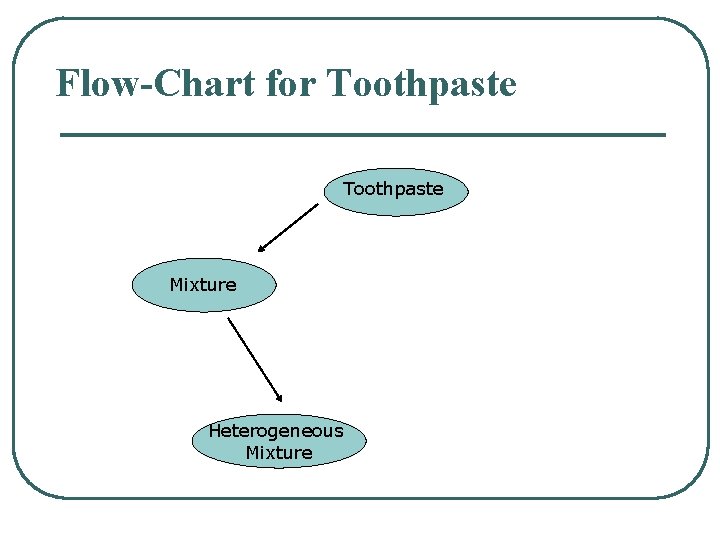
Flow-Chart for Toothpaste Mixture Heterogeneous Mixture

Classify each of the following as a homogeneous or a heterogeneous mixture?

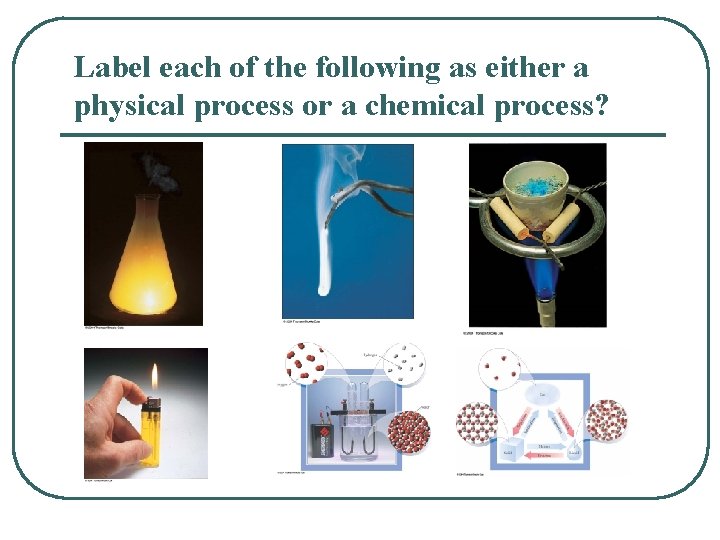
Label each of the following as either a physical process or a chemical process?

Label each of the following as either a physical process or a chemical process?

Classify each of the following as a homogeneous or a heterogeneous mixture? You do it ! • Muddy river water • Sugar dissolved in water

Reading…. Please read section 1 -12: HEAT AND TEMPERATURE

CHAPTER 2 Chemical Formulas and Composition Stoichiometry Chapter Goals 1. 2. 3. 4. 5. 6. Atoms and Molecules Chemical Formulas Ions and Ionic Compounds Names and Formulas of Some Ionic Compounds Atomic Weights The Mole

Cont… 7. 8. 9. 10. 11. 12. Formula Weights, Molecular Weights, and Moles Percent Composition and Formulas of Compounds Derivation of Formulas from Elemental Composition Determination of Molecular Formulas Some Other Interpretations of Chemical Formulas Purity of Samples

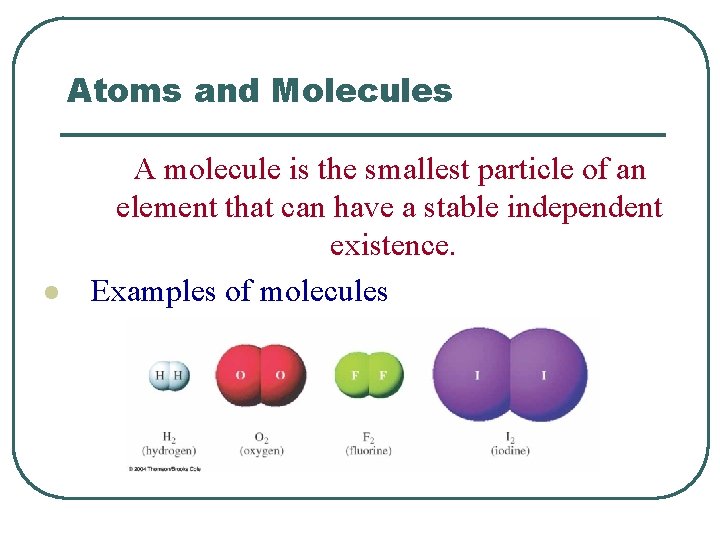
Atoms and Molecules l A molecule is the smallest particle of an element that can have a stable independent existence. Examples of molecules

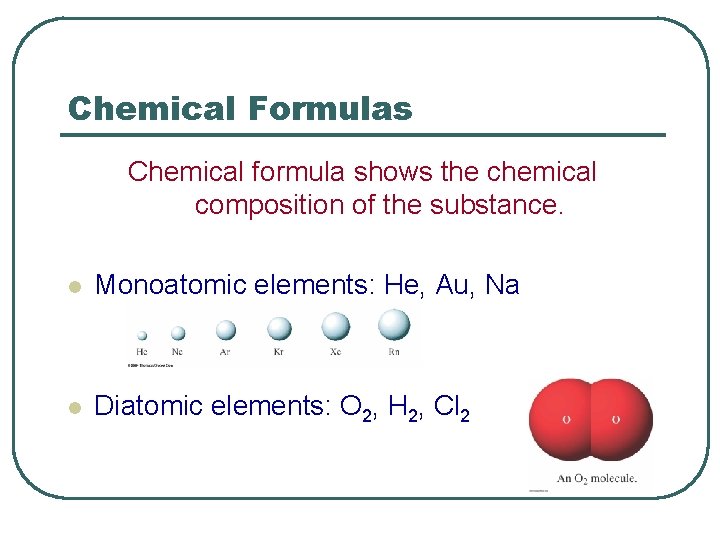
Chemical Formulas Chemical formula shows the chemical composition of the substance. l Monoatomic elements: He, Au, Na l Diatomic elements: O 2, H 2, Cl 2

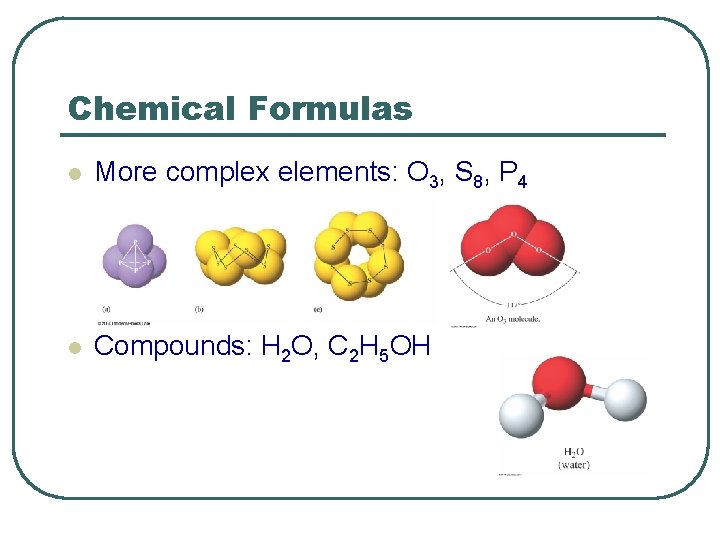
Chemical Formulas l More complex elements: O 3, S 8, P 4 l Compounds: H 2 O, C 2 H 5 OH

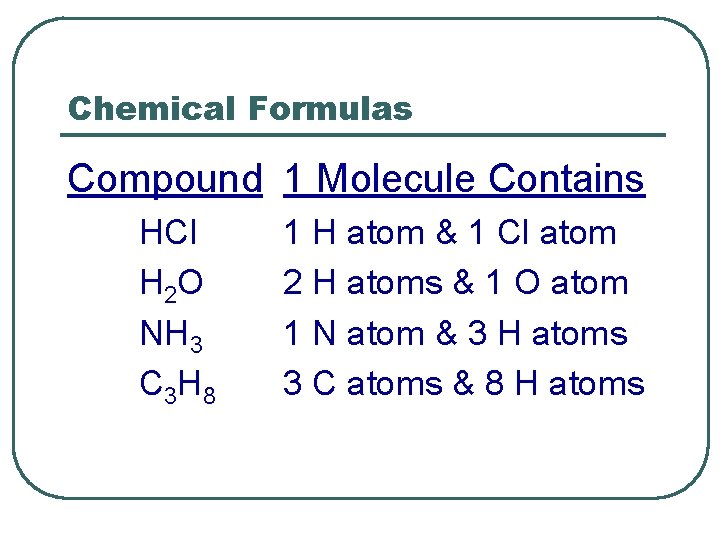
Chemical Formulas Compound 1 Molecule Contains HCl H 2 O NH 3 C 3 H 8 1 H atom & 1 Cl atom 2 H atoms & 1 O atom 1 N atom & 3 H atoms 3 C atoms & 8 H atoms

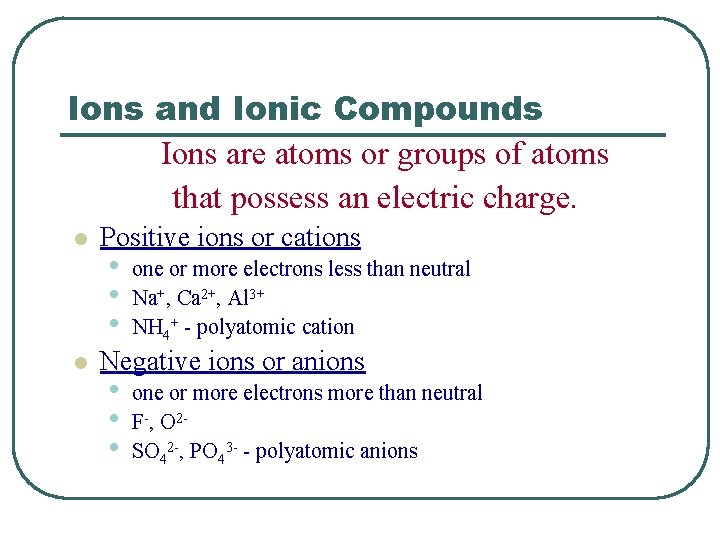
Ions and Ionic Compounds Ions are atoms or groups of atoms that possess an electric charge. l l Positive ions or cations • • • one or more electrons less than neutral Na+, Ca 2+, Al 3+ NH 4+ - polyatomic cation Negative ions or anions • • • one or more electrons more than neutral F-, O 2 SO 42 -, PO 43 - - polyatomic anions

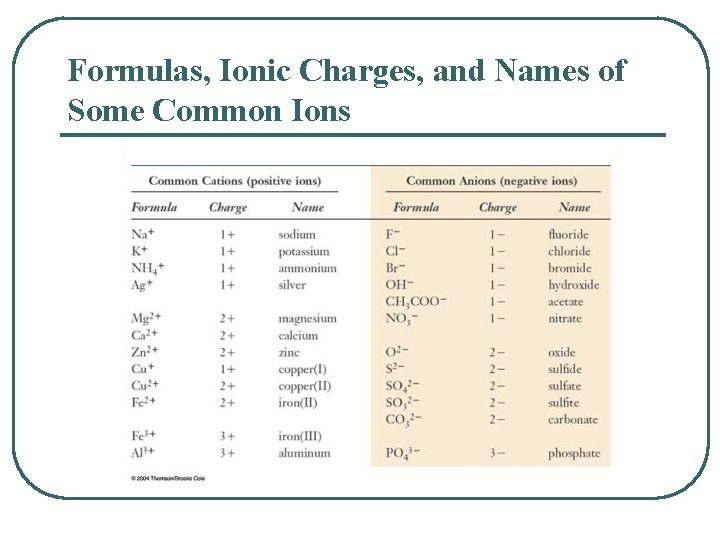
Formulas, Ionic Charges, and Names of Some Common Ions

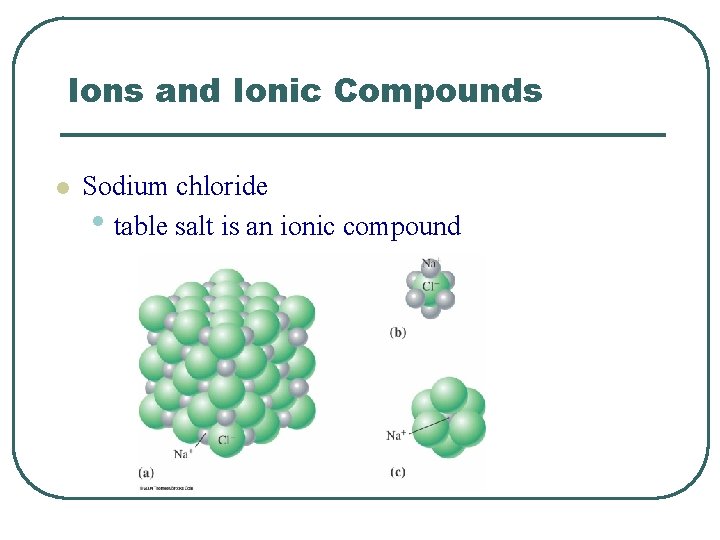
Ions and Ionic Compounds l Sodium chloride • table salt is an ionic compound

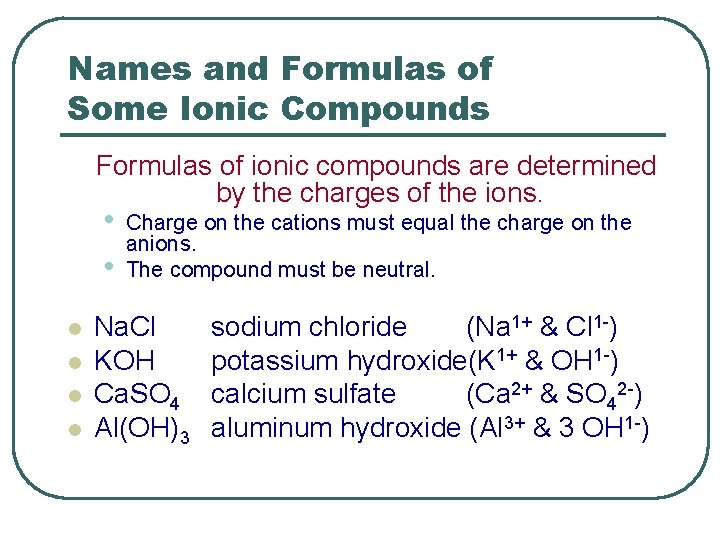
Names and Formulas of Some Ionic Compounds Formulas of ionic compounds are determined by the charges of the ions. • • l l Charge on the cations must equal the charge on the anions. The compound must be neutral. Na. Cl KOH Ca. SO 4 Al(OH)3 sodium chloride (Na 1+ & Cl 1 -) potassium hydroxide(K 1+ & OH 1 -) calcium sulfate (Ca 2+ & SO 42 -) aluminum hydroxide (Al 3+ & 3 OH 1 -)

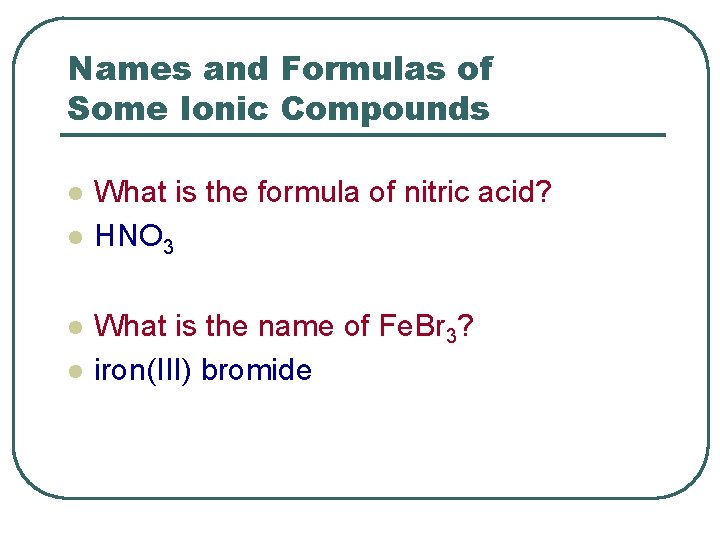
Names and Formulas of Some Ionic Compounds l l What is the formula of nitric acid? HNO 3 What is the name of Fe. Br 3? iron(III) bromide

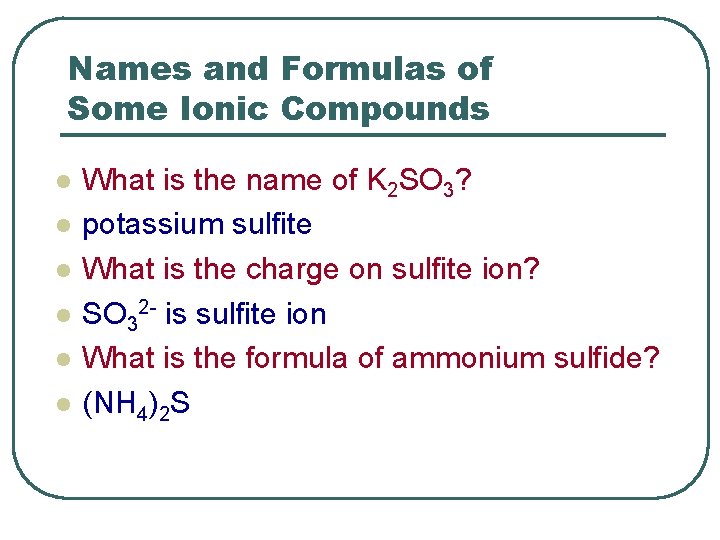
Names and Formulas of Some Ionic Compounds l l l What is the name of K 2 SO 3? potassium sulfite What is the charge on sulfite ion? SO 32 - is sulfite ion What is the formula of ammonium sulfide? (NH 4)2 S

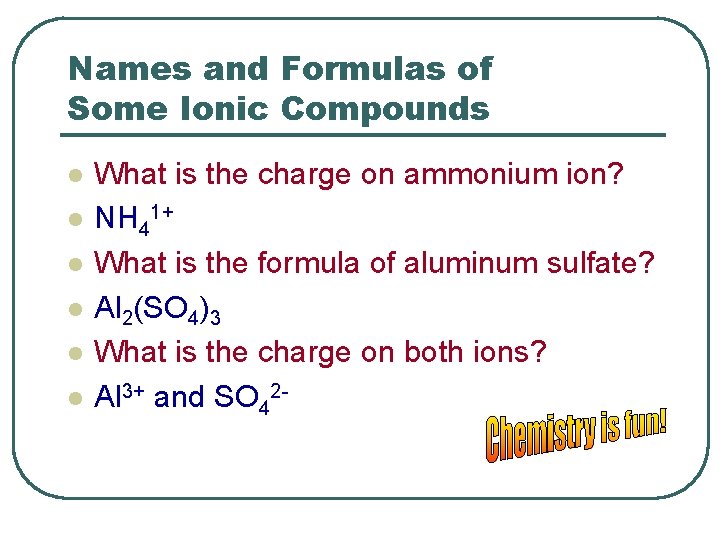
Names and Formulas of Some Ionic Compounds l l l What is the charge on ammonium ion? NH 41+ What is the formula of aluminum sulfate? Al 2(SO 4)3 What is the charge on both ions? Al 3+ and SO 42 -