Gel Filtration Chromatography The method mostly involves the












- Slides: 29

Gel Filtration Chromatography The method mostly involves the separation of the proteins based on its molecular size. This method is also known as Size exclusion chromatography Related LOs: Column preparation, Chromatographic technique > Prior Viewing – IDD-6. Extraction of serum protein, IDD-42. Liquid chromatography - affinity chromatography > Future Viewing – IDD-38. Stable isotope labeling using amino acids in cell culture (SILAC), IDD-37. Isotope-coded affinity tags (ICAT) , IDD-39. LC-MSMS data analysis Course Name: Gel Filtration Chromatography Level(UG/PG): UG Author(s): Dinesh Raghu, Vinayak Pachapur Mentor: Dr. Sanjeeva Srivastava *The contents in this ppt are licensed under Creative Commons Attribution-Non. Commercial-Share. Alike 2. 5 India license

1 2 Learning objectives After interacting with this learning object, the learner will be able to: 1. 3 2. Prepare elution buffers for the experiments 3. Analyse the mechanism behind the protein purification 4. 4 5 Define the column preparation for the chromatographic technique Assess the troubleshooting steps involved in the experiments

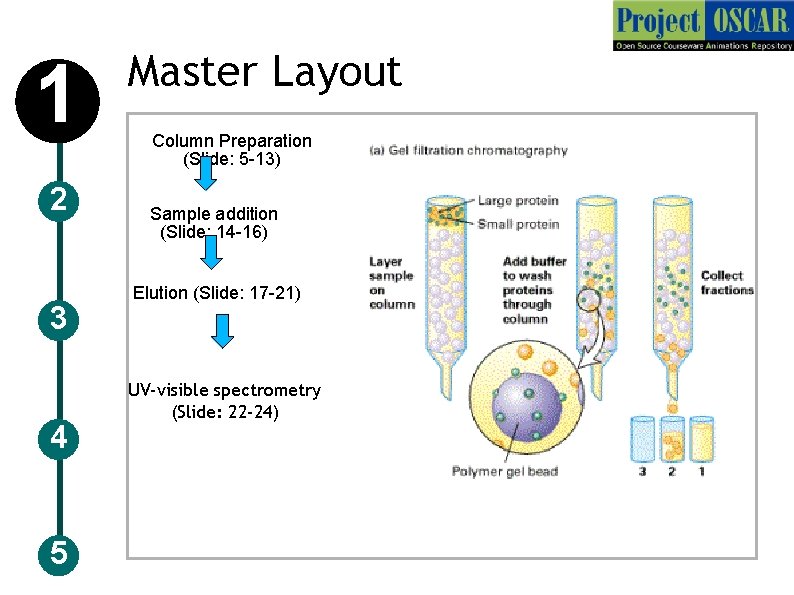
1 2 3 4 5 Master Layout Column Preparation (Slide: 5 -13) Sample addition (Slide: 14 -16) Elution (Slide: 17 -21) UV-visible spectrometry (Slide: 22 -24)

1 2 3 4 5 Definitions and Keywords 1. Gel filtration chromatography: The protein separation is based on the molecular size of the protein, gel packing in the column and the molecular filtration efficiency of the gel 2. Size exclusion beads: The manufactured beads that has pore size depending on the molecular weight/size of the protein to be purified. These beads act as stationary phase 3. Elution buffer: Elution buffer consists of, 0. 1% SDS, 50 m. M TRIS that can be used as the mobile phase to elute out the protein from the column

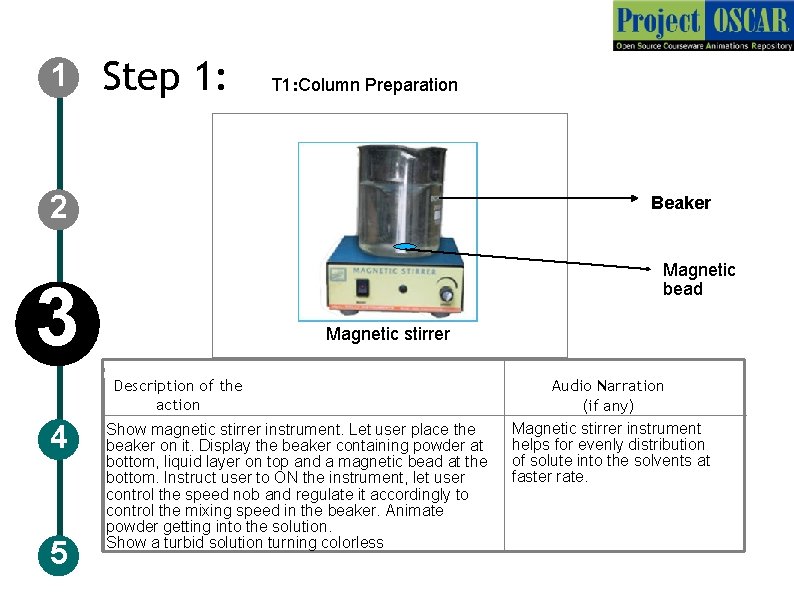
1 Step 1: T 1: Column Preparation 2 Beaker Magnetic bead 3 Magnetic stirrer Description of the action 4 5 Show magnetic stirrer instrument. Let user place the beaker on it. Display the beaker containing powder at bottom, liquid layer on top and a magnetic bead at the bottom. Instruct user to ON the instrument, let user control the speed nob and regulate it accordingly to control the mixing speed in the beaker. Animate powder getting into the solution. Show a turbid solution turning colorless Audio Narration (if any) Magnetic stirrer instrument helps for evenly distribution of solute into the solvents at faster rate.

1 Step 1: T 1: Column Preparation 2 3 4 5 Measuring balance Description of the action Audio Narration (if any) Show a measuring balance, with display, ON, OFF and TARE/0 buttons on it. let When measuing user ON it, display reading as 0. 000 g, let with paper, the user picks up the paper from the rack, weight of the makes 1/10 of folding on the sides and paper need to be places it on the balance. Now the display tared from actual reading changes to 0. 003 g. Instruct user reading. to TARE the reading. And animate to click the tare button. Once user clicks it, reading must show ” 0”

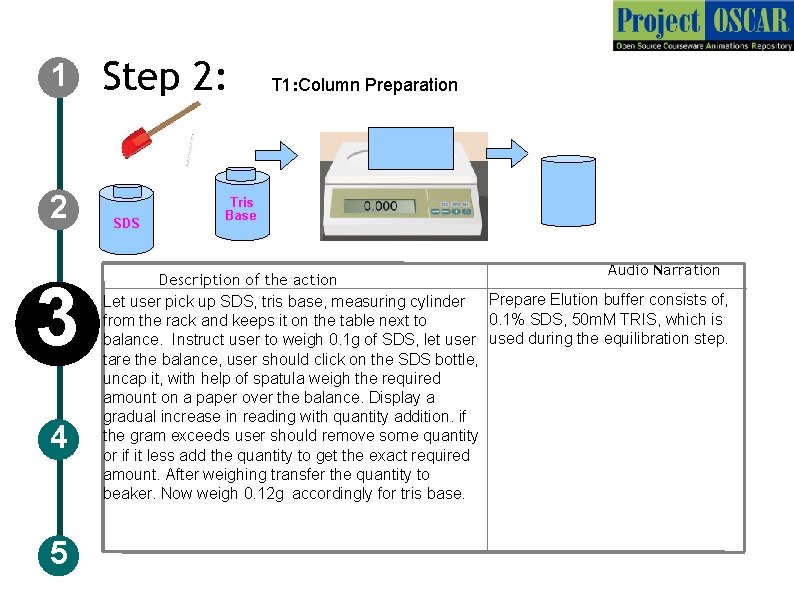
1 2 3 4 5 Step 2: SDS T 1: Column Preparation Tris Base Audio Narration Description of the action Let user pick up SDS, tris base, measuring cylinder Prepare Elution buffer consists of, 0. 1% SDS, 50 m. M TRIS, which is from the rack and keeps it on the table next to balance. Instruct user to weigh 0. 1 g of SDS, let user used during the equilibration step. tare the balance, user should click on the SDS bottle, uncap it, with help of spatula weigh the required amount on a paper over the balance. Display a gradual increase in reading with quantity addition. if the gram exceeds user should remove some quantity or if it less add the quantity to get the exact required amount. After weighing transfer the quantity to beaker. Now weigh 0. 12 g accordingly for tris base.

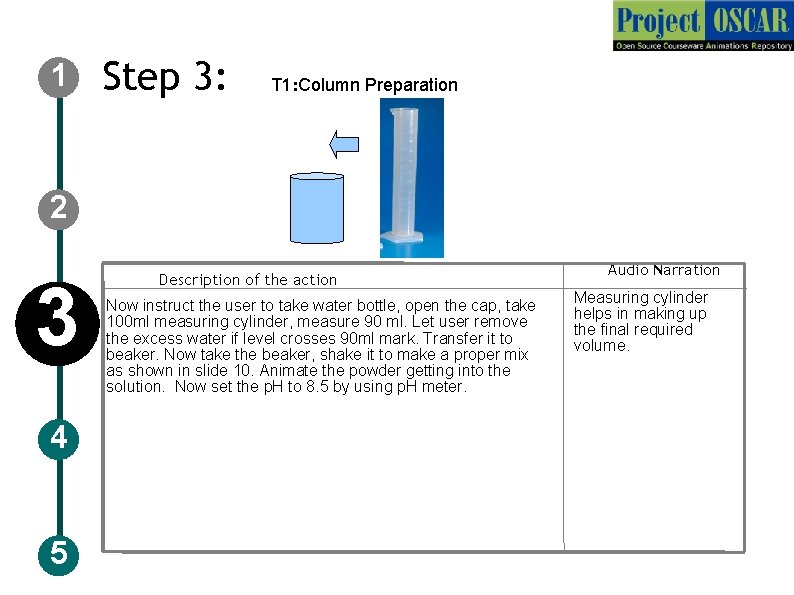
1 Step 3: T 1: Column Preparation 2 3 4 5 Description of the action Now instruct the user to take water bottle, open the cap, take 100 ml measuring cylinder, measure 90 ml. Let user remove the excess water if level crosses 90 ml mark. Transfer it to beaker. Now take the beaker, shake it to make a proper mix as shown in slide 10. Animate the powder getting into the solution. Now set the p. H to 8. 5 by using p. H meter. Audio Narration Measuring cylinder helps in making up the final required volume.

1 Step 4: T 1: Column Preparation 2 3 4 5 STD 1 Description of the action Display standard p. H bottles and p. H instrument and deionized water, discard bottle placed on a table. Instruct user to caliberate the instrument. Let user ON the instrument. Initially for the p. H rod is dipped in in dipped in 3 M KCl. Now show like user taking out the rod and washing it with deionized-water, let user cleans the rod with tissue. Now pick the STD 1 , uncap it, dip the cleaned rod into the solution, user must click read button with display showing “ 7”. Now clean the rod and repeat the step to note down the reading for STD 2 and now the display should show “ 10” STD 2 Audio Narration Before the p. H reading, p. H instrument need to be calibrated with standards. Once with STD 1 at p. H 7 and with STD 2 at p. H 10.

1 Step 5: T 1: Column Preparation 2 Na. OH HCl 3 4 5 Description of the action Instruct user to set the p. H for labeling buffer p. H at 8. 5. Now take the Elution buffer bottle, uncap it, dip the cleaned p. H rod into the solution. User need to click on read button. Initially display must show a reading 6. now instruct user to add Na. OH to adjust the p. H. Now allow the user to click on Na. OH bottle so that drops of Na. OH should be added with filler, user need to mix the solution with glass rod, click on read button and the reading should anywhere near 11. let user keeps adding the Na. OH drop till the p. H display shows 8. 5 and later transfer the beaker solution to 100 ml measuring cylinder to makeup the volume to 100 ml by clicking on water and adding it to that. All action should happen when the user clicks the hand image. Audio Narration Prepare elution buffer of p. H 8. 5.

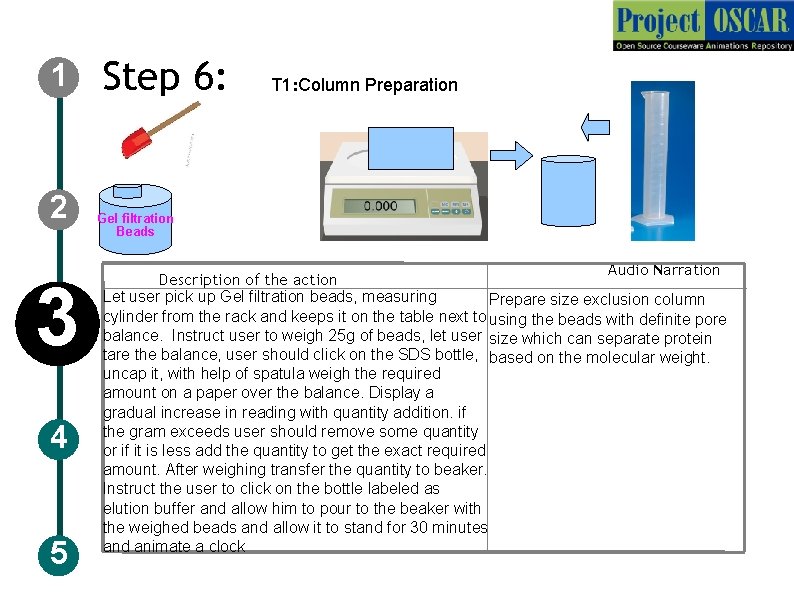
1 2 3 4 5 Step 6: T 1: Column Preparation Gel filtration Beads Audio Narration Description of the action Let user pick up Gel filtration beads, measuring Prepare size exclusion column cylinder from the rack and keeps it on the table next to using the beads with definite pore balance. Instruct user to weigh 25 g of beads, let user size which can separate protein tare the balance, user should click on the SDS bottle, based on the molecular weight. uncap it, with help of spatula weigh the required amount on a paper over the balance. Display a gradual increase in reading with quantity addition. if the gram exceeds user should remove some quantity or if it is less add the quantity to get the exact required amount. After weighing transfer the quantity to beaker. Instruct the user to click on the bottle labeled as elution buffer and allow him to pour to the beaker with the weighed beads and allow it to stand for 30 minutes and animate a clock

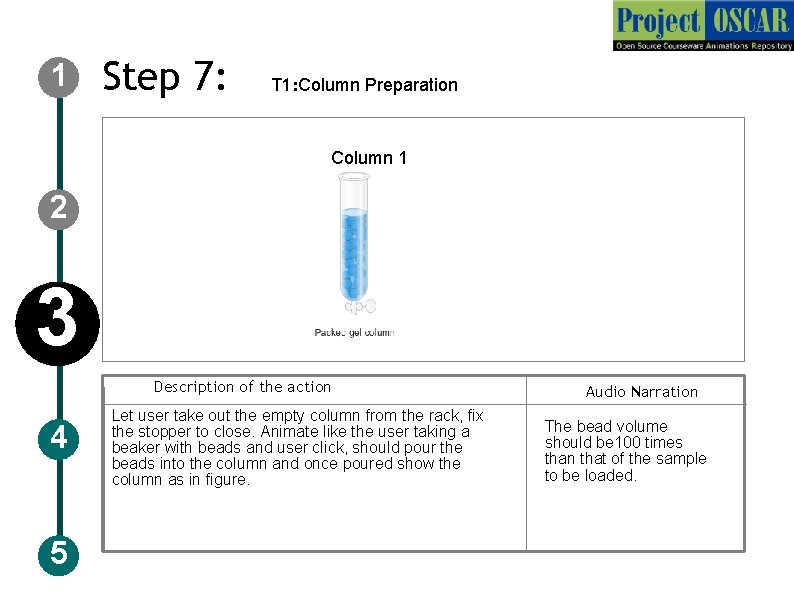
1 Step 7: T 1: Column Preparation AE DE Coulmn Column 1 2 3 Description of the action 4 5 Let user take out the empty column from the rack, fix the stopper to close. Animate like the user taking a beaker with beads and user click, should pour the beads into the column and once poured show the column as in figure. Audio Narration The bead volume should be 100 times than that of the sample to be loaded.

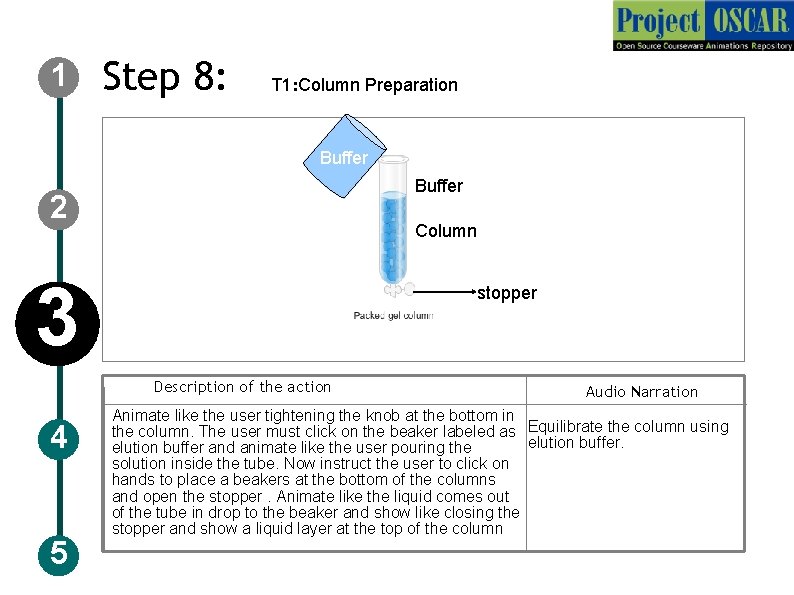
1 Step 8: T 1: Column Preparation Buffer 2 Column 3 stopper Description of the action 4 5 Audio Narration Animate like the user tightening the knob at the bottom in the column. The user must click on the beaker labeled as Equilibrate the column using elution buffer and animate like the user pouring the solution inside the tube. Now instruct the user to click on hands to place a beakers at the bottom of the columns and open the stopper. Animate like the liquid comes out of the tube in drop to the beaker and show like closing the stopper and show a liquid layer at the top of the column

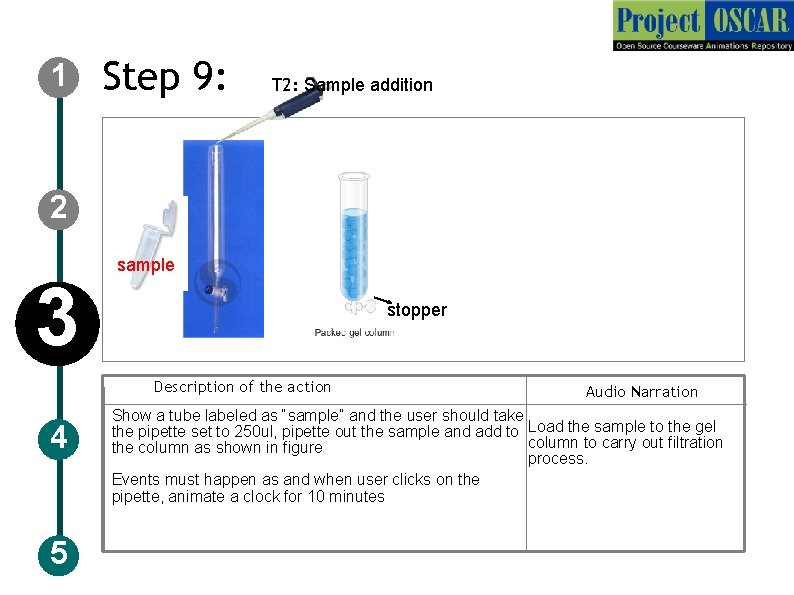
1 Step 9: T 2: Sample addition 2 3 sample stopper Description of the action 4 5 Audio Narration Show a tube labeled as “sample” and the user should take the pipette set to 250 ul, pipette out the sample and add to Load the sample to the gel column to carry out filtration the column as shown in figure process. Events must happen as and when user clicks on the pipette, animate a clock for 10 minutes

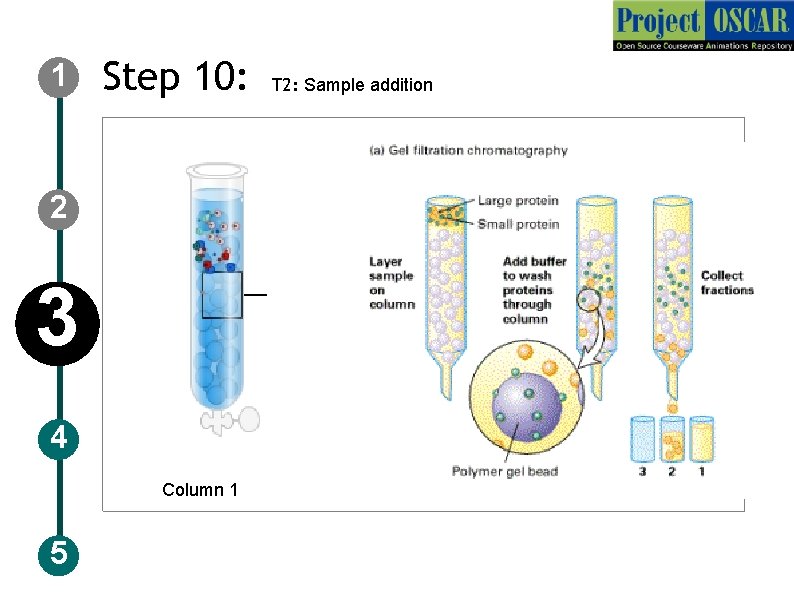
1 Step 10: 2 3 4 Column 1 5 T 2: Sample addition

1 Step 10: T 2: Sample addition Description of the action 2 3 4 5 Animate like rings of different color with some small and large circles. the larger circle rings must move at faster rate while the small circle rings must move slowly by passing through the column. Please re-draw the previous figure. Audio Narration The separation is based on the molecular weight of the sample, higher molecular weight proteins will be washed first while the proteins of lower molecular weight moves slower and takes time to elute out as it passes through the pores of the column.

1 Step 11: T 3: Gel filtration Elution 2 3 4 5 Elution buffer

1 Step 11: T 3: Gel filtration Elution Description of the action 2 3 4 5 Now instruct the user to take the pipette set 1000 ul and take the elution buffer and add to the column show the increase in the volume in the column and the large/small circle movement as described in slide 20. Events must happen when the user clicks on it Audio Narration Pour the elution buffer to elute out the proteins at faster rate.

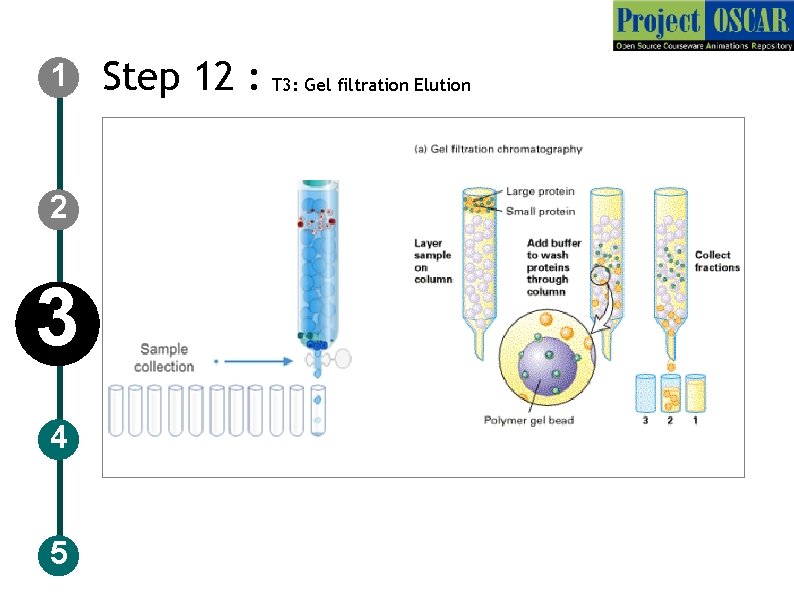
1 2 3 4 5 Step 12 : T 3: Gel filtration Elution

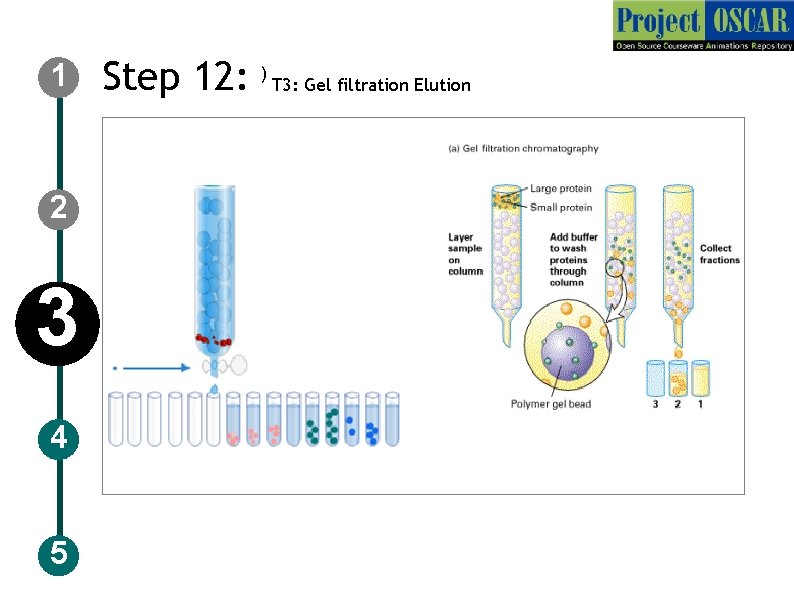
1 2 3 4 5 Step 12: ) T 3: Gel filtration Elution

1 2 3 4 5 Step 13: T 3: Gel filtration Elution Description of the action Show the collection tubes in a row and the solution dropping into it. Show the tube: 1 with only solution, tube: 2 with some large rings, user should click on it and a tab should appear labeled as “high molecular weight proteins” and tube: 3 with more of small rings tube: 4 with some small rings user should click on it and a tab should appear labeled as ” Low molecular weight proteins” and decreased amount of small rings in tube: 5, 6 and less of small size rings in tube: 7, 8 and only solution in tube: 9 Audio Narration The larger sized molecules tend to pass through the openings between the beads, while the small molecules pass through the gel beads taking a lot of time to elute out.

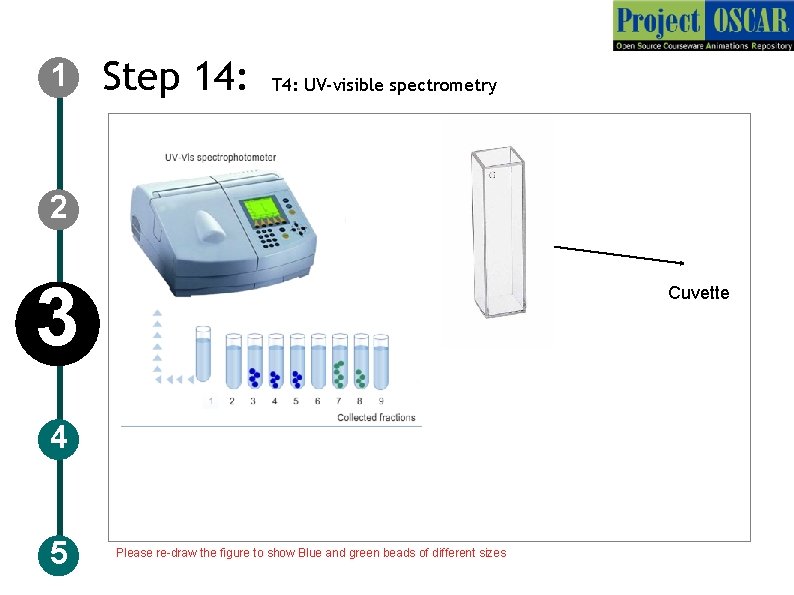
1 Step 14: T 4: UV-visible spectrometry 2 3 Cuvette 4 5 Please re-draw the figure to show Blue and green beads of different sizes

1 Step 14: T 4: UV-visible spectrometry Description of the action 2 3 4 5 Show a instrument labeled as “UV –visible spectrometry” and the samples in the stand as shown in figure. Animate buttons like “start, auto zero, absorbance, stop” on the instrument Now instruct the user to switch on the instrument, set the wavelength to 595 nm by pressing on numbers-open the lid of the instrument and take a cuvette as in figure and click on phosphate buffer to take it into cuvette and animate like keeping it inside the UV Visible spectrometry and press “auto zero” Display a value on the system as “ 0. 000” animate like the user opening the lid and taking out the cuvette and discarding the solution, now animate like the user taking the sample 1 and adding to the cuvette, keeping it inside, closing the lid and press absorbance show the values as in next slide for each sample follow the same for (2 -9) Audio Narration Detect the presence of protein of using the UV – visible spectrometry. The high absorbance reading indicate the presence of protein. For more information on the UVVisible spectrometry please go through IDD-50 basic instrumentation.

1 2 3 4 5 Step 14: T 4: UV-visible spectrometry Sample absorbance Volume of sample 1 0. 12 2 2 0. 229 2 3 0. 303 2 4 0. 457 2 5 0. 533 2 6 0. 681 2 7 0. 71 2 8 9 0. 62 0. 65 2 2

Slide 513 Tab 01 Slide 22 -24 Slide 17 -16 -21 Tab 02 Tab 03 Tab 04 Tab 05 Tab 06 Tab 07 Name of the section/stage Interactivity area Animation area Slide 13 Let the user remove the buffer entirely from the column, show like the column drying. Instruction Button 01 Button 02 Button 03 Instruct the user to add the buffer to the column and close the stopper, show like some buffer still remains in the top of the column. Instructions/ Working area Credits

APPENDIX 1 Questionnaire: Question 1 In gel filtration chromatography separation is based on a) Molecular size b) Molecular structure c) Confirmation d) Stereochemistry Question 2 Gel filtration chromatography also known as a) b) c) d) Size exclusion chromatography Ion exchange chromatography Affinity chromatography Chromatofocussing Question 3: In gel Filtration chromatography mobile phase is a) Buffer containing acid b) Buffer containing Base c) Buffer Containing salt d) Size exclusion beads

APPENDIX 1 Questionnaire: Question 4: In gel Filtration chromatography stationary phase is a) Buffer containing acid b) Buffer containing Base c) Buffer Containing salt d) Size exclusion beads Question 5: Volume of sample to be loaded in the column is a) b) c) d) Sample volume=100*column size Column size=100*sample volume Samplevolume=column size/100 Sample volume= column size

APPENDIX 2 Links for further reading 1. 2. Reference websites: http: //www. mnstate. edu/provost/sizeexclusionprotocol. pdf http: //kirschner. med. harvard. edu/files/protocols/GE_gelfiltr ation. pdf

APPENDIX 3 Summary Size exclusion chromatography involves separation based on molecular size of the protein. The size of the gel beads used in the column preparation plays a very important role in separation of molecules.