Gene Therapy for Primary Immunodeficiency Phase lll studies















- Slides: 49

Gene Therapy for Primary Immunodeficiency Phase l/ll studies at ICH/GOS X-SCID (9+1 patients) ADA SCID (1 patient) X-CGD (2 patients)

X-linked severe combined immunodeficiency (SCIDX 1) 1 in 50 -100, 000 live births Major form of SCID Severe diarrhoea, pneumonia, septicaemia, fungal infection, failure to thrive, death usually within first year of life.


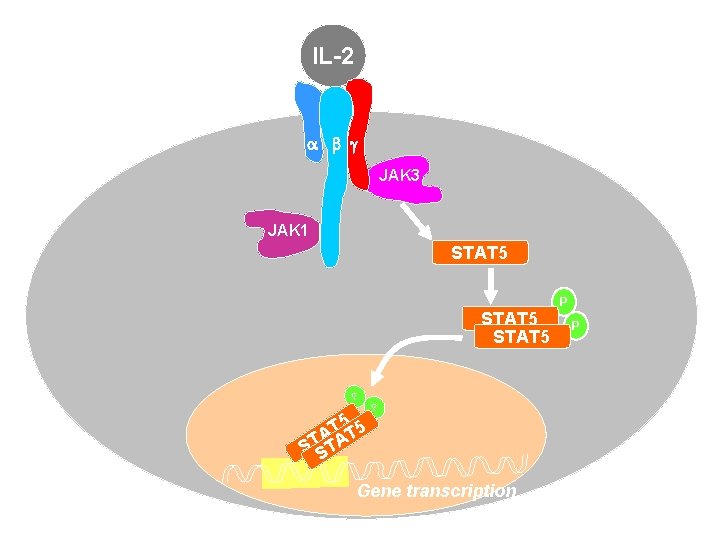
IL-2 a b g JAK 3 JAK 1 STAT 5 P T 5 T 5 A STSTA P Gene transcription P P


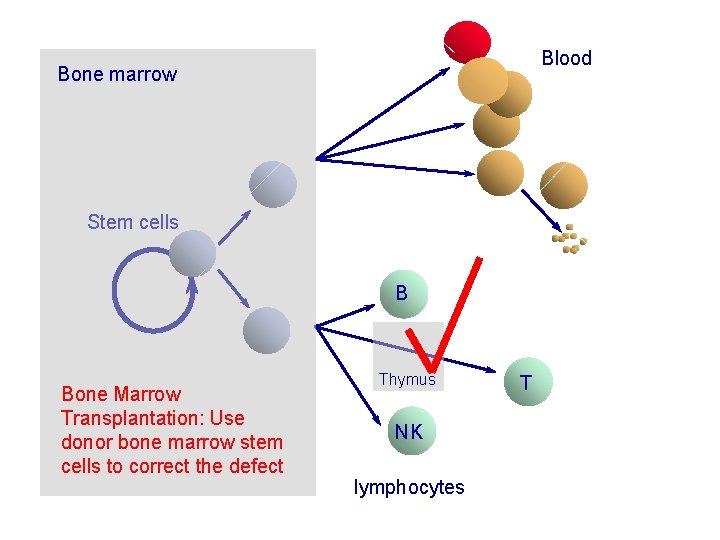
Blood Bone marrow Stem cells B Bone Marrow Transplantation: Use donor bone marrow stem cells to correct the defect Thymus NK lymphocytes T

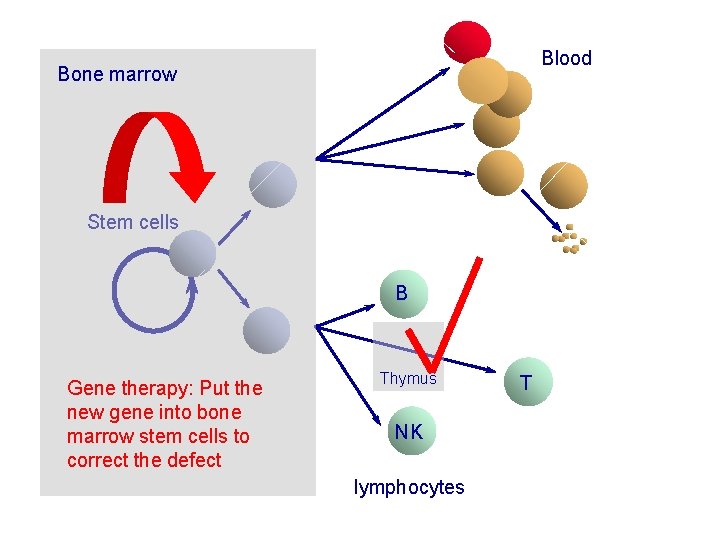
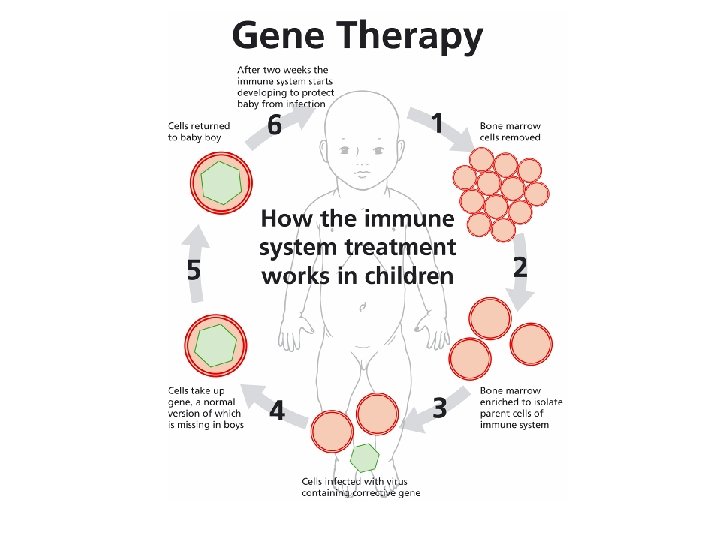
Blood Bone marrow Stem cells B Gene therapy: Put the new gene into bone marrow stem cells to correct the defect Thymus NK lymphocytes T

Treatment by bone marrow transplantation u Use of matched donors successful in 90% of cases u for 60% of cases, no matched donors have to use mis-matched donors, reduces success rate to <60% u need to improve success for children with no matched donor

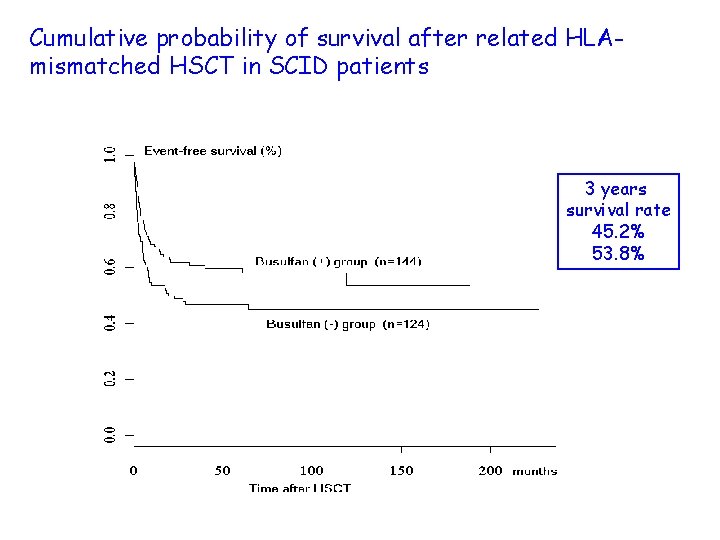
Cumulative probability of survival after related HLAmismatched HSCT in SCID patients 3 years survival rate 45. 2% 53. 8%

SCIDX 1 morbidity and mortality following HLAmismatched transplantation… 20% 1 year mortality Long term effects related to chemotherapy, usually with alkylating agents (growth, fertility, secondary malignancy, neuropsychological, hypodontia) Incomplete immunological reconstitution

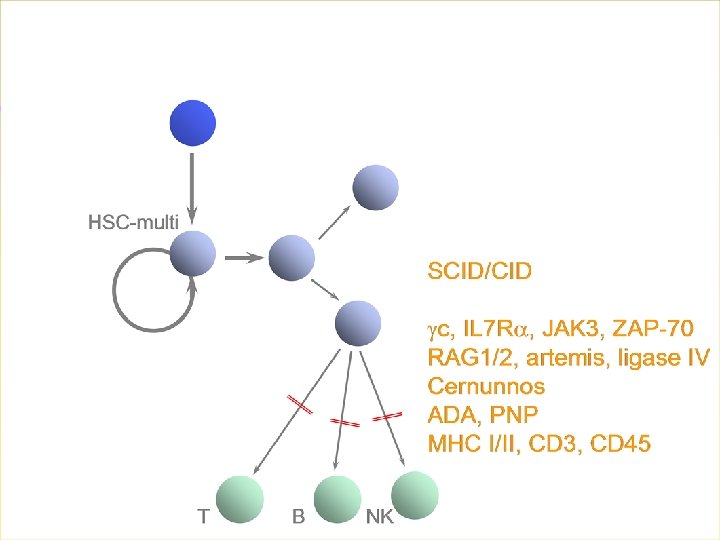
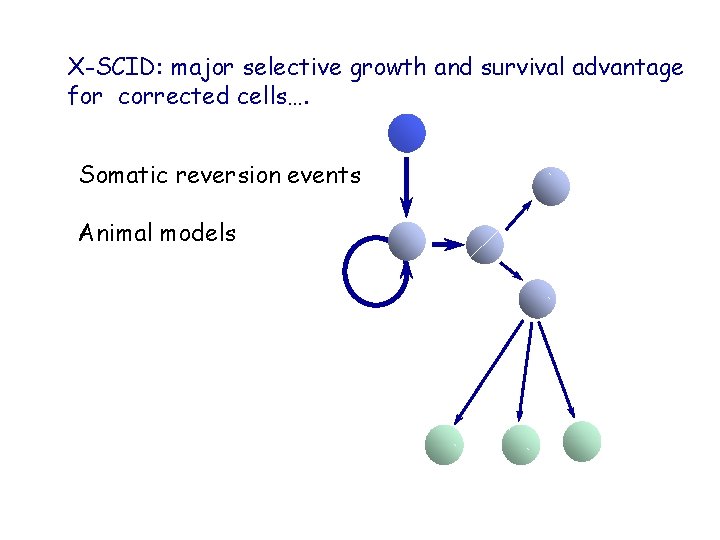
X-SCID: major selective growth and survival advantage for corrected cells…. Somatic reversion events Animal models



Y+

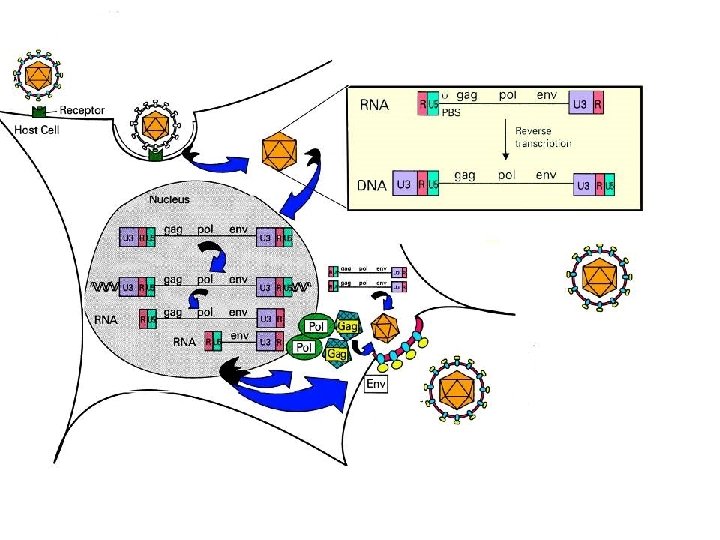
Gene therapy for X-SCID: phase I study Criteria for entry: No matched sibling donor Molecularly confirmed diagnosis Common gamma chain vector: PG 13 producer cells (GALV envelope) titre approximately 1 x 10 e 6 transducing units per ml


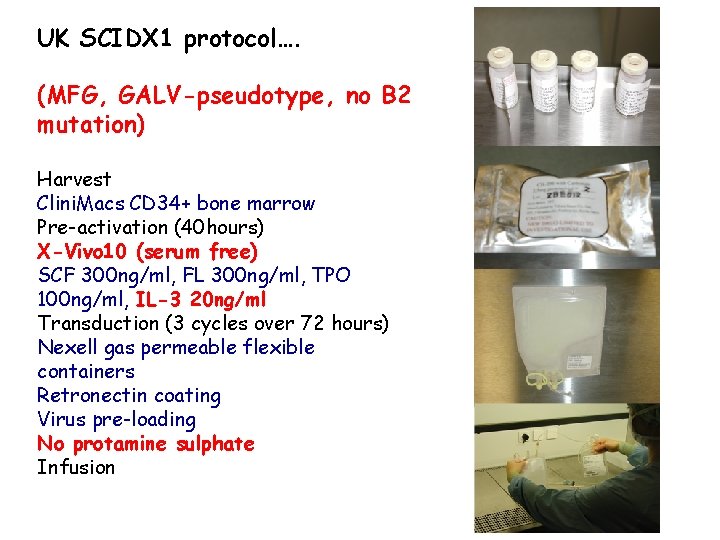
Transduction protocol – SCIDX 1 Harvest Clini. Macs CD 34+ bone marrow Pre-activation (40 hours) X-Vivo 10 (serum free) SCF 300 ng/ml, FL 300 ng/ml, TPO 100 ng/ml, IL-3 20 ng/ml Transduction (3 cycles over 72 hours) Nexell gas permeable flexible containers Retronectin coating Virus pre-loading Infusion

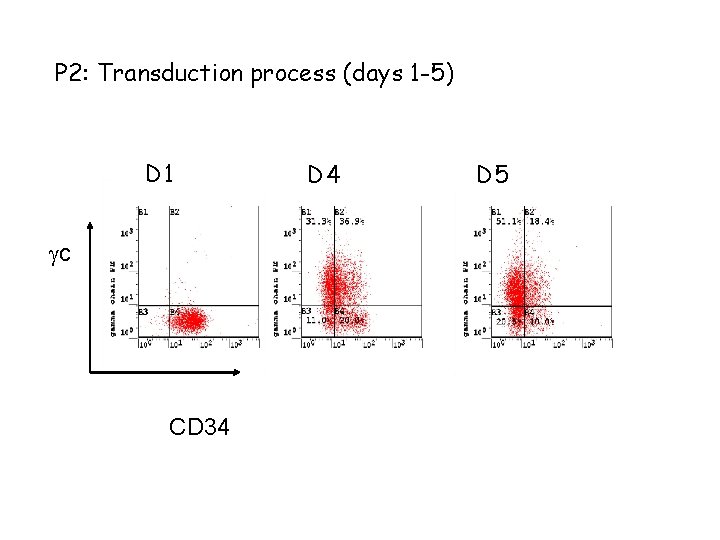
P 2: Transduction process (days 1 -5) D 1 c CD 34 D 5



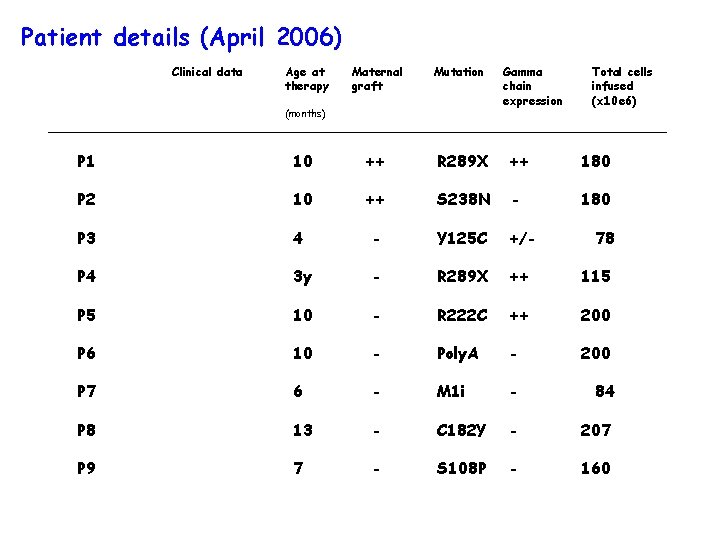
Patient details (April 2006) Clinical data Age at therapy Maternal graft Mutation (months) Gamma chain expression Total cells infused (x 10 e 6) P 1 10 ++ R 289 X ++ 180 P 2 10 ++ S 238 N - 180 P 3 4 - Y 125 C +/- P 4 3 y - R 289 X ++ 115 P 5 10 - R 222 C ++ 200 P 6 10 - Poly. A - 200 P 7 6 - M 1 i - 84 P 8 13 - C 182 Y - 207 P 9 7 - S 108 P - 160 78

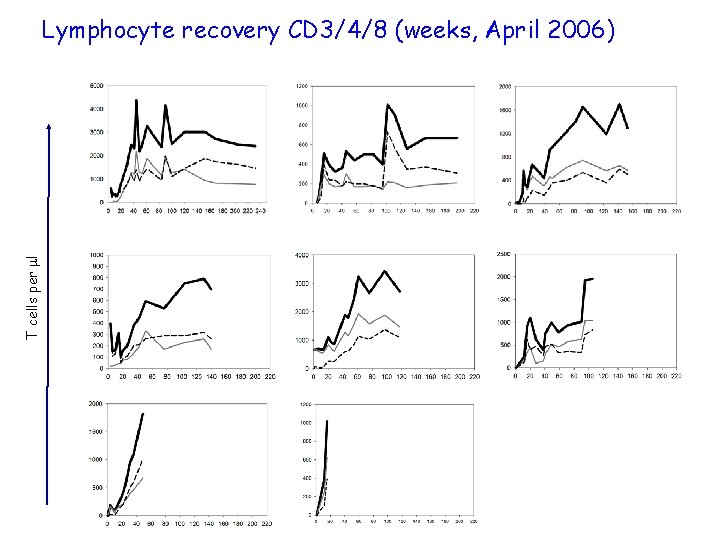
Lymphocyte recovery CD 3/4/8 (weeks, April 2006) P 2 P 3 P 4 P 5 P 6 T cells per l P 1 P 7 P 8

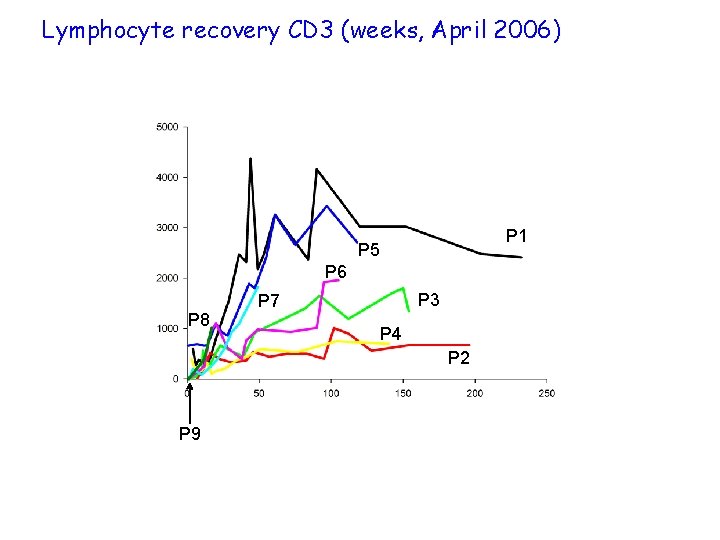
Lymphocyte recovery CD 3 (weeks, April 2006) P 1 P 5 P 6 P 8 P 3 P 7 P 4 P 2 P 9

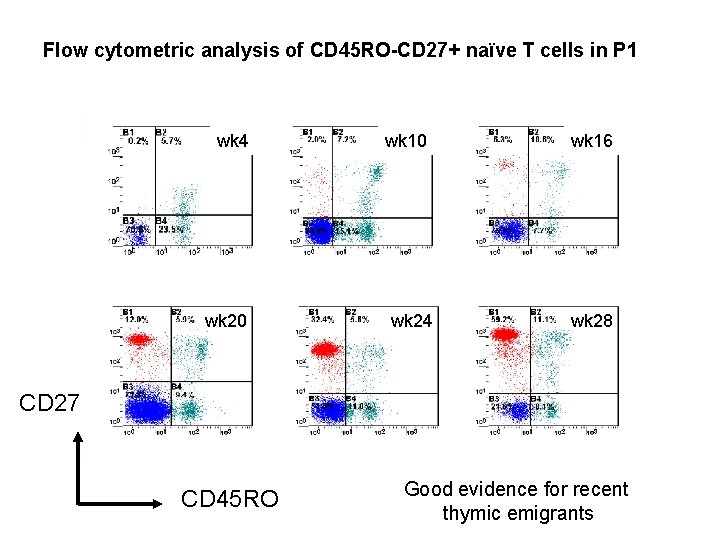
Flow cytometric analysis of CD 45 RO-CD 27+ naïve T cells in P 1 wk 4 wk 20 wk 10 wk 24 wk 16 wk 28 CD 27 CD 45 RO Good evidence for recent thymic emigrants

P 1 Immunoglobulin levels……… sc. Ig

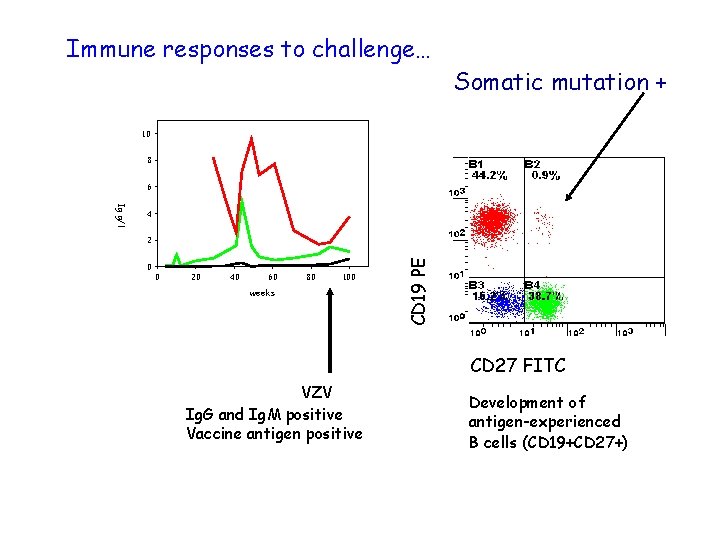
Immune responses to challenge… Somatic mutation + 10 8 6 2 0 0 20 40 60 80 100 weeks CD 19 PE Ig g/l 4 CD 27 FITC VZV Ig. G and Ig. M positive Vaccine antigen positive Development of antigen-experienced B cells (CD 19+CD 27+)

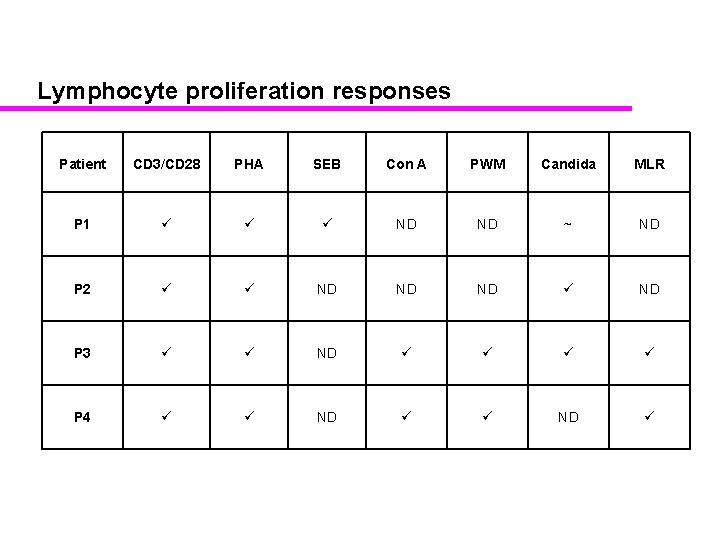
Lymphocyte proliferation responses Patient CD 3/CD 28 PHA SEB Con A PWM Candida MLR P 1 ND ND ~ ND P 2 ND ND P 3 ND P 4 ND


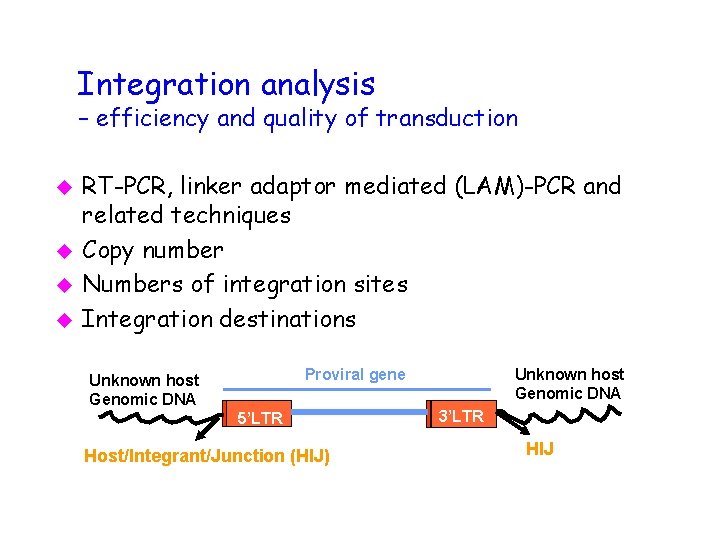
Integration analysis – efficiency and quality of transduction u u RT-PCR, linker adaptor mediated (LAM)-PCR and related techniques Copy number Numbers of integration sites Integration destinations Unknown host Genomic DNA Proviral gene Unknown host Genomic DNA 5’LTR Host/Integrant/Junction (HIJ) 3’LTR HIJ

Efficiency of gene transfer…. . CD 3 CD 19 CD 33 <3 copies 0. 01 -0. 2 copies 0. 001 -0. 1 copies CD 34 +ve (P 1, P 2, P 3)

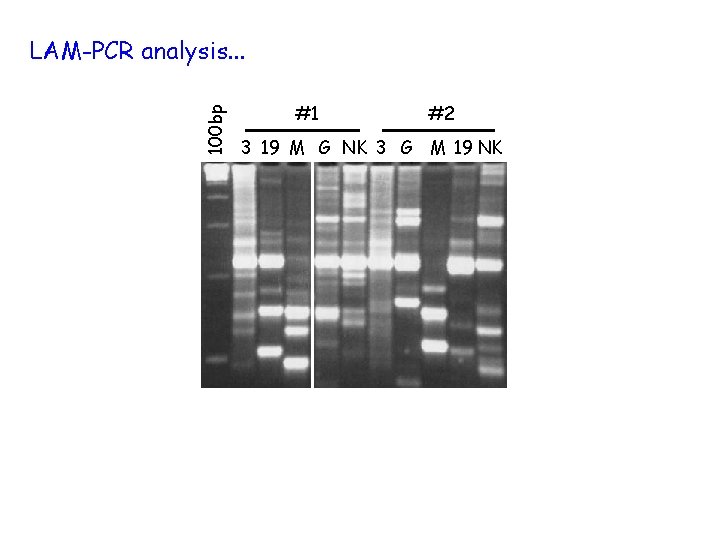
100 bp LAM-PCR analysis. . . #1 #2 3 19 M G NK 3 G M 19 NK


Summary of results: u Immunological reconstitution u Immune cell function u Immunisation u Children at home, off therapy

Similar case with another adult patient in Paris

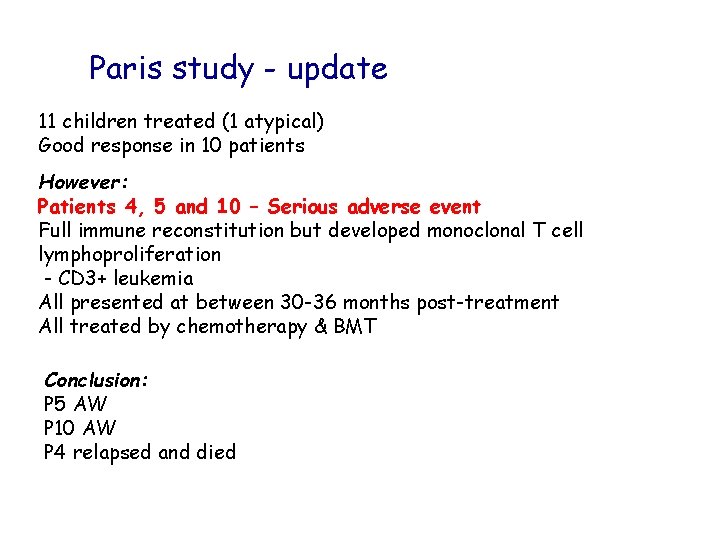
Paris study - update 11 children treated (1 atypical) Good response in 10 patients However: Patients 4, 5 and 10 – Serious adverse event Full immune reconstitution but developed monoclonal T cell lymphoproliferation - CD 3+ leukemia All presented at between 30 -36 months post-treatment All treated by chemotherapy & BMT Conclusion: P 5 AW P 10 AW P 4 relapsed and died

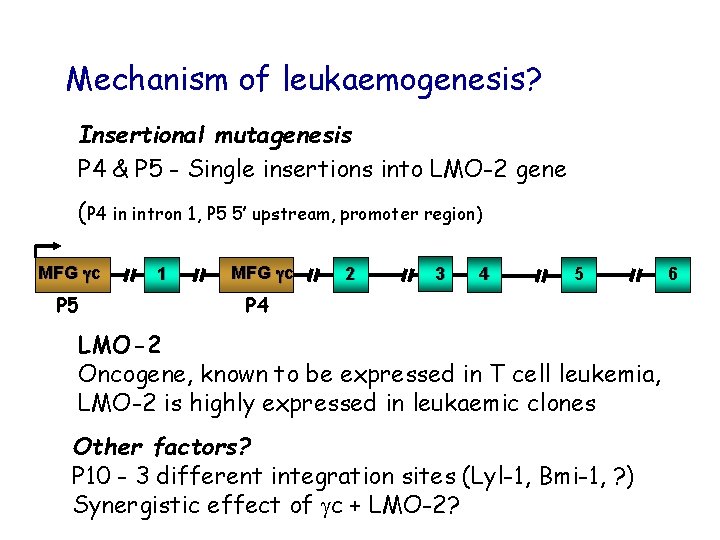
Mechanism of leukaemogenesis? Insertional mutagenesis P 4 & P 5 - Single insertions into LMO-2 gene (P 4 in intron 1, P 5 5’ upstream, promoter region) MFG gc P 5 1 MFG gc 2 3 4 5 P 4 LMO-2 Oncogene, known to be expressed in T cell leukemia, LMO-2 is highly expressed in leukaemic clones Other factors? P 10 - 3 different integration sites (Lyl-1, Bmi-1, ? ) Synergistic effect of c + LMO-2? 6

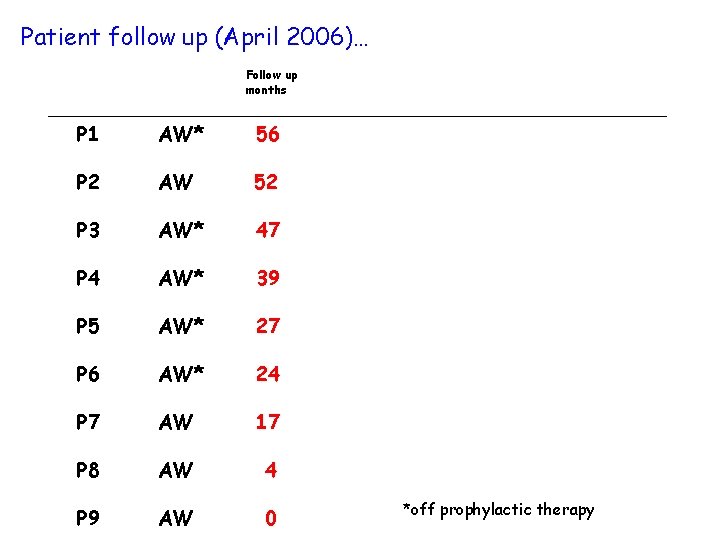
Patient follow up (April 2006)… P 1 Follow up months AW* 56 P 2 AW 52 P 3 AW* 47 P 4 AW* 39 P 5 AW* 27 P 6 AW* 24 P 7 AW 17 P 8 AW 4 P 9 AW 0 *off prophylactic therapy

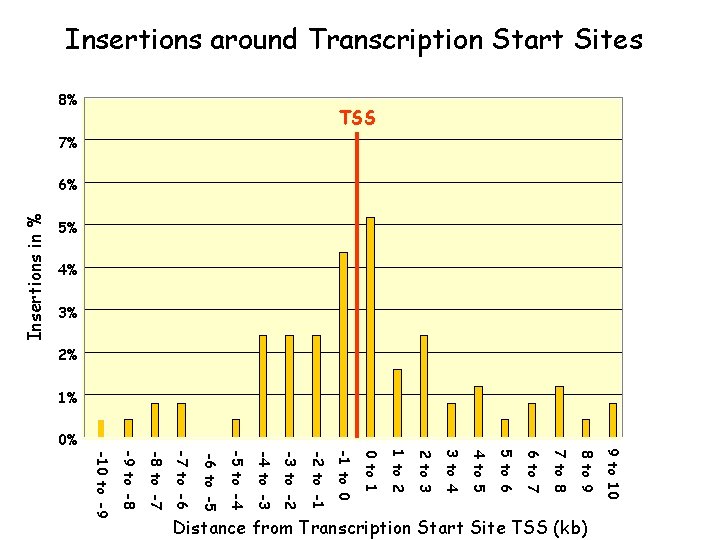
5% 4% 3% Insertions in % Insertions around Transcription Start Sites TSS 8% 7% 6% 2% 1% 9 to 10 8 to 9 7 to 8 6 to 7 5 to 6 4 to 5 3 to 4 2 to 3 1 to 2 0 to 1 Distance from Transcription Start Site TSS (kb) -10 to -9 -9 to -8 -8 to -7 -7 to -6 -6 to -5 -5 to -4 -4 to -3 -3 to -2 -2 to -1 -1 to 0 0%

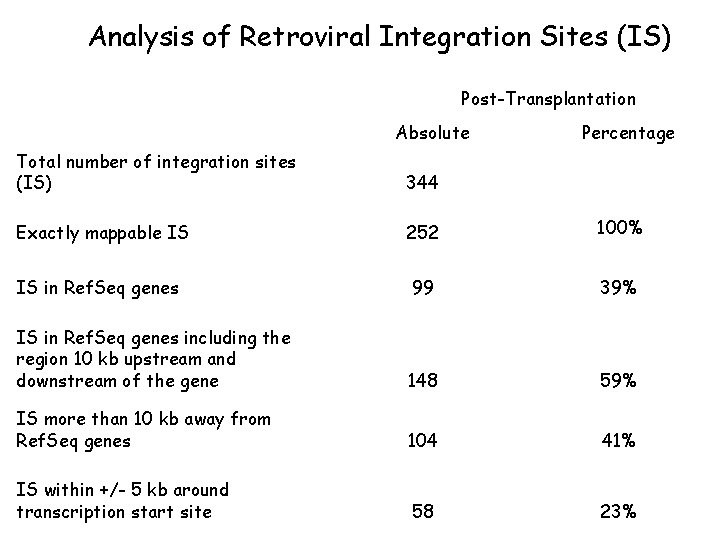
Analysis of Retroviral Integration Sites (IS) Post-Transplantation Absolute Percentage Total number of integration sites (IS) 344 Exactly mappable IS 252 100% IS in Ref. Seq genes 99 39% IS in Ref. Seq genes including the region 10 kb upstream and downstream of the gene 148 59% IS more than 10 kb away from Ref. Seq genes 104 41% IS within +/- 5 kb around transcription start site 58 23%

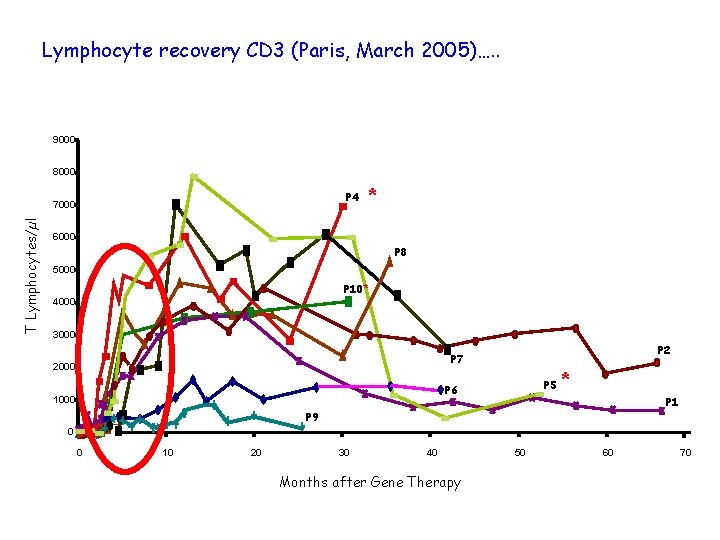
Lymphocyte recovery CD 3 (Paris, March 2005)…. . 9000 8000 P 4 T Lymphocytes/µl 7000 * 6000 P 8 5000 P 10* 4000 3000 P 2 P 7 2000 P 5 P 6 1000 * P 1 P 9 0 0 10 20 30 40 Months after Gene Therapy 50 60 70

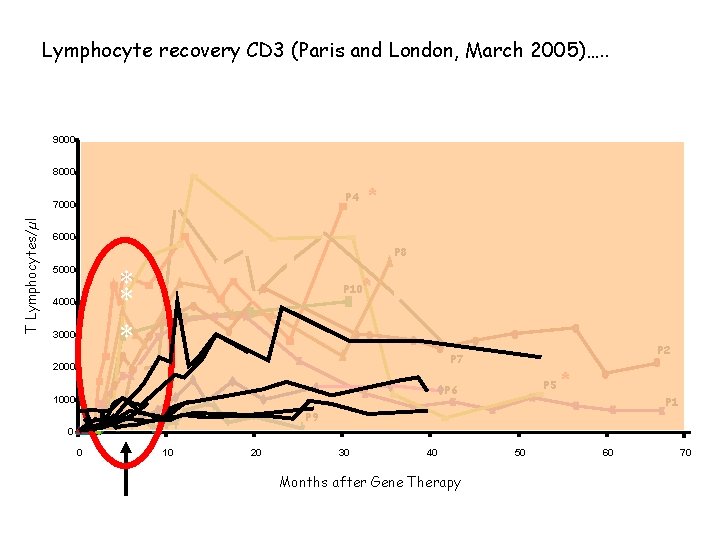
Lymphocyte recovery CD 3 (Paris and London, March 2005)…. . 9000 8000 T Lymphocytes/µl * P 4 7000 6000 P 8 * * * 5000 4000 3000 * P 10 P 2 P 7 2000 P 5 P 6 1000 * P 1 P 9 0 0 10 20 30 40 Months after Gene Therapy 50 60 70

UK SCIDX 1 protocol…. (MFG, GALV-pseudotype, no B 2 mutation) Harvest Clini. Macs CD 34+ bone marrow Pre-activation (40 hours) X-Vivo 10 (serum free) SCF 300 ng/ml, FL 300 ng/ml, TPO 100 ng/ml, IL-3 20 ng/ml Transduction (3 cycles over 72 hours) Nexell gas permeable flexible containers Retronectin coating Virus pre-loading No protamine sulphate Infusion

Will it happen to children treated in London? We don’t know, but: 1. At least 4 of our patients are out of the “time-frame” 2. We used a slightly different virus 3. We used a different protocol Development of safer vectors

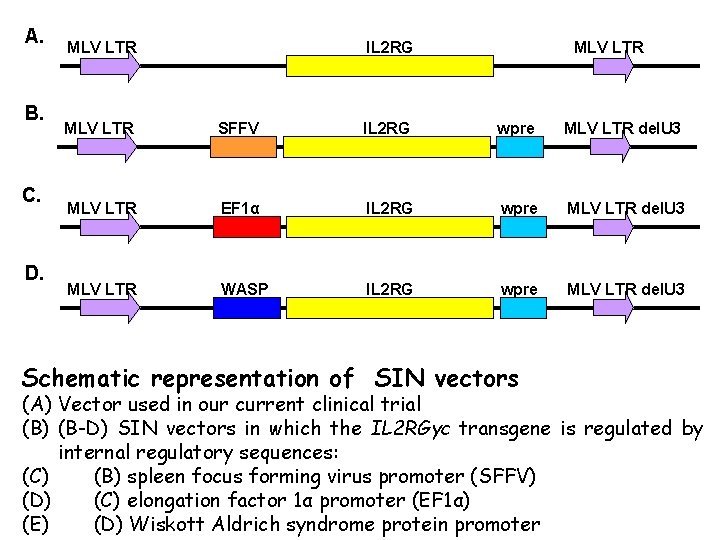
New Improved Retroviral Vectors u Self-inactivating (SIN) vectors - promoter and enhancer element deletion in 3’LTR - should have no LTR-directed transcription - should reduce the risk of insertional mutagenesis u Tissue specific promoters - viral promoters, SFFV LTR is active in stem and progenitor cells - constitutive eukaryotic promoters, EF 1α - haematopoietic cell specific promoters, WASp

A. B. C. D. MLV LTR IL 2 RG MLV LTR SFFV IL 2 RG wpre MLV LTR del. U 3 MLV LTR EF 1α IL 2 RG wpre MLV LTR del. U 3 MLV LTR WASP IL 2 RG wpre MLV LTR del. U 3 Schematic representation of SIN vectors (A) Vector used in our current clinical trial (B) (B-D) SIN vectors in which the IL 2 RGγc transgene is regulated by internal regulatory sequences: (C) (B) spleen focus forming virus promoter (SFFV) (D) (C) elongation factor 1α promoter (EF 1α) (E) (D) Wiskott Aldrich syndrome protein promoter

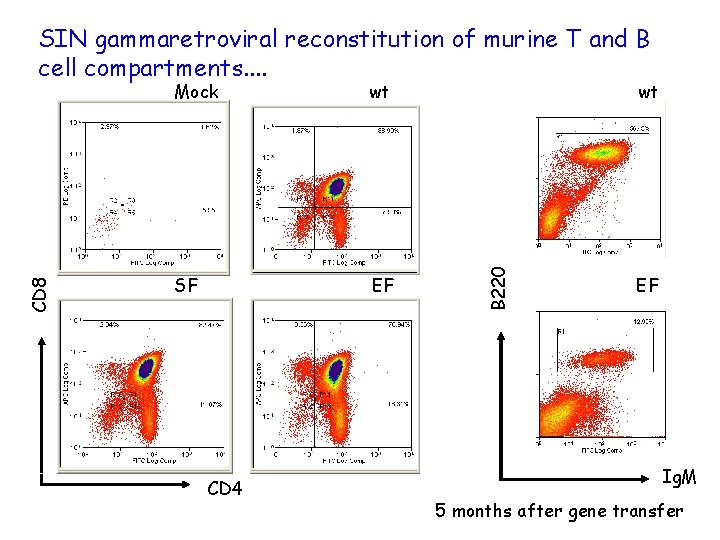
Mock wt SF EF CD 4 wt B 220 CD 8 SIN gammaretroviral reconstitution of murine T and B cell compartments. . EF Ig. M 5 months after gene transfer

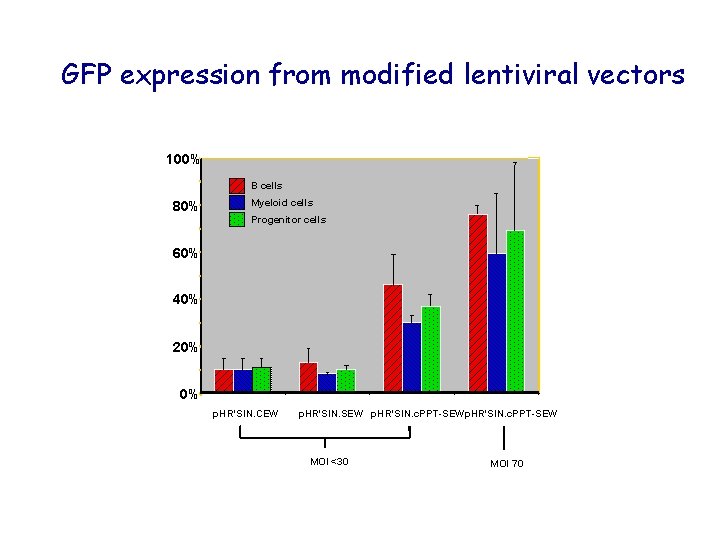
GFP expression from modified lentiviral vectors 100% B cells 80% Myeloid cells Progenitor cells 60% 40% 20% 0% p. HR'SIN. CEW p. HR'SIN. SEW p. HR'SIN. c. PPT-SEW MOI <30 MOI 70

Future gene therapy: Other primary immunodeficiency disorders -ADA-SCID (1 patient, 3 further planned) -X-CGD (2 patients) -WAS (hope to start 2007? ) Primary haematological disorders, including leukemia Stem cell protection strategies

The Molecular Immunology Unit at ICH Kate Parsley Kimberly Gilmour Jo Sinclair Steve Howe Doug King Suzy Bailey Fang Zhang Aris Giannakopoulos Meera Ulaganathan Mohammed Osman Mike Blundell Graham Davies Christine Kinnon Bobby Gaspar Adrian Thrasher Funded by: The Wellcome Trust, MRC, BBSRC, Department of Healt CGD Trust, Primary Immunodeficiency Association, CF Trus EU, LRF, ARC, SPARKS and others… GOS Nursing Staff Pharmacy Staff EUFETS Klaus Khulke Cincinatti/ Freiberg Manfred Schmidt Christof Von Kalle Frankfurt Manuel Grez Stefan Stein Hannover Christopher Baum