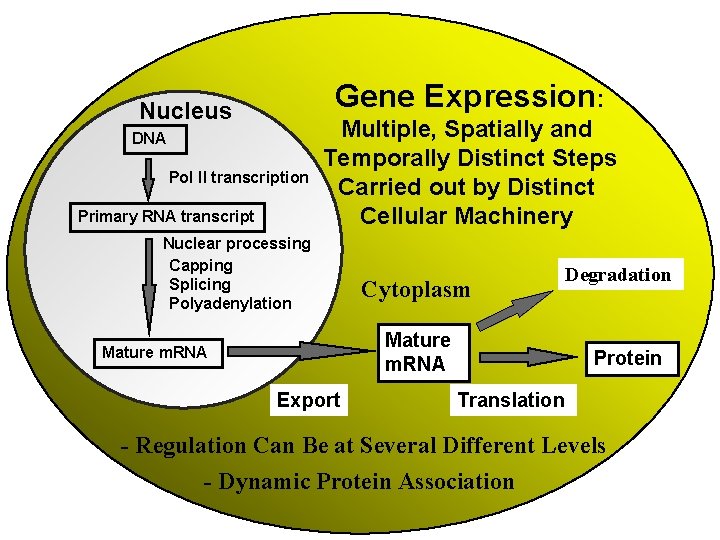
Gene Expression Nucleus DNA Pol II transcription Primary







- Slides: 66

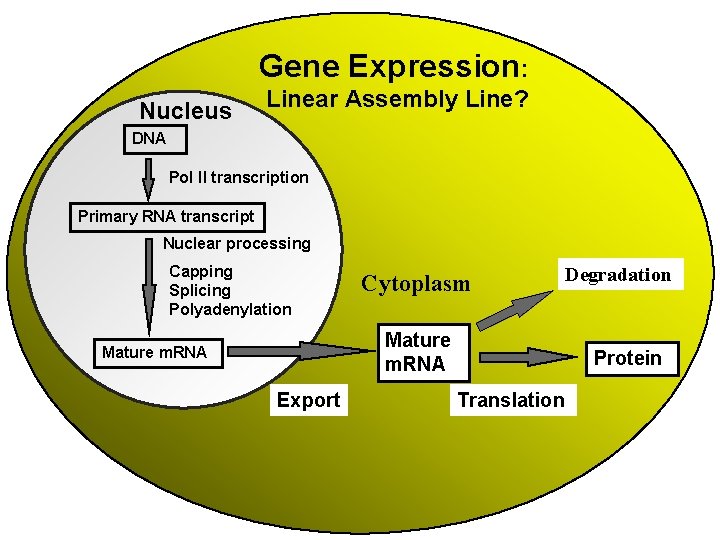
Gene Expression: Nucleus DNA Pol II transcription Primary RNA transcript Multiple, Spatially and Temporally Distinct Steps Carried out by Distinct Cellular Machinery Nuclear processing Capping Splicing Polyadenylation Cytoplasm Mature m. RNA Export Degradation Protein Translation - Regulation Can Be at Several Different Levels - Dynamic Protein Association

How can the cell distinguish between (1) intron-containing pre-m. RNAs (2) spliced m. RNAs (3) intronless m. RNAs to ensure that (1) are retained in the nucleus while (2 and 3) are exported to the cytoplasm?

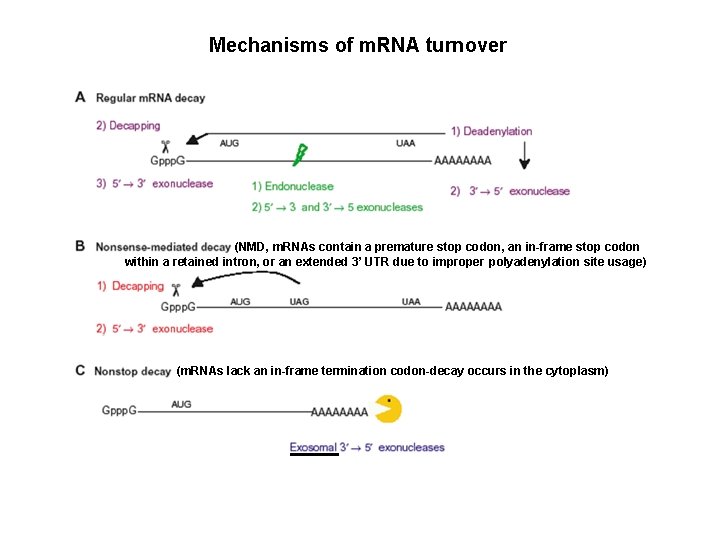
Mechanisms of m. RNA turnover (NMD, m. RNAs contain a premature stop codon, an in-frame stop codon within a retained intron, or an extended 3’ UTR due to improper polyadenylation site usage) (m. RNAs lack an in-frame termination codon-decay occurs in the cytoplasm)

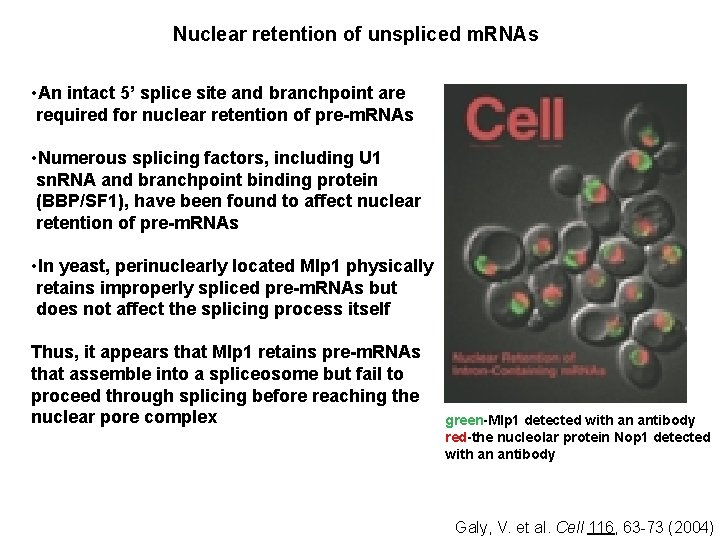
Nuclear retention of unspliced m. RNAs • An intact 5’ splice site and branchpoint are required for nuclear retention of pre-m. RNAs • Numerous splicing factors, including U 1 sn. RNA and branchpoint binding protein (BBP/SF 1), have been found to affect nuclear retention of pre-m. RNAs • In yeast, perinuclearly located Mlp 1 physically retains improperly spliced pre-m. RNAs but does not affect the splicing process itself Thus, it appears that Mlp 1 retains pre-m. RNAs that assemble into a spliceosome but fail to proceed through splicing before reaching the nuclear pore complex green-Mlp 1 detected with an antibody red-the nucleolar protein Nop 1 detected with an antibody Galy, V. et al. Cell 116, 63 -73 (2004)

Coupling of Transcriptional and Post-Transcriptional Events m. RNA Surveillance RNA-Mediated Gene Silencing

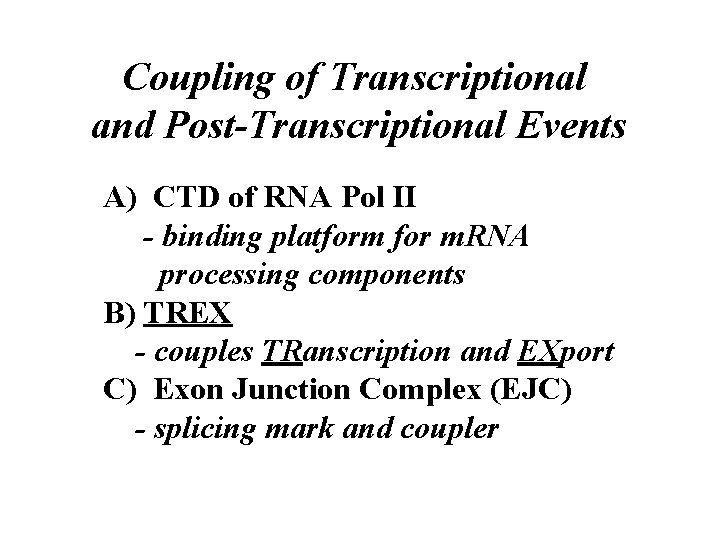
Coupling of Transcriptional and Post-Transcriptional Events A) CTD of RNA Pol II - binding platform for m. RNA processing components B) TREX - couples TRanscription and EXport C) Exon Junction Complex (EJC) - splicing mark and coupler

m. RNA Surveillance A) Quality Control Mechanism B) - ‘Process vs. Discard’ C) B) Nonsense Mediated Decay D) - Elimination of m. RNAs with E) Premature Stop Codons (PTCs)

RNA-Mediated Gene Silencing A) Post-Transcriptional Gene Silencing B) C) D) (PTGS or RNA Interference) - m. RNA degradation - translation block B) Transcriptional Gene Silencing (TGS) - DNA methylation - Heterochromatin formation - DNA rearrangement/elimination

Gene Expression: Nucleus Linear Assembly Line? DNA Pol II transcription Primary RNA transcript Nuclear processing Capping Splicing Polyadenylation Cytoplasm Mature m. RNA Export Degradation Protein Translation

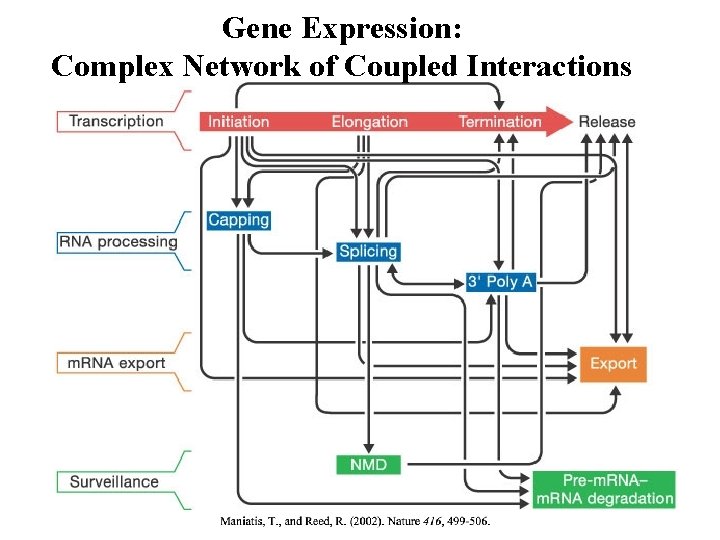
Gene Expression: Complex Network of Coupled Interactions

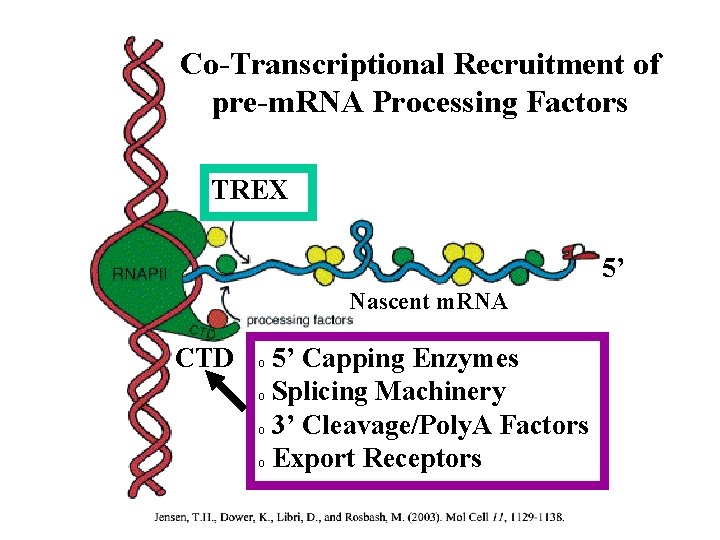
Co-Transcriptional Recruitment of pre-m. RNA Processing Factors TREX 5’ Nascent m. RNA CTD 5’ Capping Enzymes o Splicing Machinery o 3’ Cleavage/Poly. A Factors o Export Receptors o

Transcription Export T-REX Transcription Elongation Factors (THO Complex: Hpr 1 p, Tho 2 p, Mft 1 p, Thp 2 p) Export Factors (Yra, Sub 2) Tex 1 (unknown function) http: //home. wxs. nl/~vrie 0388/trex. JPG

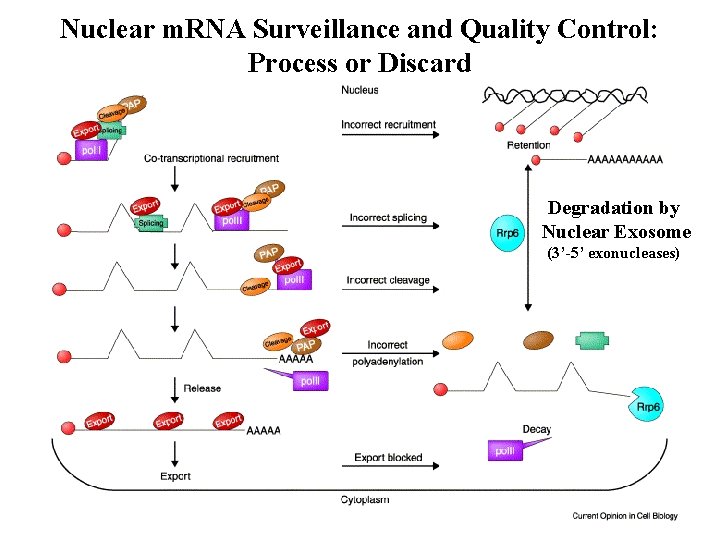
Nuclear m. RNA Surveillance and Quality Control: Process or Discard Degradation by Nuclear Exosome (3’-5’ exonucleases)

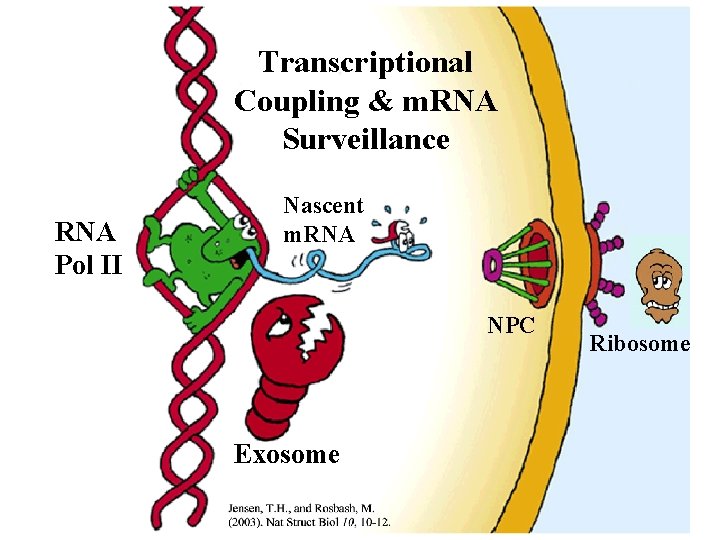
Transcriptional Coupling & m. RNA Surveillance RNA Pol II Nascent m. RNA NPC Exosome Ribosome

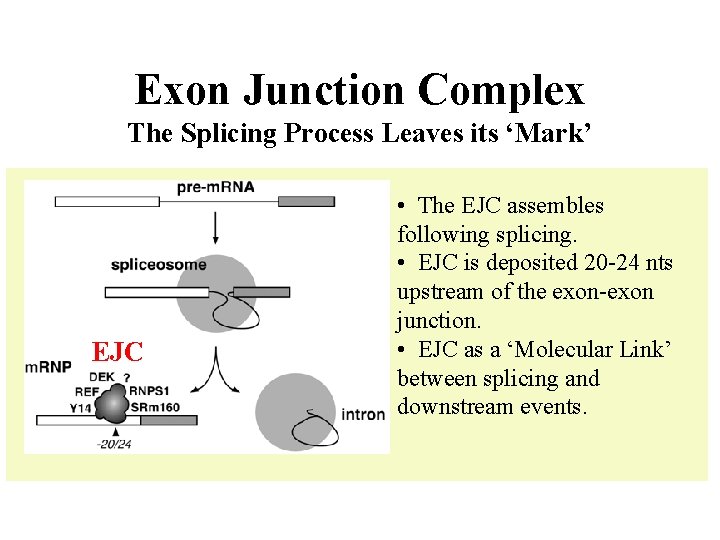
Exon Junction Complex The Splicing Process Leaves its ‘Mark’ EJC • The EJC assembles following splicing. • EJC is deposited 20 -24 nts upstream of the exon-exon junction. • EJC as a ‘Molecular Link’ between splicing and downstream events.

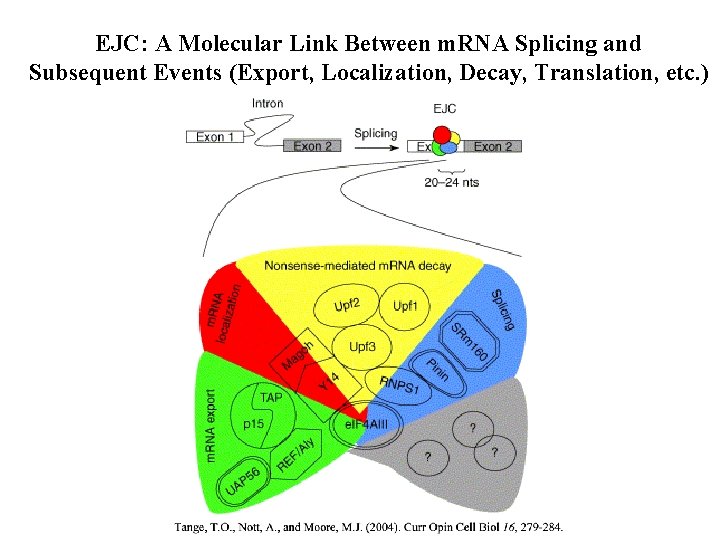
EJC: A Molecular Link Between m. RNA Splicing and Subsequent Events (Export, Localization, Decay, Translation, etc. )

RNA-Mediated Gene Silencing RNA Interference (PTGS) Transcriptional Gene Silencing (TGS) Common Trigger:

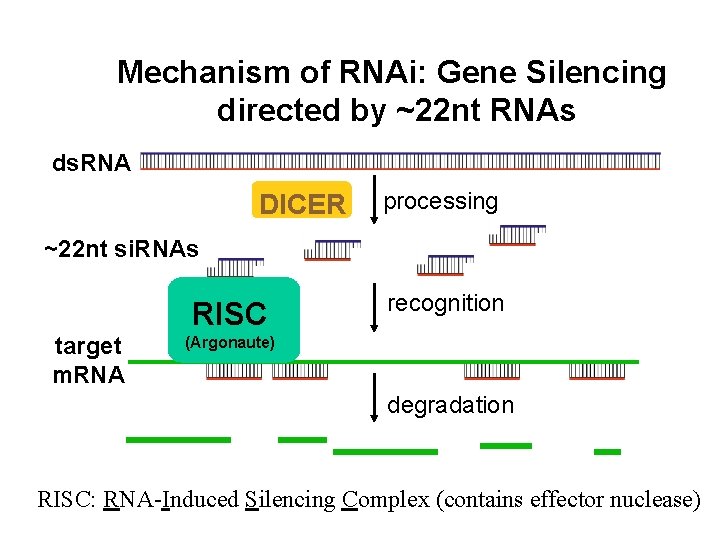
Mechanism of RNAi: Gene Silencing directed by ~22 nt RNAs ds. RNA DICER processing ~22 nt si. RNAs RISC target m. RNA recognition (Argonaute) degradation RISC: RNA-Induced Silencing Complex (contains effector nuclease)

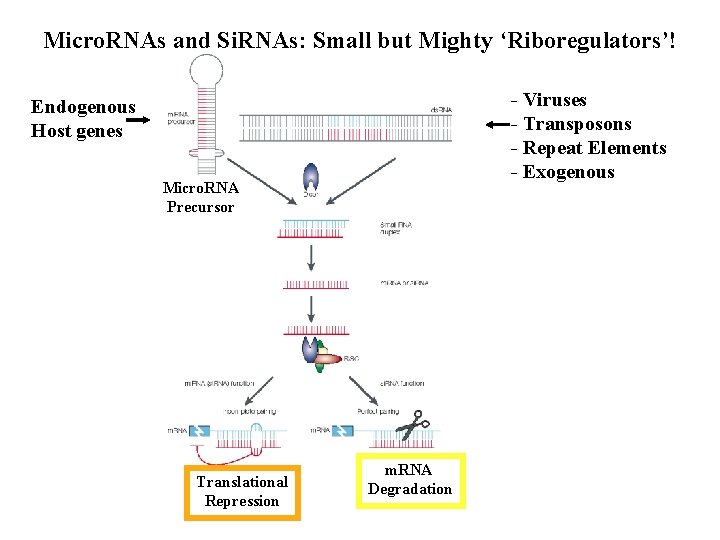
Micro. RNAs and Si. RNAs: Small but Mighty ‘Riboregulators’! - Viruses - Transposons - Repeat Elements - Exogenous Endogenous Host genes Micro. RNA Precursor Translational Repression m. RNA Degradation

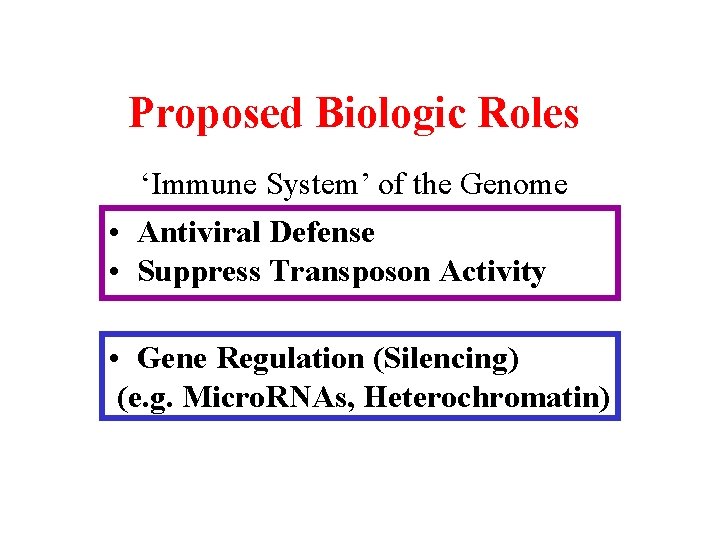
Proposed Biologic Roles ‘Immune System’ of the Genome • Antiviral Defense • Suppress Transposon Activity • Gene Regulation (Silencing) (e. g. Micro. RNAs, Heterochromatin)

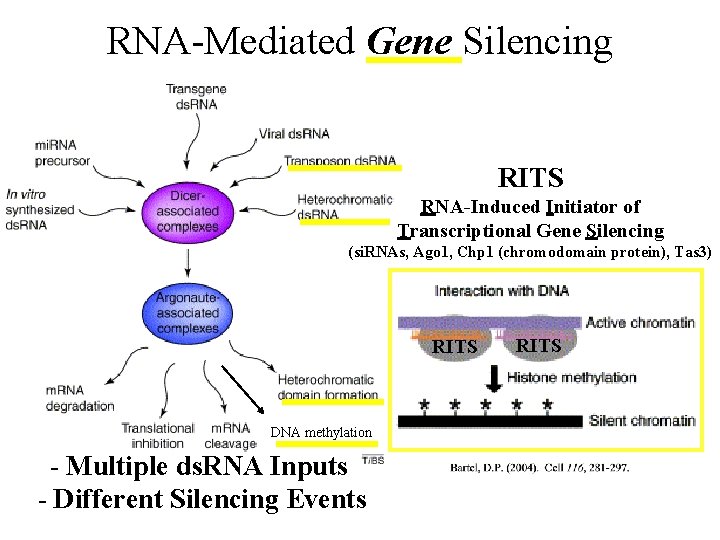
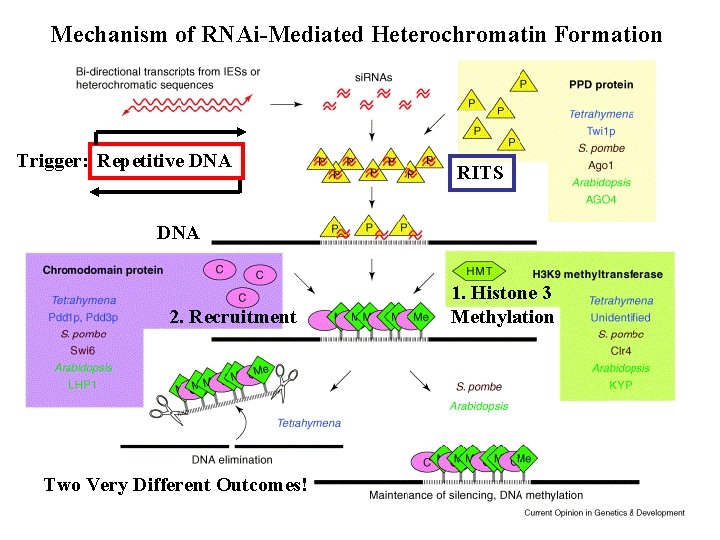
RNA-Mediated Gene Silencing RITS RNA-Induced Initiator of Transcriptional Gene Silencing (si. RNAs, Ago 1, Chp 1 (chromodomain protein), Tas 3) RITS DNA methylation - Multiple ds. RNA Inputs - Different Silencing Events RITS

Mechanism of RNAi-Mediated Heterochromatin Formation Trigger: Repetitive DNA RITS DNA 2. Recruitment Two Very Different Outcomes! 1. Histone 3 Methylation

TRANSLATION

Molecular Biology Familiarity with basic concepts is assumed, including: w nature of the genetic code w maintenance of genes through DNA replication w transcription of information from DNA to m. RNA w translation of m. RNA into protein.

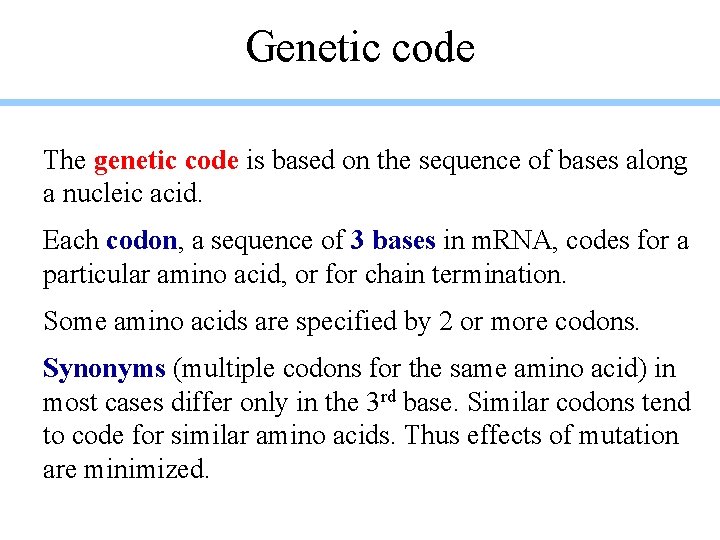
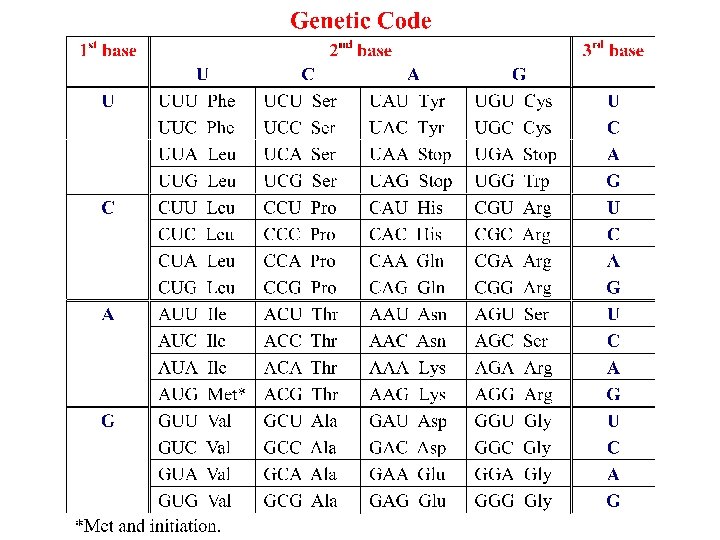
Genetic code The genetic code is based on the sequence of bases along a nucleic acid. Each codon, a sequence of 3 bases in m. RNA, codes for a particular amino acid, or for chain termination. Some amino acids are specified by 2 or more codons. Synonyms (multiple codons for the same amino acid) in most cases differ only in the 3 rd base. Similar codons tend to code for similar amino acids. Thus effects of mutation are minimized.


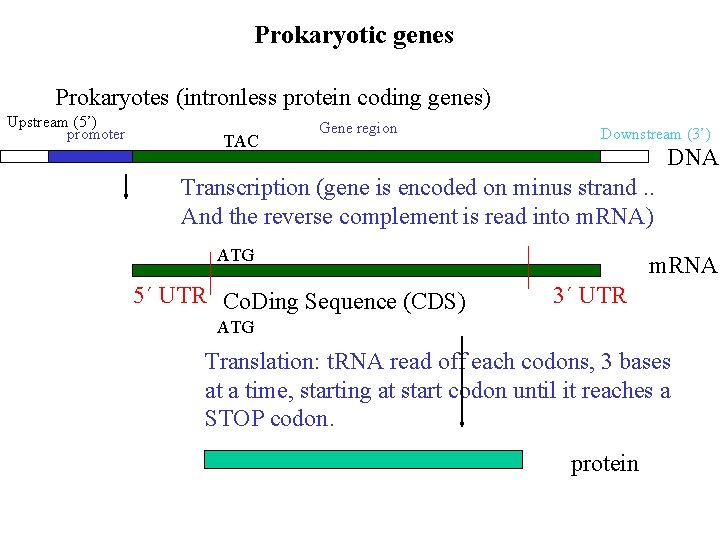
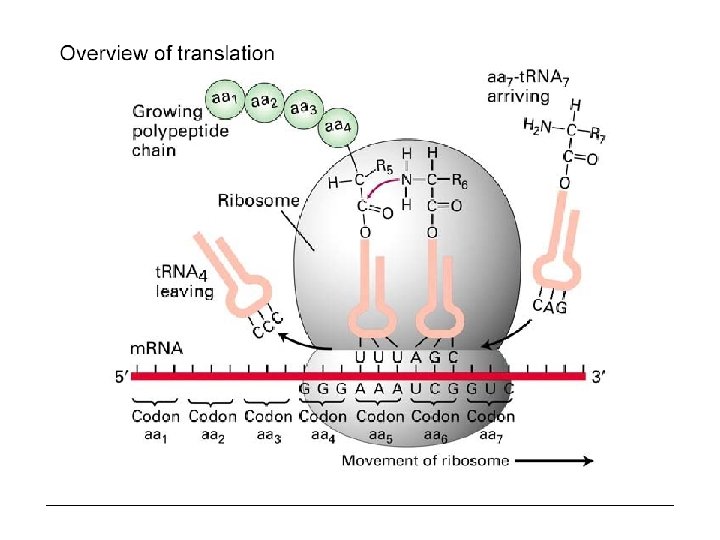
Prokaryotic genes Prokaryotes (intronless protein coding genes) Upstream (5’) promoter TAC Gene region Downstream (3’) DNA Transcription (gene is encoded on minus strand. . And the reverse complement is read into m. RNA) ATG 5´ UTR Co. Ding Sequence (CDS) m. RNA 3´ UTR ATG Translation: t. RNA read off each codons, 3 bases at a time, starting at start codon until it reaches a STOP codon. protein

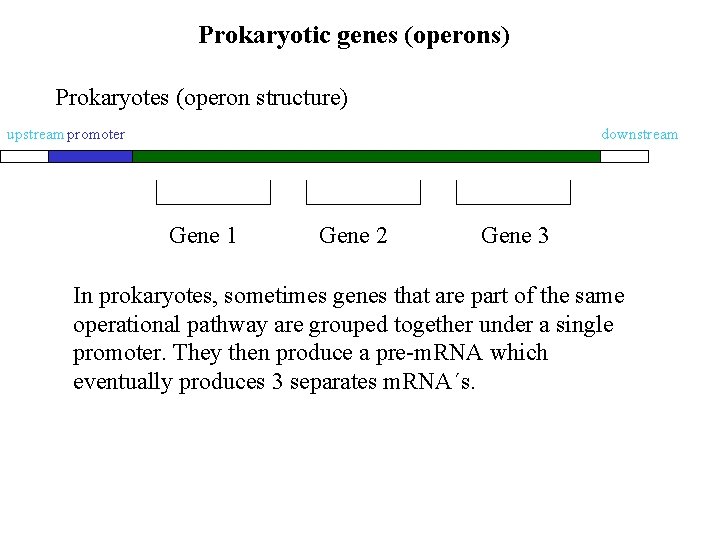
Prokaryotic genes (operons) Prokaryotes (operon structure) upstream promoter downstream Gene 1 Gene 2 Gene 3 In prokaryotes, sometimes genes that are part of the same operational pathway are grouped together under a single promoter. They then produce a pre-m. RNA which eventually produces 3 separates m. RNA´s.

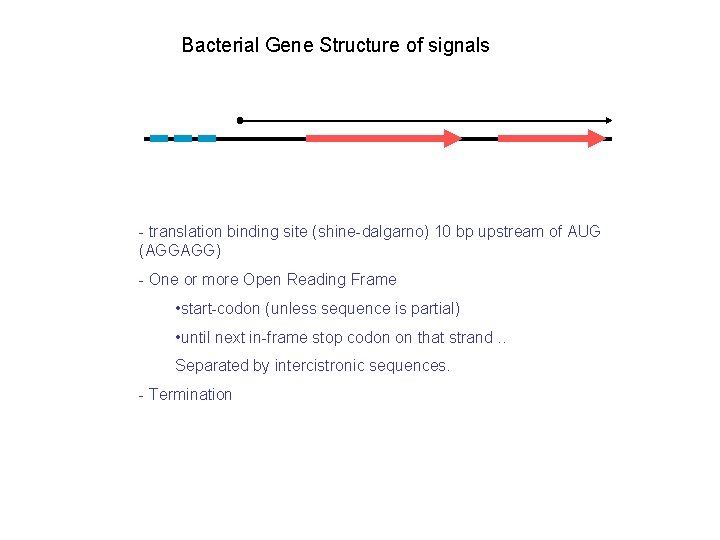
Bacterial Gene Structure of signals - translation binding site (shine-dalgarno) 10 bp upstream of AUG (AGGAGG) - One or more Open Reading Frame • start-codon (unless sequence is partial) • until next in-frame stop codon on that strand. . Separated by intercistronic sequences. - Termination

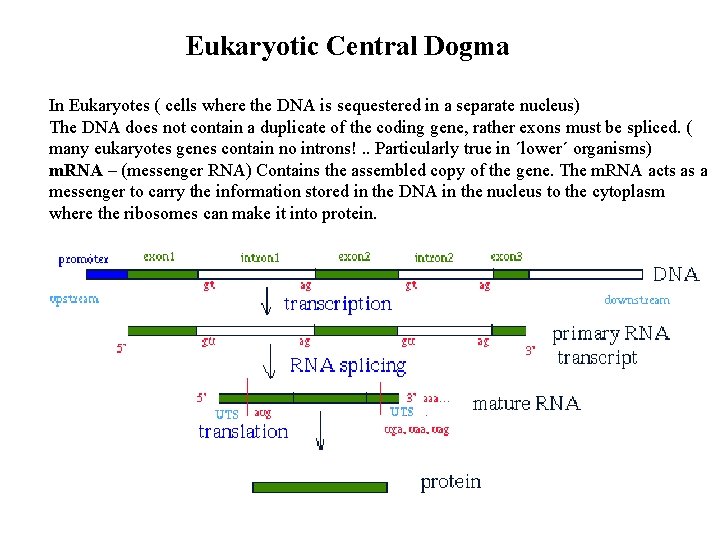
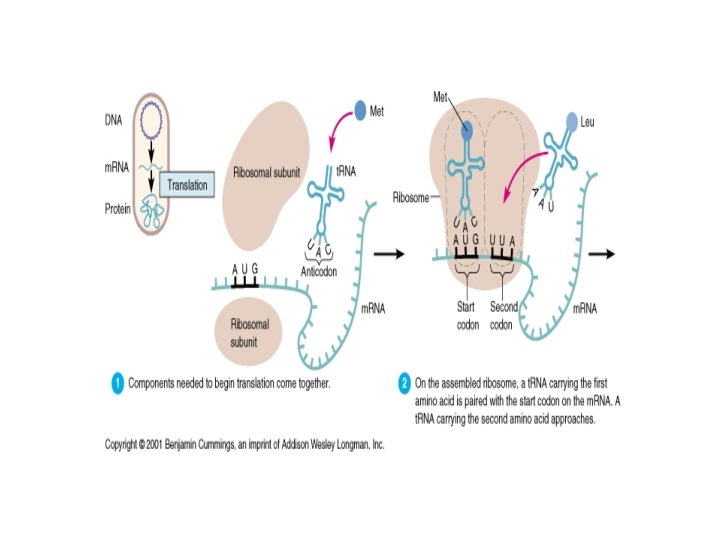
Eukaryotic Central Dogma In Eukaryotes ( cells where the DNA is sequestered in a separate nucleus) The DNA does not contain a duplicate of the coding gene, rather exons must be spliced. ( many eukaryotes genes contain no introns!. . Particularly true in ´lower´ organisms) m. RNA – (messenger RNA) Contains the assembled copy of the gene. The m. RNA acts as a messenger to carry the information stored in the DNA in the nucleus to the cytoplasm where the ribosomes can make it into protein.

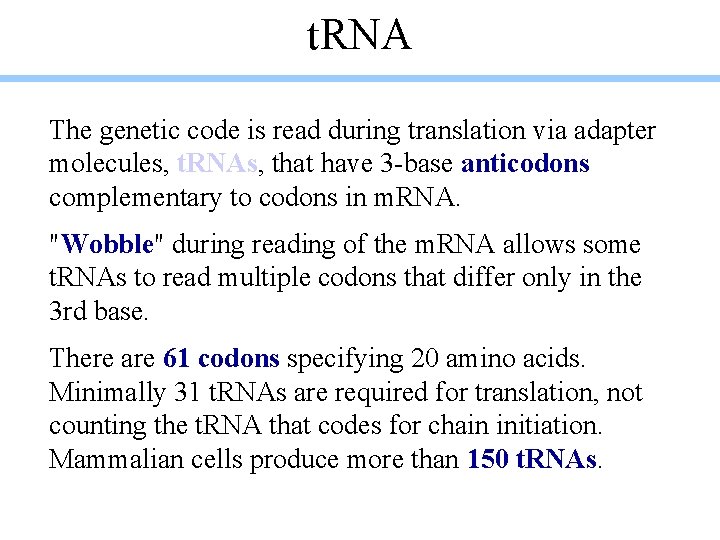
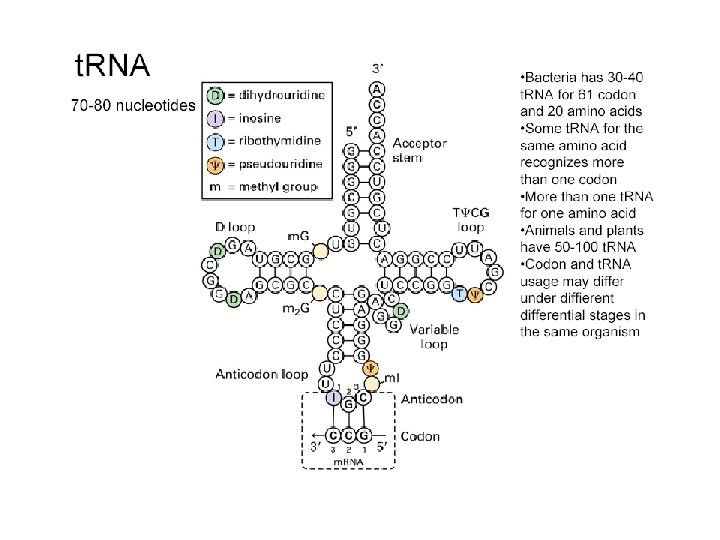
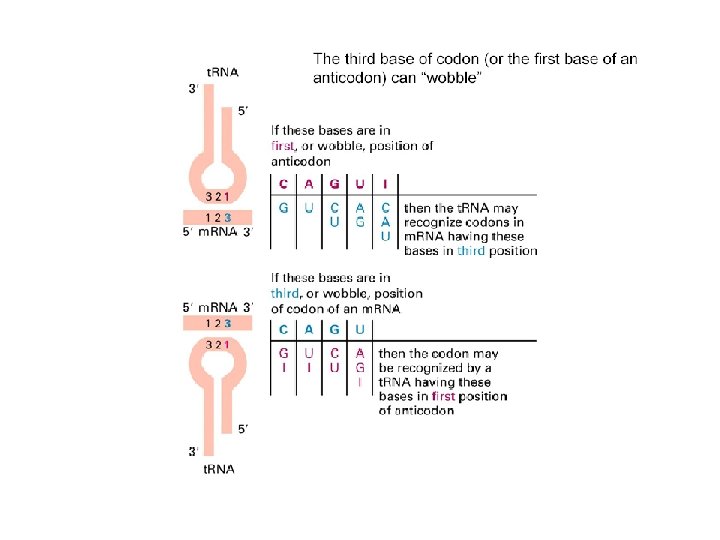
t. RNA The genetic code is read during translation via adapter molecules, t. RNAs, that have 3 -base anticodons complementary to codons in m. RNA. "Wobble" during reading of the m. RNA allows some t. RNAs to read multiple codons that differ only in the 3 rd base. There are 61 codons specifying 20 amino acids. Minimally 31 t. RNAs are required for translation, not counting the t. RNA that codes for chain initiation. Mammalian cells produce more than 150 t. RNAs.

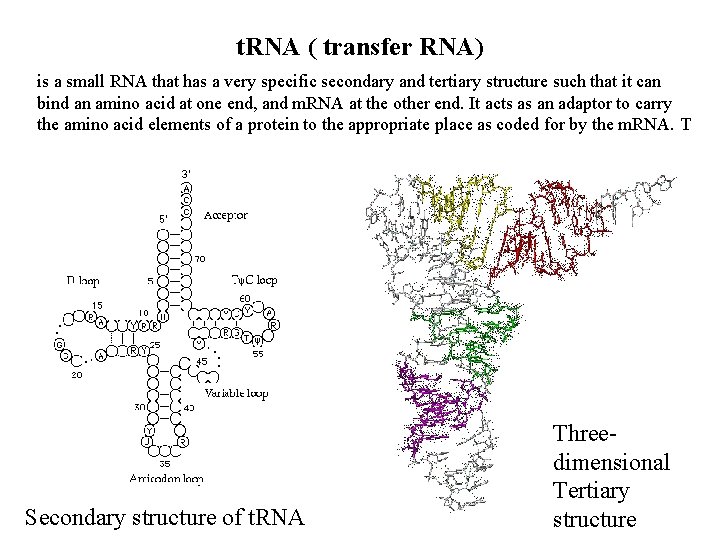
t. RNA ( transfer RNA) is a small RNA that has a very specific secondary and tertiary structure such that it can bind an amino acid at one end, and m. RNA at the other end. It acts as an adaptor to carry the amino acid elements of a protein to the appropriate place as coded for by the m. RNA. T Secondary structure of t. RNA Threedimensional Tertiary structure


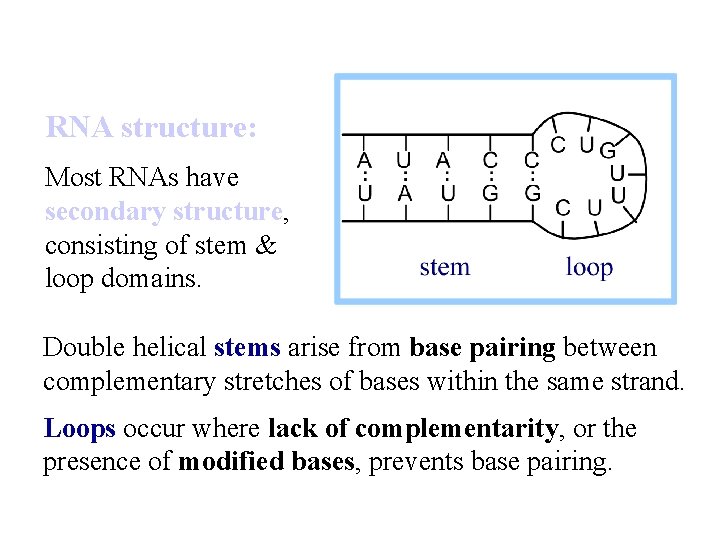
RNA structure: Most RNAs have secondary structure, consisting of stem & loop domains. Double helical stems arise from base pairing between complementary stretches of bases within the same strand. Loops occur where lack of complementarity, or the presence of modified bases, prevents base pairing.

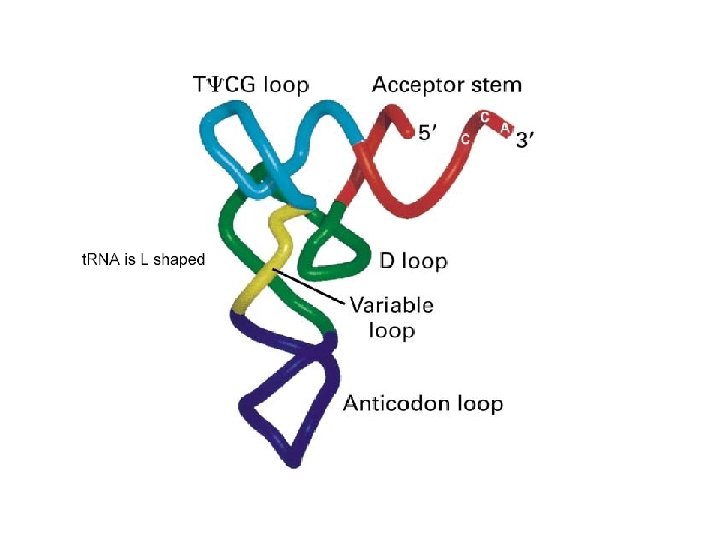
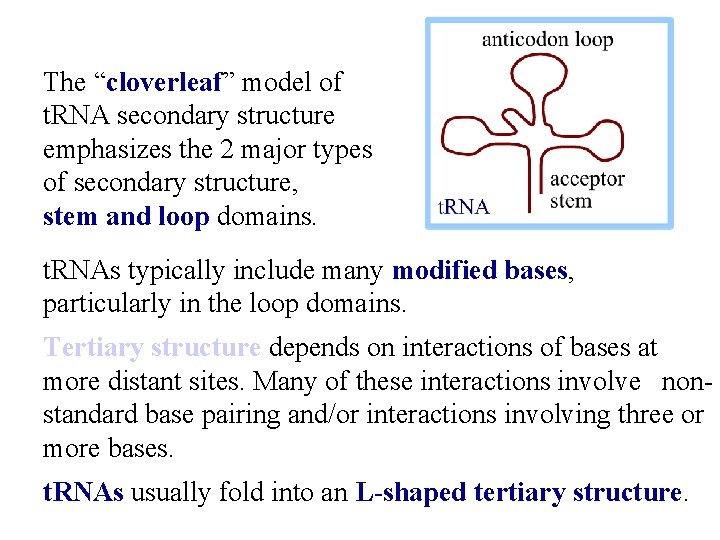
The “cloverleaf” model of t. RNA secondary structure emphasizes the 2 major types of secondary structure, stem and loop domains. t. RNAs typically include many modified bases, particularly in the loop domains. Tertiary structure depends on interactions of bases at more distant sites. Many of these interactions involve nonstandard base pairing and/or interactions involving three or more bases. t. RNAs usually fold into an L-shaped tertiary structure.


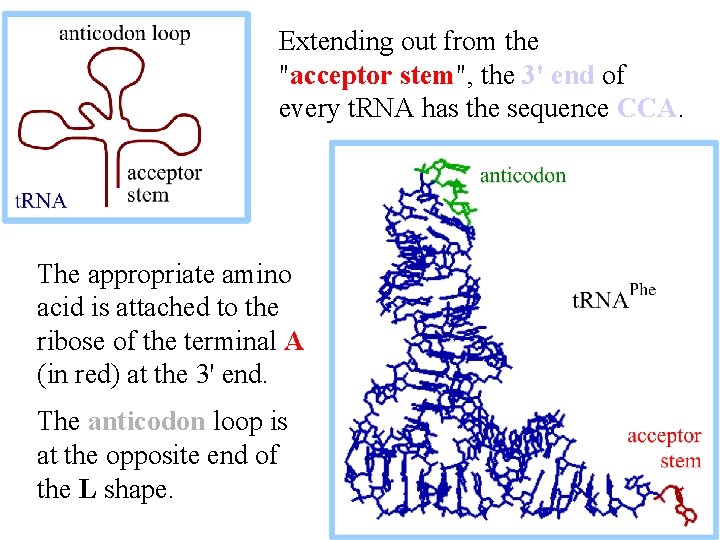
Extending out from the "acceptor stem", the 3' end of every t. RNA has the sequence CCA. The appropriate amino acid is attached to the ribose of the terminal A (in red) at the 3' end. The anticodon loop is at the opposite end of the L shape.

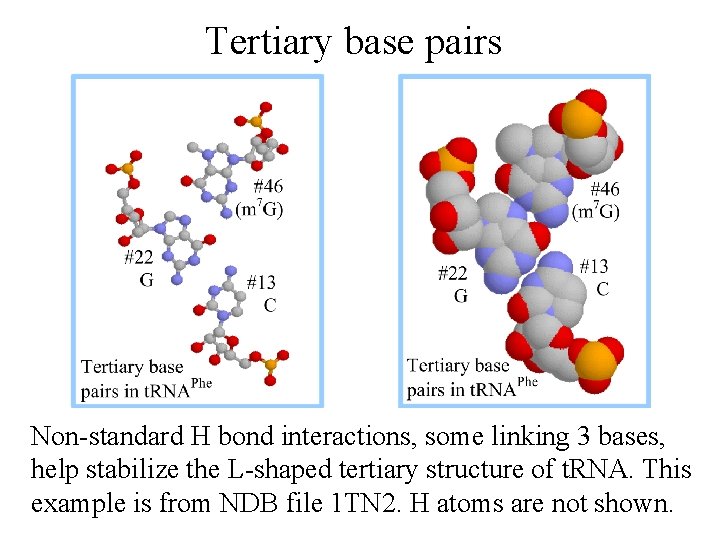
Tertiary base pairs Non-standard H bond interactions, some linking 3 bases, help stabilize the L-shaped tertiary structure of t. RNA. This example is from NDB file 1 TN 2. H atoms are not shown.

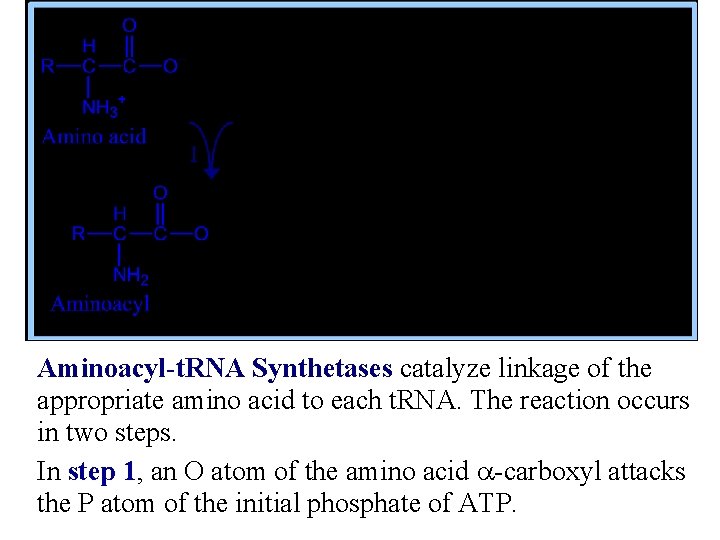
Aminoacyl-t. RNA Synthetases catalyze linkage of the appropriate amino acid to each t. RNA. The reaction occurs in two steps. In step 1, an O atom of the amino acid a-carboxyl attacks the P atom of the initial phosphate of ATP.

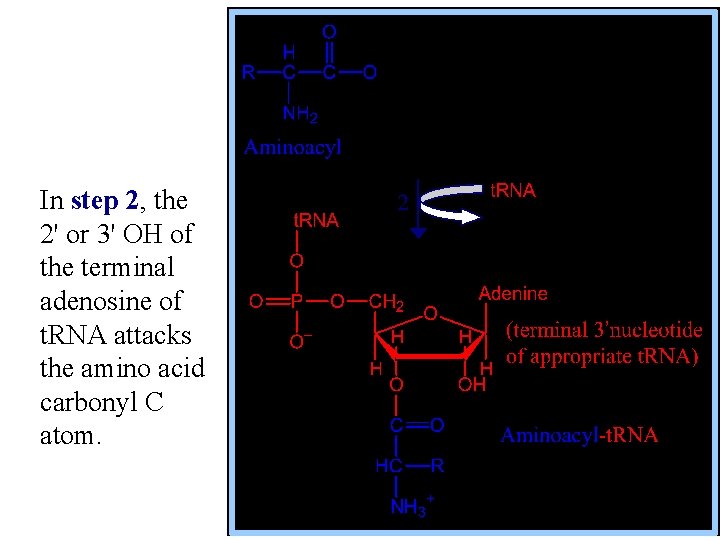
In step 2, the 2' or 3' OH of the terminal adenosine of t. RNA attacks the amino acid carbonyl C atom.

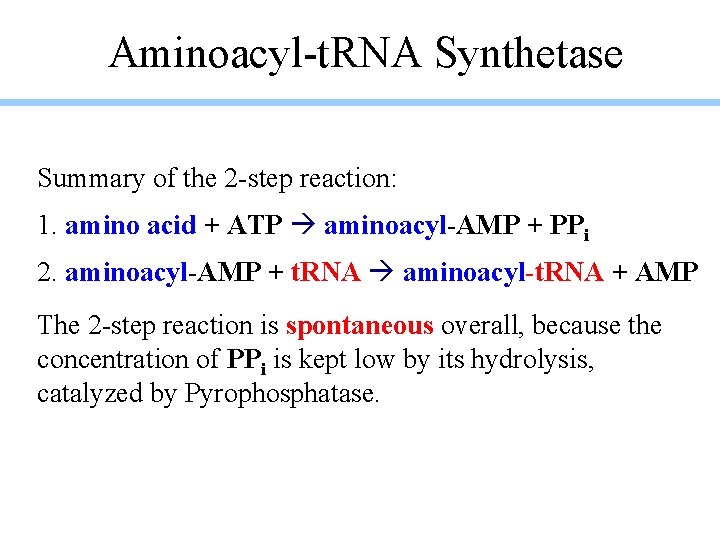
Aminoacyl-t. RNA Synthetase Summary of the 2 -step reaction: 1. amino acid + ATP aminoacyl-AMP + PPi 2. aminoacyl-AMP + t. RNA aminoacyl-t. RNA + AMP The 2 -step reaction is spontaneous overall, because the concentration of PPi is kept low by its hydrolysis, catalyzed by Pyrophosphatase.

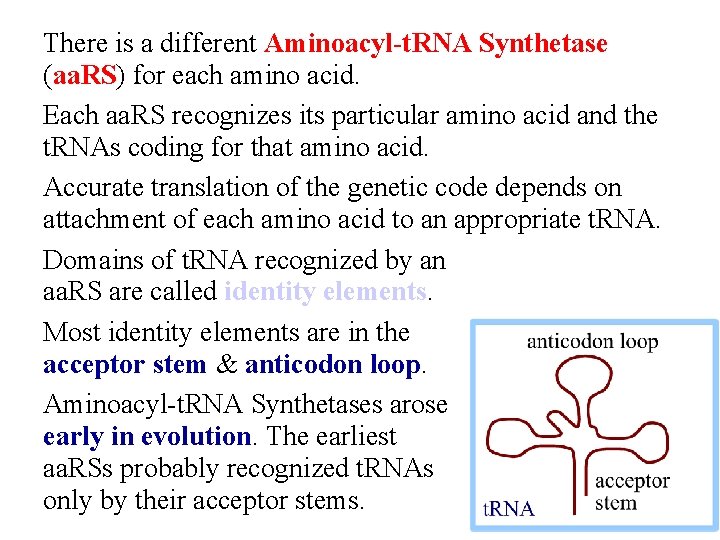
There is a different Aminoacyl-t. RNA Synthetase (aa. RS) for each amino acid. Each aa. RS recognizes its particular amino acid and the t. RNAs coding for that amino acid. Accurate translation of the genetic code depends on attachment of each amino acid to an appropriate t. RNA. Domains of t. RNA recognized by an aa. RS are called identity elements. Most identity elements are in the acceptor stem & anticodon loop. Aminoacyl-t. RNA Synthetases arose early in evolution. The earliest aa. RSs probably recognized t. RNAs only by their acceptor stems.

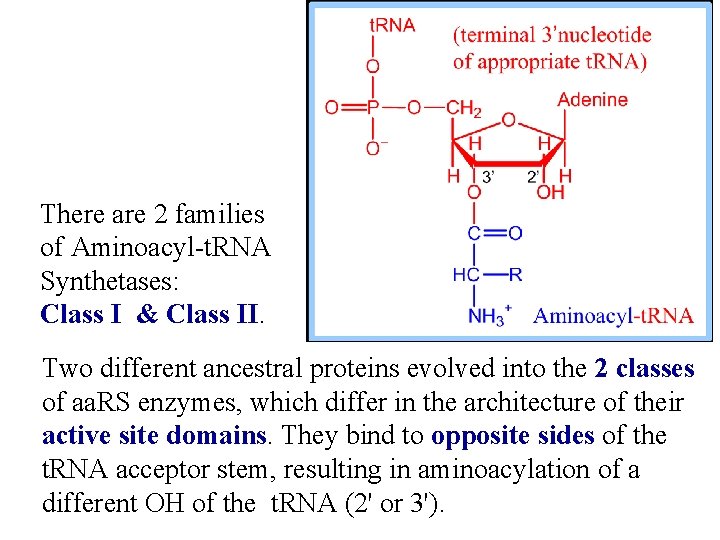
There are 2 families of Aminoacyl-t. RNA Synthetases: Class I & Class II. Two different ancestral proteins evolved into the 2 classes of aa. RS enzymes, which differ in the architecture of their active site domains. They bind to opposite sides of the t. RNA acceptor stem, resulting in aminoacylation of a different OH of the t. RNA (2' or 3').

Class I aa. RSs: Identity elements usually include residues of the anticodon loop & acceptor stem. Class I aa. RSs aminoacylate the 2'-OH of adenosine at their 3' end. Class II aa. RSs: Identity elements for some Class II enzymes do not include the anticodon domain. Class II aa. RSs tend to aminoacylate the 3'-OH of adenosine at their 3' end.

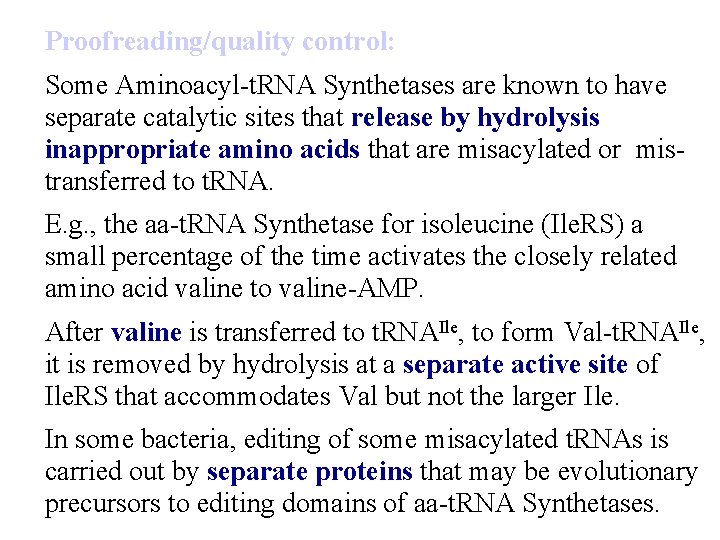
Proofreading/quality control: Some Aminoacyl-t. RNA Synthetases are known to have separate catalytic sites that release by hydrolysis inappropriate amino acids that are misacylated or mistransferred to t. RNA. E. g. , the aa-t. RNA Synthetase for isoleucine (Ile. RS) a small percentage of the time activates the closely related amino acid valine to valine-AMP. After valine is transferred to t. RNAIle, to form Val-t. RNAIle, it is removed by hydrolysis at a separate active site of Ile. RS that accommodates Val but not the larger Ile. In some bacteria, editing of some misacylated t. RNAs is carried out by separate proteins that may be evolutionary precursors to editing domains of aa-t. RNA Synthetases.

Some amino acids are modified after being linked to t. RNA. w E. g. , in prokaryotes & in mitochondria the initiator t. RNAf. Met is first charged with methionine. Methionyl-t. RNA formyltransferase then catalyzes formylation of the methionine moiety, using THF as formyl donor, to yield f. Met-t. RNAf. Met. w In some prokaryotes, a non-discriminating aa. RS loads aspartate onto t. RNAAsn. The aspartate moiety is then converted by an amidotransferase to asparagine, yielding Asn-t. RNAAsn. Glu-t. RNAGln is similarly formed and converted to Glnt. RNAGln in such organisms.

RIBOSOMES

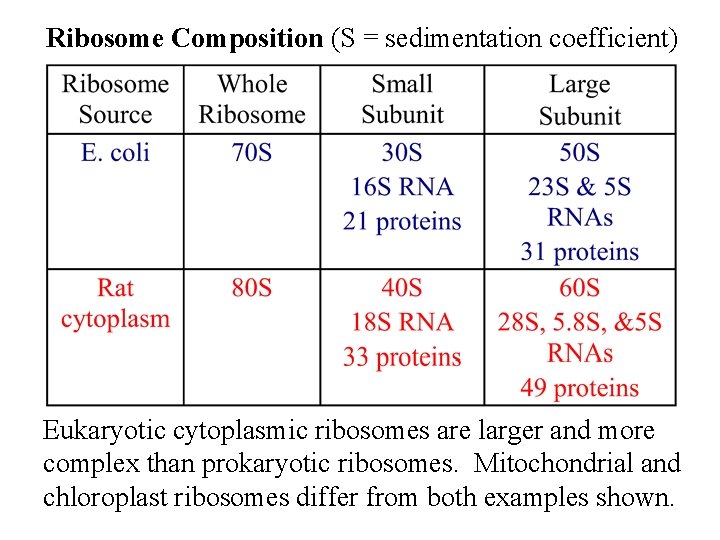
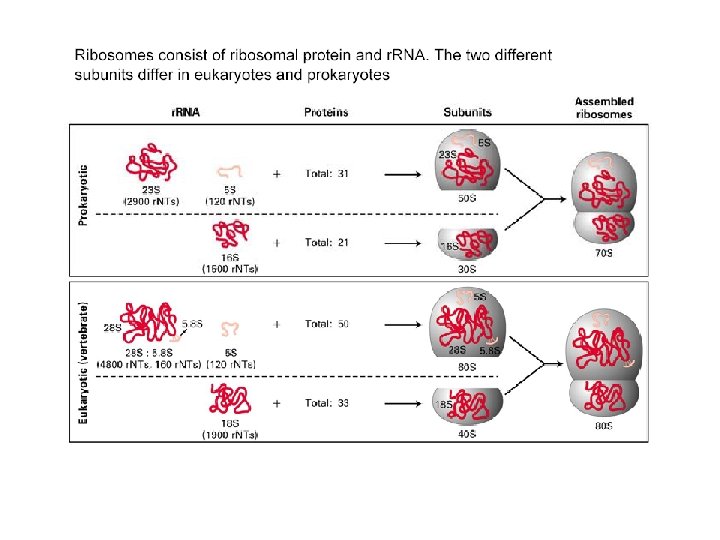
Ribosome Composition (S = sedimentation coefficient) Eukaryotic cytoplasmic ribosomes are larger and more complex than prokaryotic ribosomes. Mitochondrial and chloroplast ribosomes differ from both examples shown.


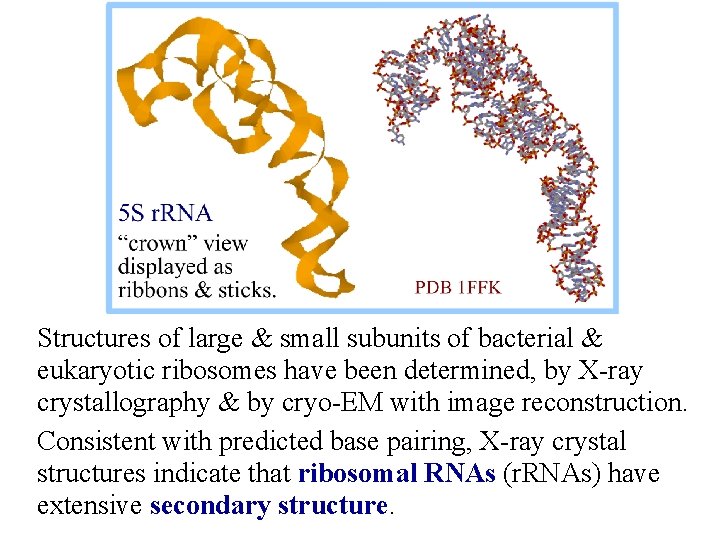
Structures of large & small subunits of bacterial & eukaryotic ribosomes have been determined, by X-ray crystallography & by cryo-EM with image reconstruction. Consistent with predicted base pairing, X-ray crystal structures indicate that ribosomal RNAs (r. RNAs) have extensive secondary structure.

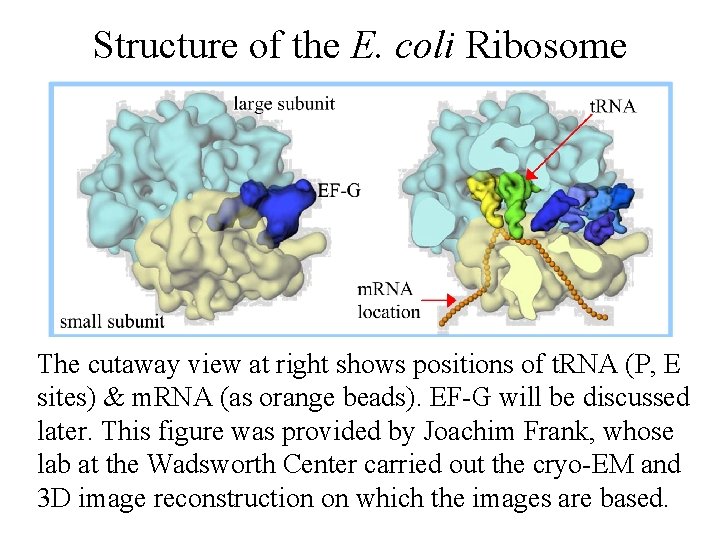
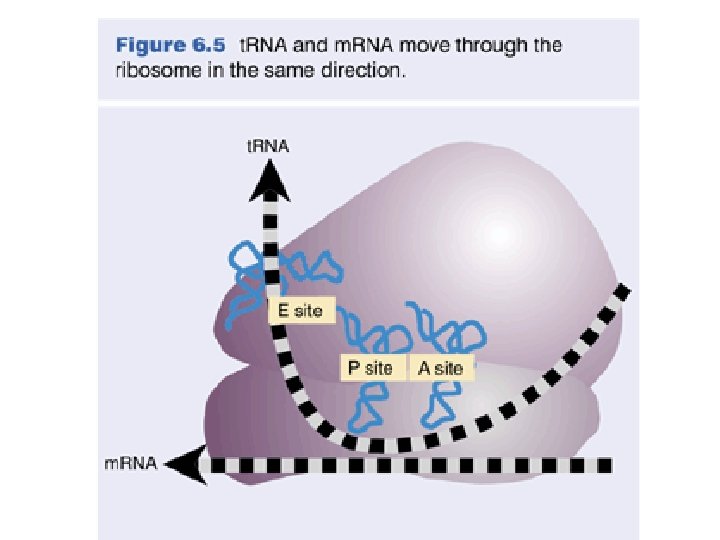
Structure of the E. coli Ribosome The cutaway view at right shows positions of t. RNA (P, E sites) & m. RNA (as orange beads). EF-G will be discussed later. This figure was provided by Joachim Frank, whose lab at the Wadsworth Center carried out the cryo-EM and 3 D image reconstruction on which the images are based.

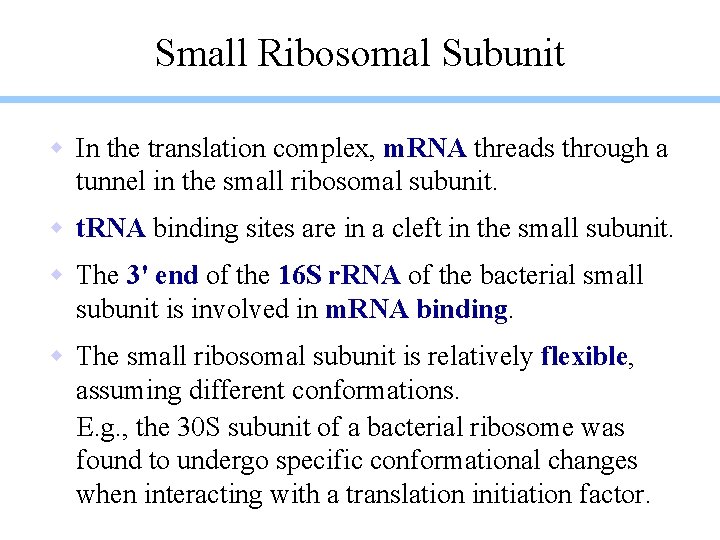
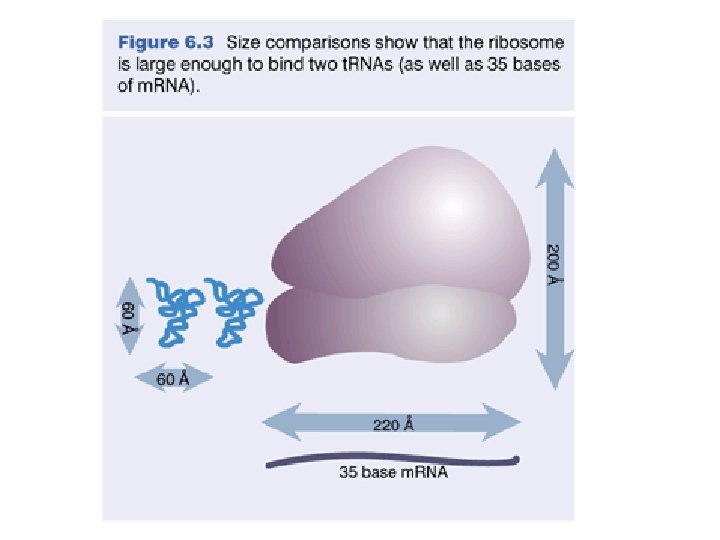
Small Ribosomal Subunit w In the translation complex, m. RNA threads through a tunnel in the small ribosomal subunit. w t. RNA binding sites are in a cleft in the small subunit. w The 3' end of the 16 S r. RNA of the bacterial small subunit is involved in m. RNA binding. w The small ribosomal subunit is relatively flexible, assuming different conformations. E. g. , the 30 S subunit of a bacterial ribosome was found to undergo specific conformational changes when interacting with a translation initiation factor.

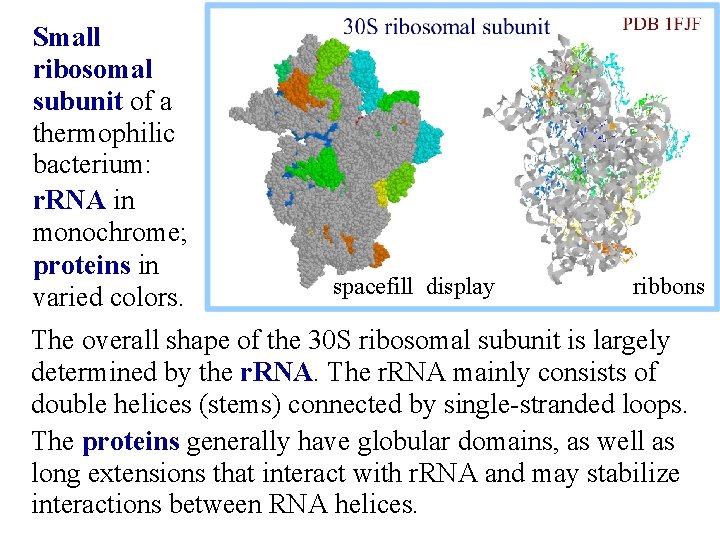
Small ribosomal subunit of a thermophilic bacterium: r. RNA in monochrome; proteins in varied colors. spacefill display ribbons The overall shape of the 30 S ribosomal subunit is largely determined by the r. RNA. The r. RNA mainly consists of double helices (stems) connected by single-stranded loops. The proteins generally have globular domains, as well as long extensions that interact with r. RNA and may stabilize interactions between RNA helices.

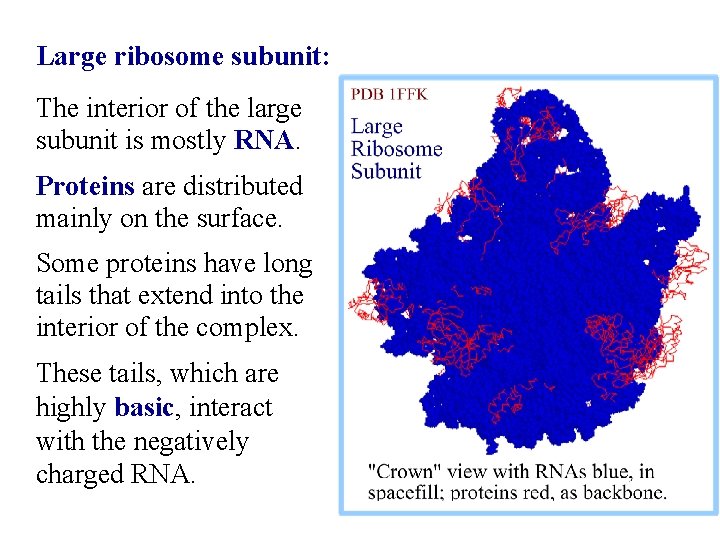
Large ribosome subunit: The interior of the large subunit is mostly RNA. Proteins are distributed mainly on the surface. Some proteins have long tails that extend into the interior of the complex. These tails, which are highly basic, interact with the negatively charged RNA.

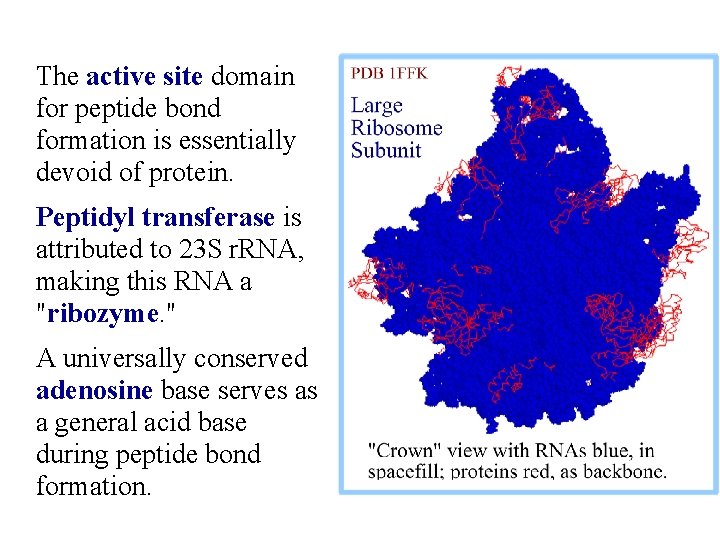
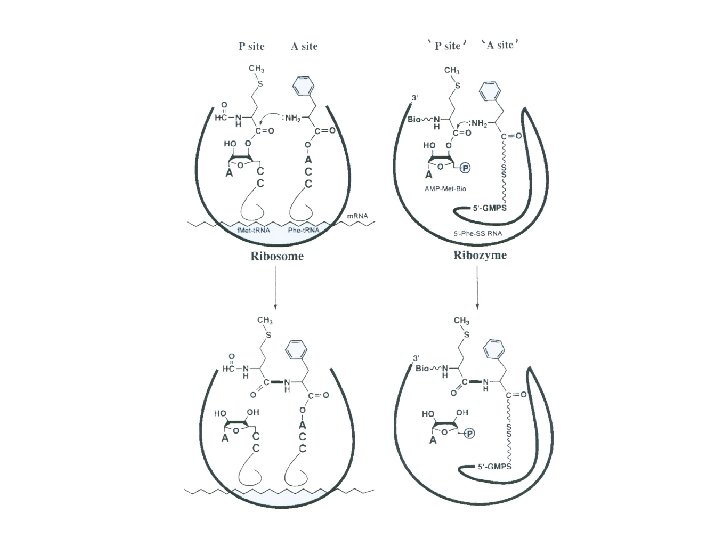
The active site domain for peptide bond formation is essentially devoid of protein. Peptidyl transferase is attributed to 23 S r. RNA, making this RNA a "ribozyme. " A universally conserved adenosine base serves as a general acid base during peptide bond formation.

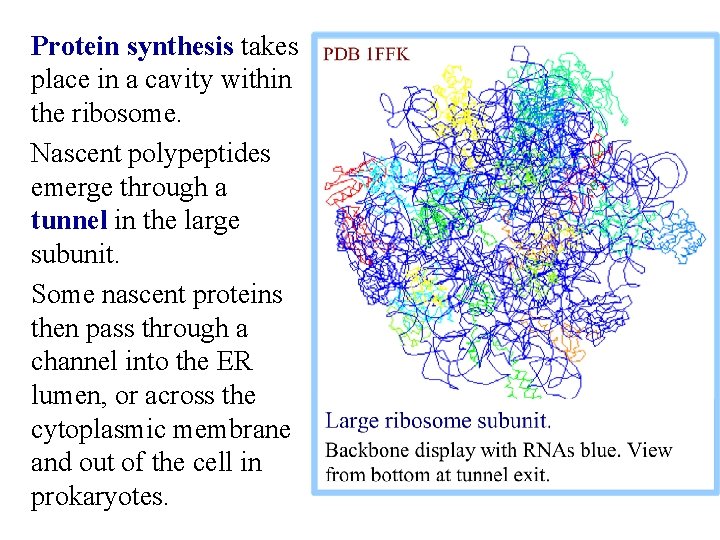
Protein synthesis takes place in a cavity within the ribosome. Nascent polypeptides emerge through a tunnel in the large subunit. Some nascent proteins then pass through a channel into the ER lumen, or across the cytoplasmic membrane and out of the cell in prokaryotes.

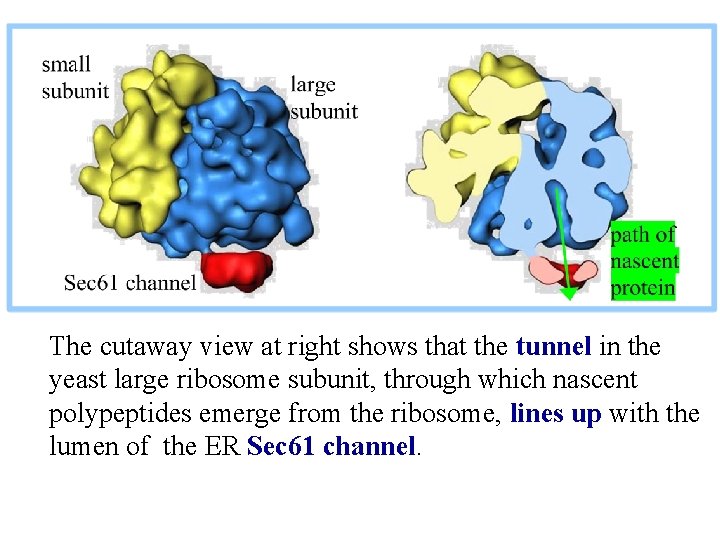
The cutaway view at right shows that the tunnel in the yeast large ribosome subunit, through which nascent polypeptides emerge from the ribosome, lines up with the lumen of the ER Sec 61 channel.








