Gel filtration Chromatography Gel filtration chromatography or molecular












- Slides: 63

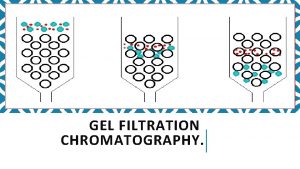
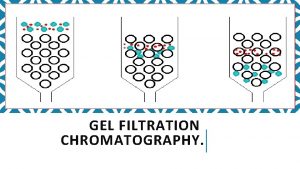
Gel filtration Chromatography Gel filtration chromatography, or molecular sieve chromatography, separates macromolecules on the bases of Stokes radius. The chromatography material is a composed of beads which contain pores. There is a distribution of pore sizes, with the average size depending on the resin. About 1/3 of the volume of liquid in a column containing gel filtration medium (Sephadex, Sepharose, and others) is contained within the beads. Proteins up to a certain size can partition into the beads. The inside of the beads is a stationary phase. Buffer flows past the beads, providing the applied force on the macromolecules to move down the column. The liquid outside the beads is a mobile phase. Proteins that do not partition into the beads because they are larger than the pores, move down the column most rapidly. Proteins that do partition into the beads are retarded in their elution because they are effectively in a larger volume.

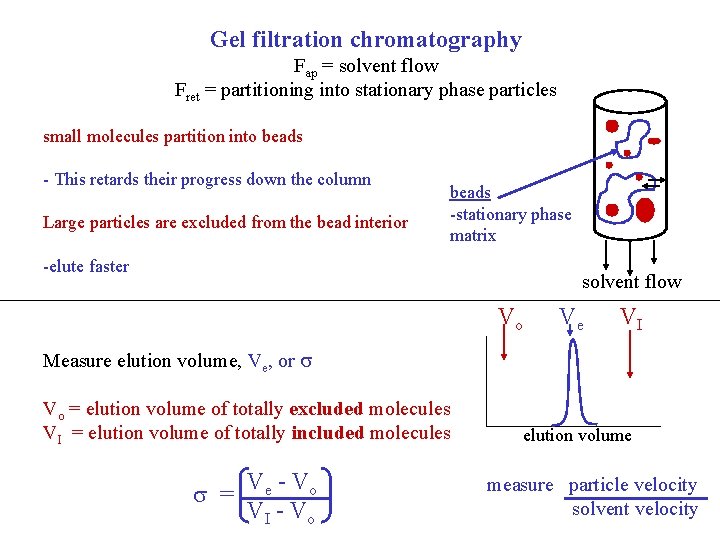
Gel filtration chromatography Fap = solvent flow Fret = partitioning into stationary phase particles small molecules partition into beads - This retards their progress down the column Large particles are excluded from the bead interior beads -stationary phase matrix -elute faster solvent flow Vo Ve VI Measure elution volume, Ve, or Vo = elution volume of totally excluded molecules VI = elution volume of totally included molecules = Ve - Vo VI - Vo elution volume measure particle velocity solvent velocity

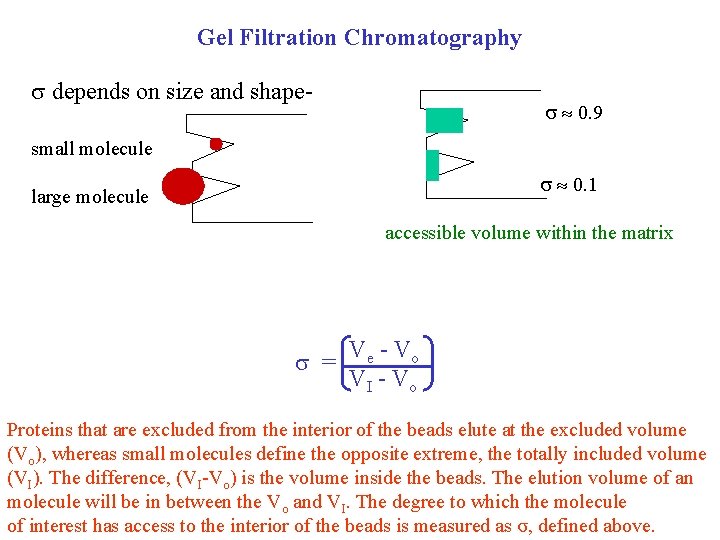
Gel Filtration Chromatography depends on size and shape- 0. 9 small molecule 0. 1 large molecule accessible volume within the matrix = Ve - Vo VI - Vo Proteins that are excluded from the interior of the beads elute at the excluded volume (Vo), whereas small molecules define the opposite extreme, the totally included volume (VI). The difference, (VI-Vo) is the volume inside the beads. The elution volume of an molecule will be in between the Vo and VI. The degree to which the molecule of interest has access to the interior of the beads is measured as σ, defined above.

The elution volume accurately measures the Stokes radius of a macromolecule. Experimentally, the value of σ or the elution value, correlates with the Stokes radius as measured by other hydrodynamics techniques. The same factors of size and shape that result in altered frictional drag through solution also determine the ease of penetration of a molecule into the bead interior in gel filtration media. Often gel filtration is calibrated using standards of known molecular weight and calibrated in terms of molecular weight rather than Stokes radius. This will be accurate only if all the standards as well as the unknown sample have the same shape and specific volume, as we described for the interpretation of sedimentation data. Hence, globular, spherical proteins can be used as a set of standards, but if the unknown protein has an elongated shape it will not penetrate the beads, and will have a large Stokes radius. If the gel filtration data are calibrated in terms of molecular weight, the answer obtained by gel filtration will be much too large, since the elongated molecule behaves as a much larger sphere.

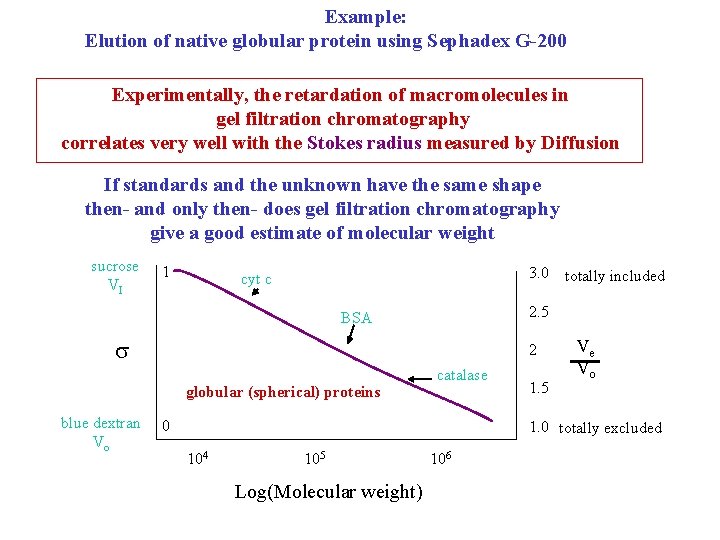
Example: Elution of native globular protein using Sephadex G-200 Experimentally, the retardation of macromolecules in gel filtration chromatography correlates very well with the Stokes radius measured by Diffusion If standards and the unknown have the same shape then- and only then- does gel filtration chromatography give a good estimate of molecular weight sucrose VI 1 3. 0 cyt c 2. 5 BSA 2 globular (spherical) proteins blue dextran Vo totally included catalase 0 1. 5 Ve Vo 1. 0 totally excluded 104 105 Log(Molecular weight) 106

The previous graph shows a plot of the elution volume of a series of globular proteins over a wide range of molecular weights for the medium Sephadex G-100. Often such graphs are used to calibrate a column and then used to obtain M for an unknown protein. This is incorrect, however, because of the unknown shape factor. Since any deviation from spherical shape will result in an increase in the Stokes radius, it can be concluded that any molecular weight obtained by such a method is a maximum value.

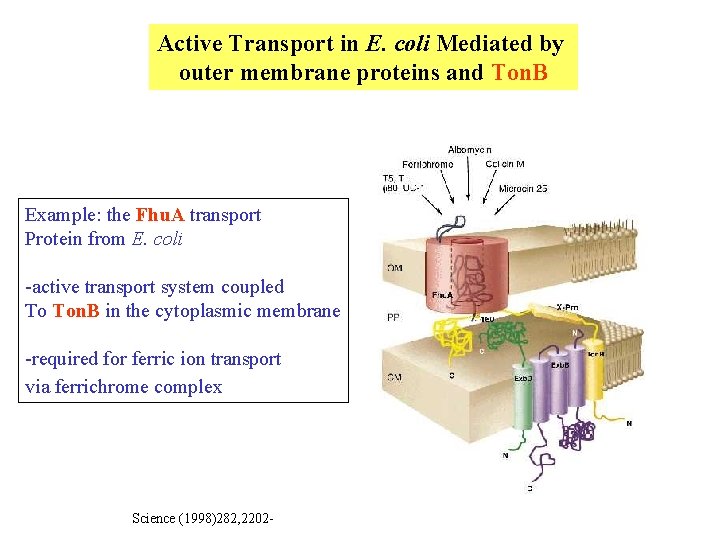
Active Transport in E. coli Mediated by outer membrane proteins and Ton. B Example: the Fhu. A transport Protein from E. coli -active transport system coupled To Ton. B in the cytoplasmic membrane -required for ferric ion transport via ferrichrome complex Science (1998)282, 2202 -

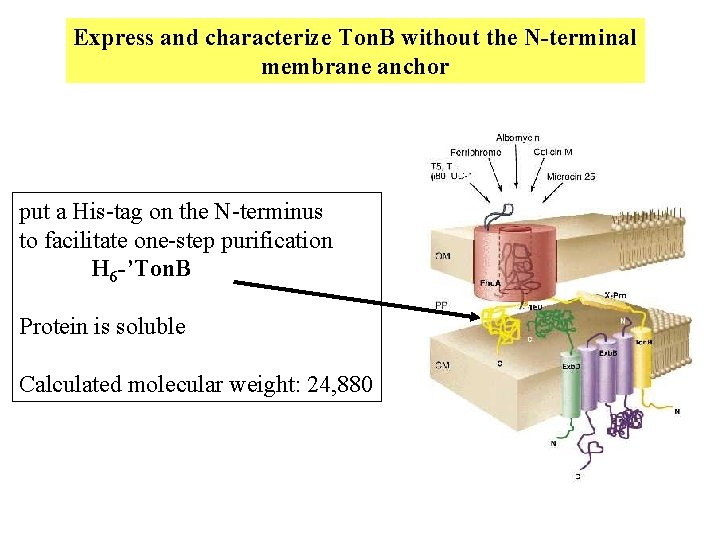
Express and characterize Ton. B without the N-terminal membrane anchor put a His-tag on the N-terminus to facilitate one-step purification H 6 -’Ton. B Protein is soluble Calculated molecular weight: 24, 880

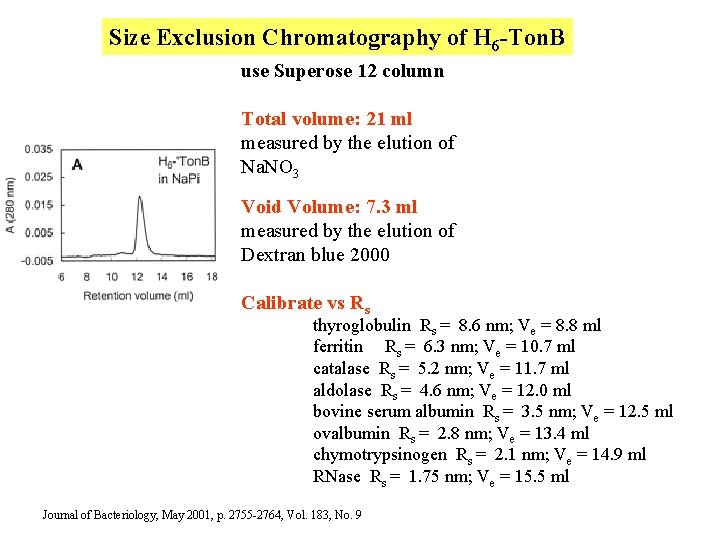
Size Exclusion Chromatography of H 6 -Ton. B use Superose 12 column Total volume: 21 ml measured by the elution of Na. NO 3 Void Volume: 7. 3 ml measured by the elution of Dextran blue 2000 Calibrate vs Rs thyroglobulin Rs = 8. 6 nm; Ve = 8. 8 ml ferritin Rs = 6. 3 nm; Ve = 10. 7 ml catalase Rs = 5. 2 nm; Ve = 11. 7 ml aldolase Rs = 4. 6 nm; Ve = 12. 0 ml bovine serum albumin Rs = 3. 5 nm; Ve = 12. 5 ml ovalbumin Rs = 2. 8 nm; Ve = 13. 4 ml chymotrypsinogen Rs = 2. 1 nm; Ve = 14. 9 ml RNase Rs = 1. 75 nm; Ve = 15. 5 ml Journal of Bacteriology, May 2001, p. 2755 -2764, Vol. 183, No. 9

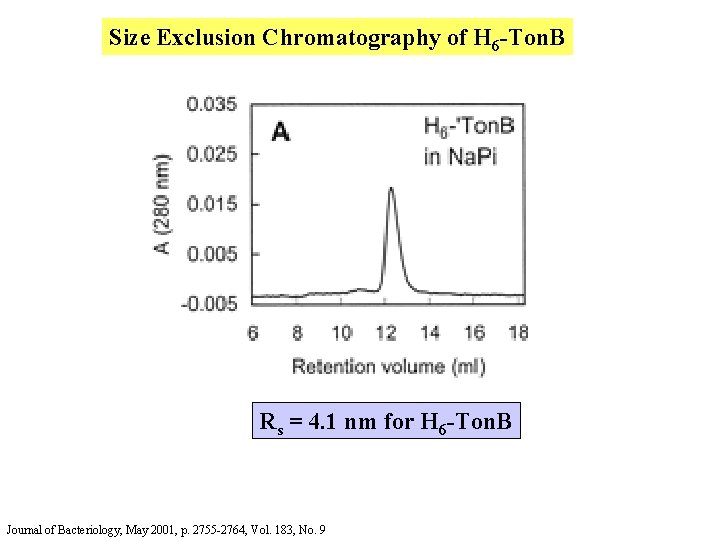
Size Exclusion Chromatography of H 6 -Ton. B Rs = 4. 1 nm for H 6 -Ton. B Journal of Bacteriology, May 2001, p. 2755 -2764, Vol. 183, No. 9

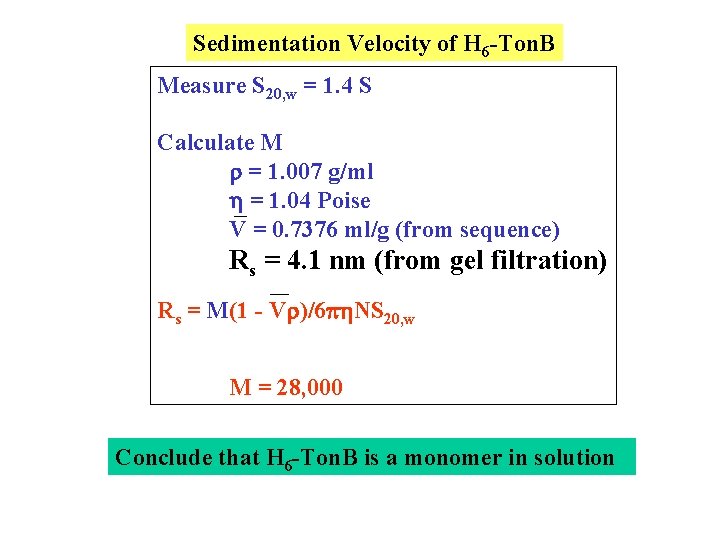
Sedimentation Velocity of H 6 -Ton. B Measure S 20, w = 1. 4 S Calculate M = 1. 007 g/ml = 1. 04 Poise V = 0. 7376 ml/g (from sequence) Rs = 4. 1 nm (from gel filtration) Rs = M(1 - V )/6 NS 20, w M = 28, 000 Conclude that H 6 -Ton. B is a monomer in solution

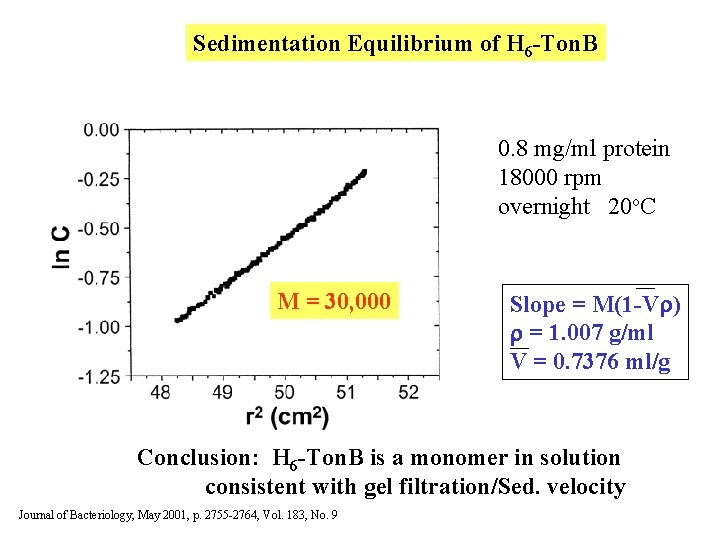
Sedimentation Equilibrium of H 6 -Ton. B 0. 8 mg/ml protein 18000 rpm overnight 20 o. C M = 30, 000 Slope = M(1 -V ) = 1. 007 g/ml V = 0. 7376 ml/g Conclusion: H 6 -Ton. B is a monomer in solution consistent with gel filtration/Sed. velocity Journal of Bacteriology, May 2001, p. 2755 -2764, Vol. 183, No. 9

Size Exclusion Chromatography of H 6 -Ton. B Rs = 4. 1 nm for H 6 -Ton. B Calculate Rmin = (3 MV/4 N)1/3 = 1. 9 nm for anhydrous sphere with M = 24, 900 from sequence Conclude: molecule is highly asymmetric Rs/Rmin = 2. 1 Journal of Bacteriology, May 2001, p. 2755 -2764, Vol. 183, No. 9

One can re-calculate Rmin assuming hydration of 0. 3 g H 2 O/g protein Rmin = 2. 0 nm so Rs/Rmin = 2 Consistent with an ellipsoid with an axial ratio of 15: 1 240 Å x 16 Å Ton. B goes from the inner to the outer bacterial membrane

Fhu. A: an integral outer membrane protein From E. coli -22 b strands in blue -“cork” domain in yellow -Bound to Fe 3+ferrichrome Science (1998)282, 2215 - Energy-driven conformational change drives Fe transport

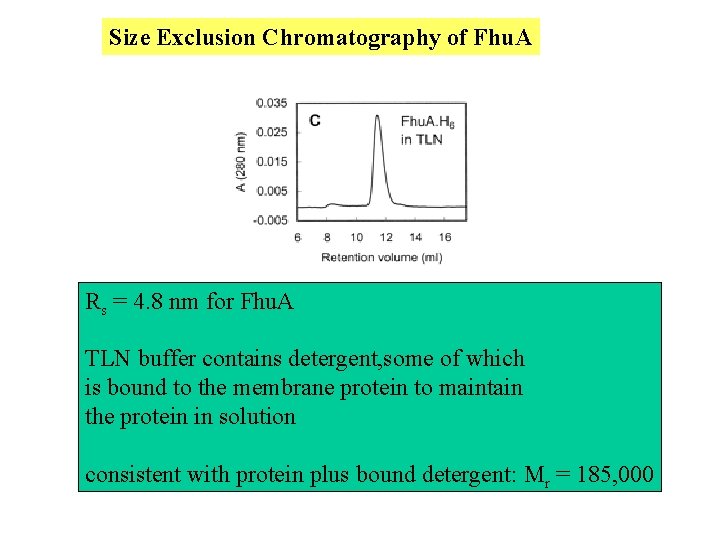
Size Exclusion Chromatography of Fhu. A Rs = 4. 8 nm for Fhu. A TLN buffer contains detergent, some of which is bound to the membrane protein to maintain the protein in solution consistent with protein plus bound detergent: Mr = 185, 000

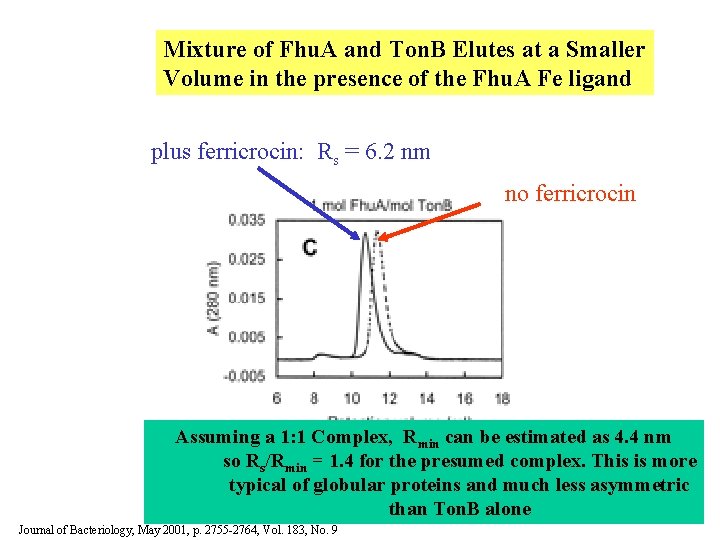
Mixture of Fhu. A and Ton. B Elutes at a Smaller Volume in the presence of the Fhu. A Fe ligand plus ferricrocin: Rs = 6. 2 nm no ferricrocin Assuming a 1: 1 Complex, Rmin can be estimated as 4. 4 nm so Rs/Rmin = 1. 4 for the presumed complex. This is more typical of globular proteins and much less asymmetric than Ton. B alone Journal of Bacteriology, May 2001, p. 2755 -2764, Vol. 183, No. 9

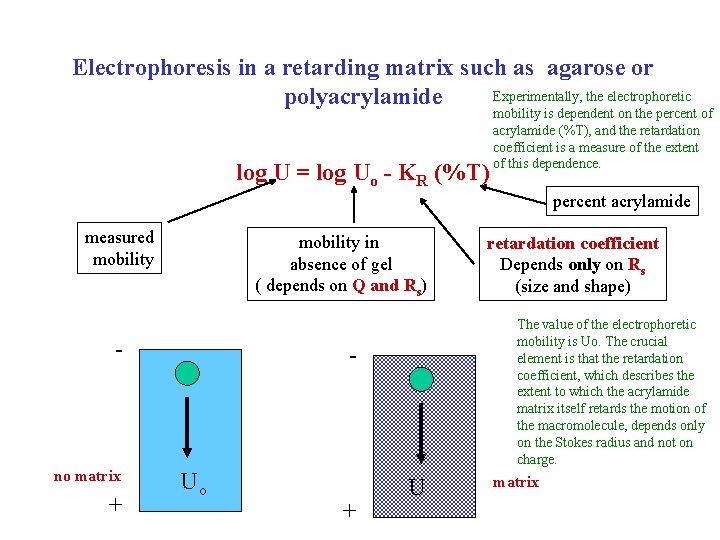
Electrophoresis The next (third) mass transport technique we will discuss is electrophoresis. In this case the applied force is due to the electric field. This will be dependent on the net charge on the macromolecule. In solution, without any retarding matrix such as polyacrylamide or agarose, the force of retardation would be proportional to the frictional coefficient or Stokes radius. The electrophoretic mobility is the velocity per unit of electric field, related to the (Q/f) or (Q/Rs). Since the electrophoretic mobility depends on both charge and size, it cannot be interpreted in any simple manner. A folded protein, for example, might have a net positive or negative charge and, therefore, might travel towards either the anode or cathode. Furthermore, the effective charge is going to be dependent on the ionic composition in solution, making this an even more complex problem.

Electrophoresis Fap Fret= f • (velocity) in steady state: applied force: electrical field retarding force: frictional drag moving through solution Fap = Q • E charge x electric field f • (velocity) = Q • E electrophoretic mobility: U = velocity Q =( ) E f depends on charge (Q) and Rs However, usually electrophoresis is done in the presence of a retarding matrix such a polyacrylamide The ability of a macromolecule to move through the retarding matrix depends on the Stokes radius

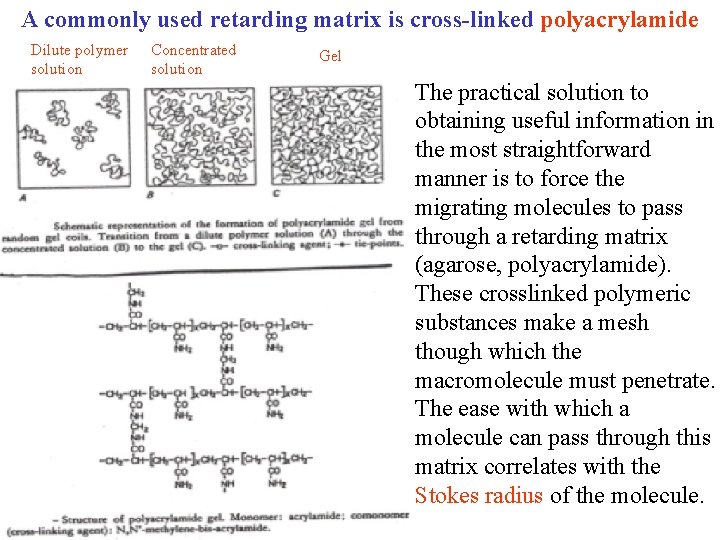
A commonly used retarding matrix is cross-linked polyacrylamide Dilute polymer solution Concentrated solution Gel The practical solution to obtaining useful information in the most straightforward manner is to force the migrating molecules to pass through a retarding matrix (agarose, polyacrylamide). These crosslinked polymeric substances make a mesh though which the macromolecule must penetrate. The ease with which a molecule can pass through this matrix correlates with the Stokes radius of the molecule.

Electrophoresis in a retarding matrix such as agarose or Experimentally, the electrophoretic polyacrylamide log U = log Uo - KR (%T) mobility is dependent on the percent of acrylamide (%T), and the retardation coefficient is a measure of the extent of this dependence. percent acrylamide measured mobility in absence of gel ( depends on Q and Rs) - no matrix + The value of the electrophoretic mobility is Uo. The crucial element is that the retardation coefficient, which describes the extent to which the acrylamide matrix itself retards the motion of the macromolecule, depends only on the Stokes radius and not on charge. - Uo + retardation coefficient Depends only on Rs (size and shape) U matrix

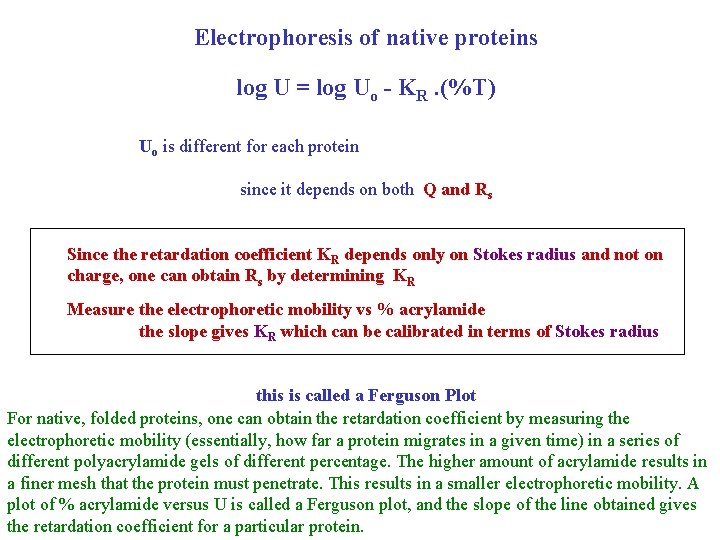
Electrophoresis of native proteins log U = log Uo - KR. (%T) Uo is different for each protein since it depends on both Q and Rs Since the retardation coefficient KR depends only on Stokes radius and not on charge, one can obtain Rs by determining KR Measure the electrophoretic mobility vs % acrylamide the slope gives KR which can be calibrated in terms of Stokes radius this is called a Ferguson Plot For native, folded proteins, one can obtain the retardation coefficient by measuring the electrophoretic mobility (essentially, how far a protein migrates in a given time) in a series of different polyacrylamide gels of different percentage. The higher amount of acrylamide results in a finer mesh that the protein must penetrate. This results in a smaller electrophoretic mobility. A plot of % acrylamide versus U is called a Ferguson plot, and the slope of the line obtained gives the retardation coefficient for a particular protein.

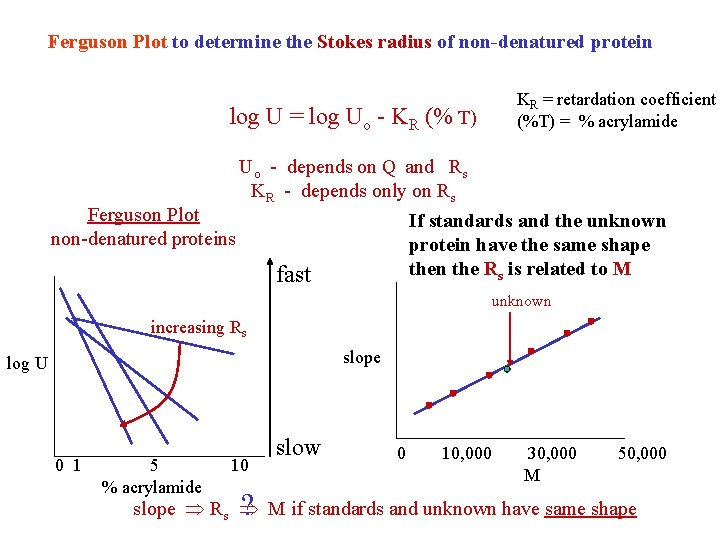
Ferguson Plot to determine the Stokes radius of non-denatured protein log U = log Uo - KR (% T) Ferguson Plot non-denatured proteins KR = retardation coefficient (%T) = % acrylamide Uo - depends on Q and Rs KR - depends only on Rs If standards and the unknown protein have the same shape then the Rs is related to M fast unknown increasing Rs slope log U 0 1 5 % acrylamide 10 ? slow 0 10, 000 30, 000 M 50, 000 slope Rs M if standards and unknown have same shape

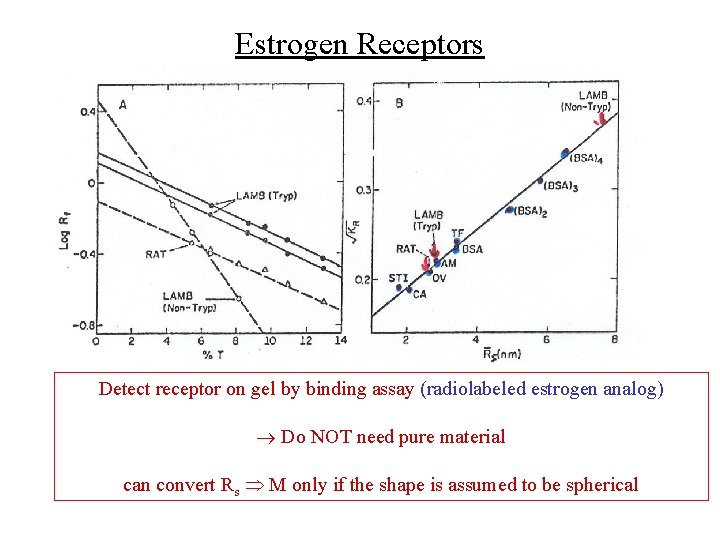
Estrogen Receptors Detect receptor on gel by binding assay (radiolabeled estrogen analog) Do NOT need pure material can convert Rs M only if the shape is assumed to be spherical

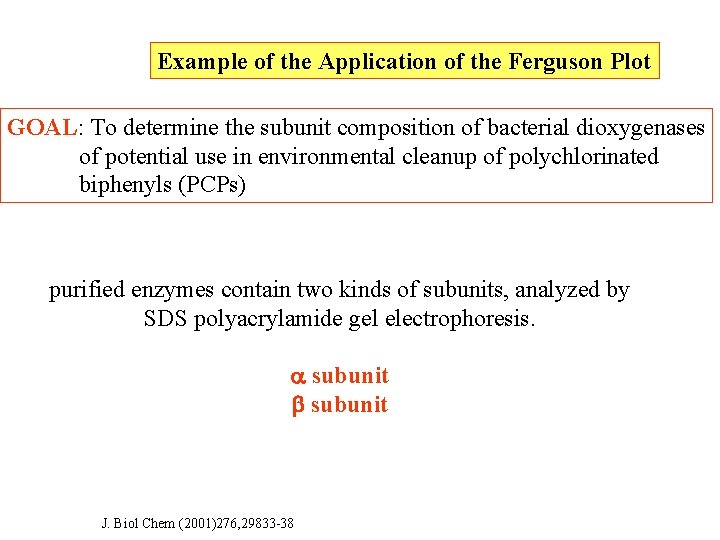
Example of the Application of the Ferguson Plot GOAL: To determine the subunit composition of bacterial dioxygenases of potential use in environmental cleanup of polychlorinated biphenyls (PCPs) purified enzymes contain two kinds of subunits, analyzed by SDS polyacrylamide gel electrophoresis. subunit J. Biol Chem (2001)276, 29833 -38

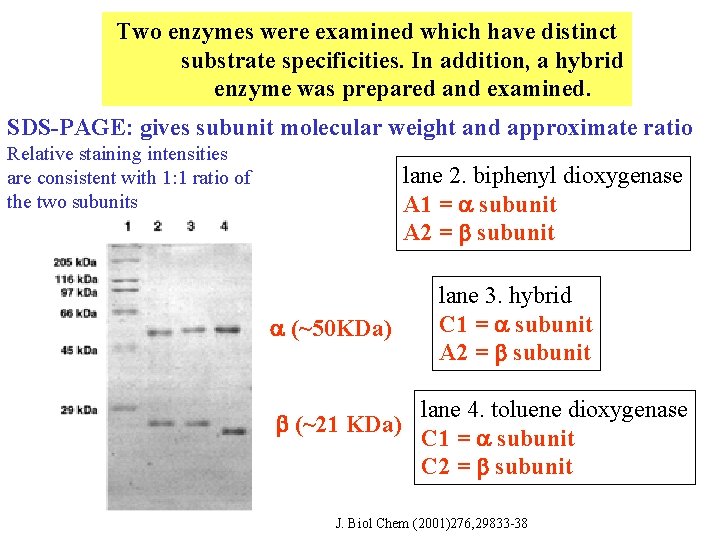
Two enzymes were examined which have distinct substrate specificities. In addition, a hybrid enzyme was prepared and examined. SDS-PAGE: gives subunit molecular weight and approximate ratio Relative staining intensities are consistent with 1: 1 ratio of the two subunits lane 2. biphenyl dioxygenase A 1 = subunit A 2 = subunit (~50 KDa) (~21 KDa) lane 3. hybrid C 1 = subunit A 2 = subunit lane 4. toluene dioxygenase C 1 = subunit C 2 = subunit J. Biol Chem (2001)276, 29833 -38

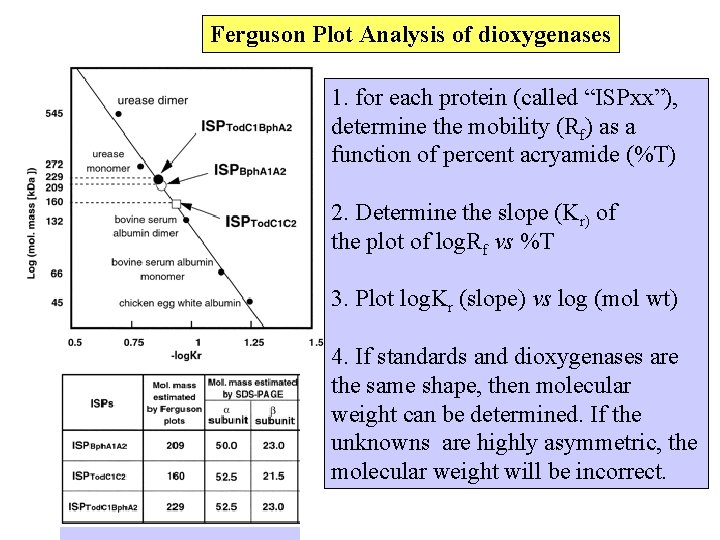
Ferguson Plot Analysis of dioxygenases 1. for each protein (called “ISPxx”), determine the mobility (Rf) as a function of percent acryamide (%T) 2. Determine the slope (Kr) of the plot of log. Rf vs %T 3. Plot log. Kr (slope) vs log (mol wt) 4. If standards and dioxygenases are the same shape, then molecular weight can be determined. If the unknowns are highly asymmetric, the molecular weight will be incorrect.

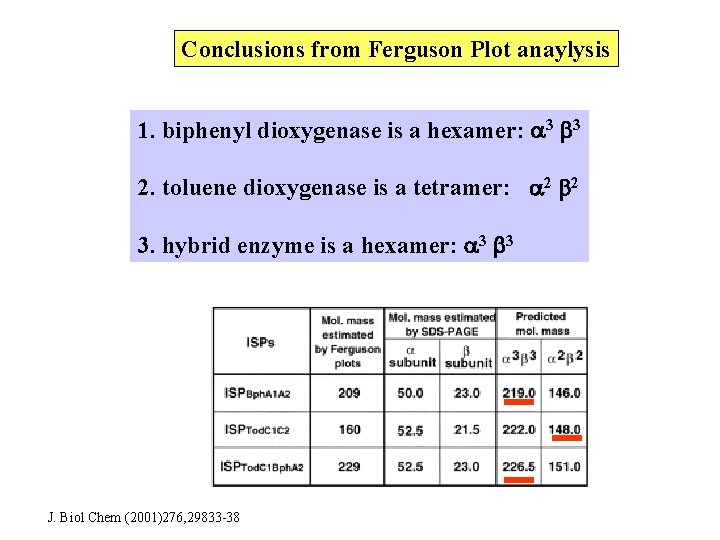
Conclusions from Ferguson Plot anaylysis 1. biphenyl dioxygenase is a hexamer: 3 3 2. toluene dioxygenase is a tetramer: 2 2 3. hybrid enzyme is a hexamer: 3 3 J. Biol Chem (2001)276, 29833 -38

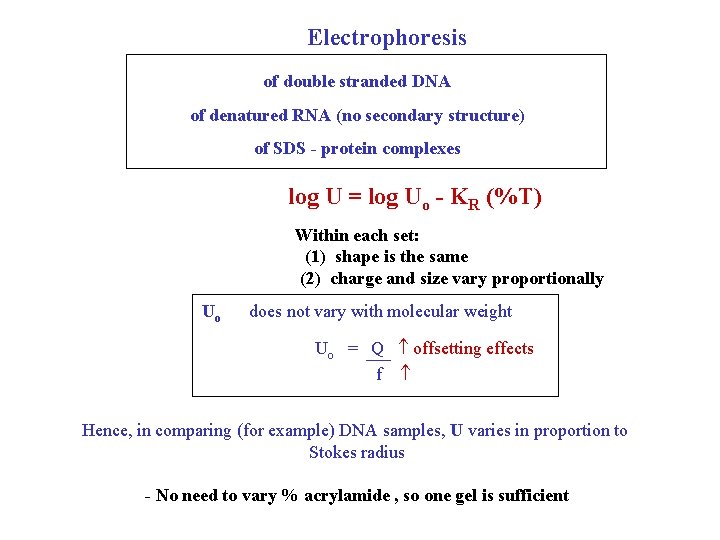
Electrophoresis of double stranded DNA of denatured RNA (no secondary structure) of SDS - protein complexes log U = log Uo - KR (%T) Within each set: (1) shape is the same (2) charge and size vary proportionally Uo does not vary with molecular weight Uo = Q offsetting effects f Hence, in comparing (for example) DNA samples, U varies in proportion to Stokes radius - No need to vary % acrylamide , so one gel is sufficient

Electrophoresis of SDS-Protein Complexes The procedure of using the Ferguson plot requires running a number of separate gels since the percent of acrylamide must be varied. Furthermore, the unknown shape factor makes it risky to interpret the results directly in terms of molecular weight. The strategy employed to simplify and improve the usefulness of electrophoresis is to use dodecyl sulfate (SDS) to denature the proteins, and then determine the electrophoretic mobility of the SDS-protein complex. In many cases, this allows one to obtain a reasonably accurate molecular weight of the polypeptide by running only one gel. Why does this work? There are two reasons.

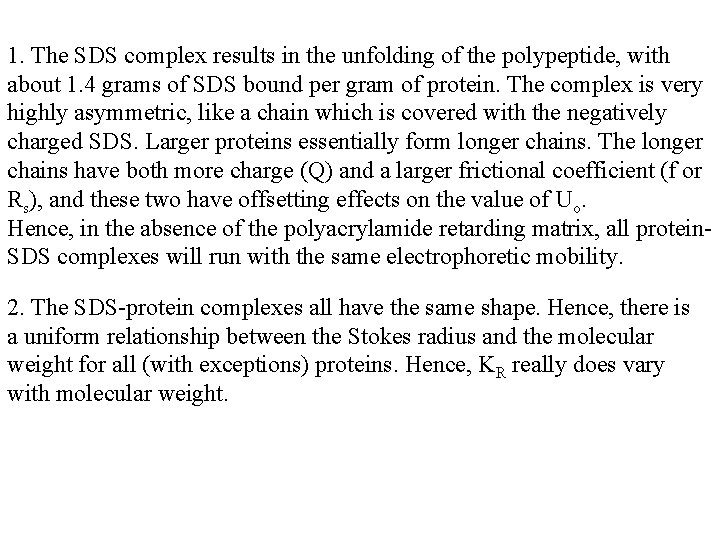
1. The SDS complex results in the unfolding of the polypeptide, with about 1. 4 grams of SDS bound per gram of protein. The complex is very highly asymmetric, like a chain which is covered with the negatively charged SDS. Larger proteins essentially form longer chains. The longer chains have both more charge (Q) and a larger frictional coefficient (f or Rs), and these two have offsetting effects on the value of Uo. Hence, in the absence of the polyacrylamide retarding matrix, all protein. SDS complexes will run with the same electrophoretic mobility. 2. The SDS-protein complexes all have the same shape. Hence, there is a uniform relationship between the Stokes radius and the molecular weight for all (with exceptions) proteins. Hence, KR really does vary with molecular weight.

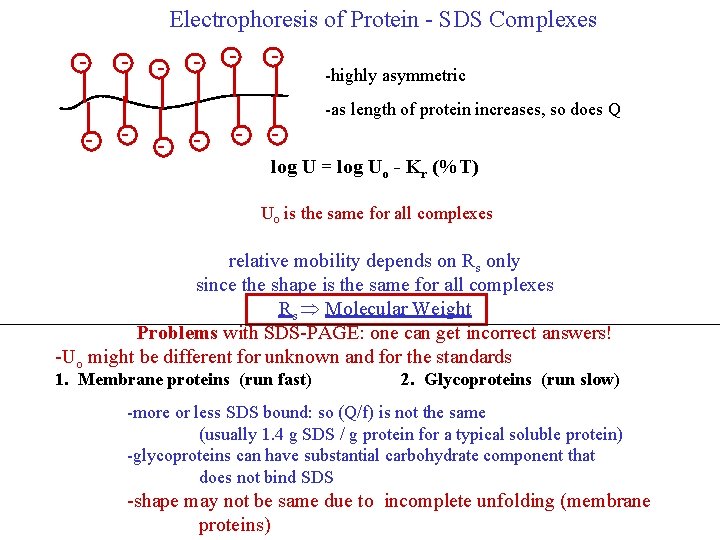
Constant value of Uo for SDS-Protein Complexes allows the Rs to be determined by the electrophoretic mobility on a single gel and comparing to standards Cannot get Rs from A single gel SAME UO Only one gel is Needed to get Rs fast increasing Rs 0 1 5 % acrylamide 10 NATIVE PROTEINS increasing Rs log U slow 0 1 5 % acrylamide 10 SDS-PROTEIN COMPLEXES Since the SDS-Protein complexes all have the same shape: Rs correlates well with molecular weight (M)

Electrophoresis of Protein - SDS Complexes - - - -highly asymmetric -as length of protein increases, so does Q log U = log Uo - Kr (%T) - - - Uo is the same for all complexes relative mobility depends on Rs only since the shape is the same for all complexes Rs Molecular Weight Problems with SDS-PAGE: one can get incorrect answers! -Uo might be different for unknown and for the standards 1. Membrane proteins (run fast) 2. Glycoproteins (run slow) -more or less SDS bound: so (Q/f) is not the same (usually 1. 4 g SDS / g protein for a typical soluble protein) -glycoproteins can have substantial carbohydrate component that does not bind SDS -shape may not be same due to incomplete unfolding (membrane proteins)

There are two major classes of proteins that present problems with this approach. 1) Membrane proteins are very hydrophobic and may not unfold completely, even in the presence of SDS. Furthermore, the very hydrophobic nature of the side chains may result in abnormal (excess) binding of SDS. Hence, the assumption that Uo is the same for the unknown as for the standards may not be correct. These effects cause hydrophobic membrane proteins to run faster in SDS-PAGE than they should. As a result, the molecular weights are often underestimated very significantly. 2) The second class of proteins that don’t behave similarly as the standards for calibration, are glycoproteins. These have covalently attached carbohydrate residues, which interfere with the binding to SDS. Hence, glycoproteins usually run slower than they would do otherwise in SDS-PAGE and the molecular weights are overestimated.

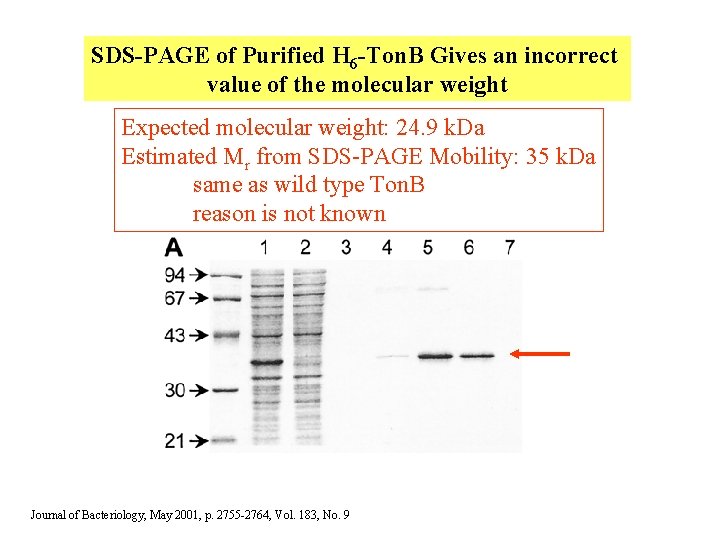
SDS-PAGE of Purified H 6 -Ton. B Gives an incorrect value of the molecular weight Expected molecular weight: 24. 9 k. Da Estimated Mr from SDS-PAGE Mobility: 35 k. Da same as wild type Ton. B reason is not known Journal of Bacteriology, May 2001, p. 2755 -2764, Vol. 183, No. 9

Chemical Crosslinking of Fhu. A and His 6 -Ton. B Shows the presence of a 1: 1 Complex Enhanced by ferricrocin and inhibited by 1 M Na. Cl Journal of Bacteriology, May 2001, p. 2755 -2764, Vol. 183, No. 9

Electrophoresis of Small Double Strand DNA and of RNA It is standard procedure to separate linear, double strand DNA species according to the number of base pairs by polyacrylamide or agarose electrophoresis. Only a single gel is necessary, and given standards run along side of an unknown, to determine the molecular weight (or number of base pairs) of DNA. The reason this can be done is the same as with SDS-protein complexes. 1. Different lengths of DNA all have the same value of Uo since both charge and size vary proportionately. 2. All the DNA molecules have the same shape if they are linear, so the Stokes radius varies in proportion to the number of base pairs. This will not work if the DNA is circular or supercoiled. These arguments do not hold for RNA, which has considerable tertiary structure. Hence, the molecular weight of native RNA cannot be determined as for DNA, but presents the same complications as native, folded proteins. As a result of this it is necessary to denature RNA during the electrophoresis. Using alkaline conditions, or solvents like dimethylformamide, which disrupt the tertiary structure and secondary structure of RNA. Under these conditions, as with double strand DNA, and as with SDS -protein complexes, the only difference between RNA species that results in different electrophoretic mobilities is the length of the RNA strand.

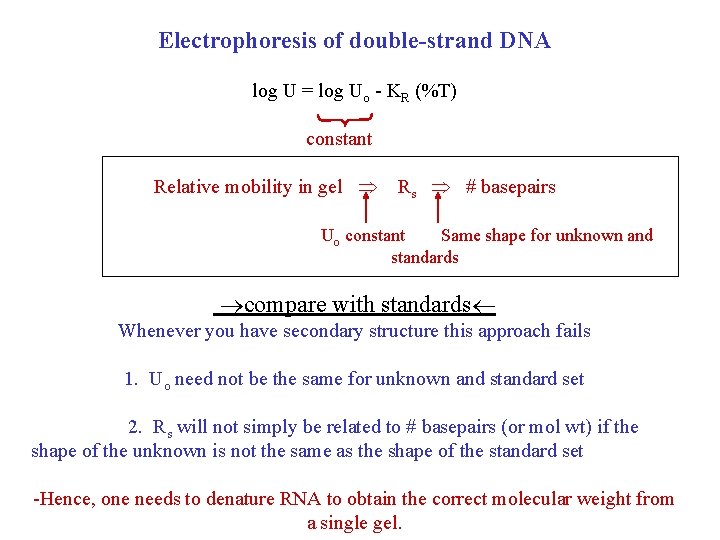
Electrophoresis of double-strand DNA log U = log Uo - KR (%T) constant Relative mobility in gel Rs # basepairs Uo constant Same shape for unknown and standards compare with standards Whenever you have secondary structure this approach fails 1. Uo need not be the same for unknown and standard set 2. Rs will not simply be related to # basepairs (or mol wt) if the shape of the unknown is not the same as the shape of the standard set -Hence, one needs to denature RNA to obtain the correct molecular weight from a single gel.

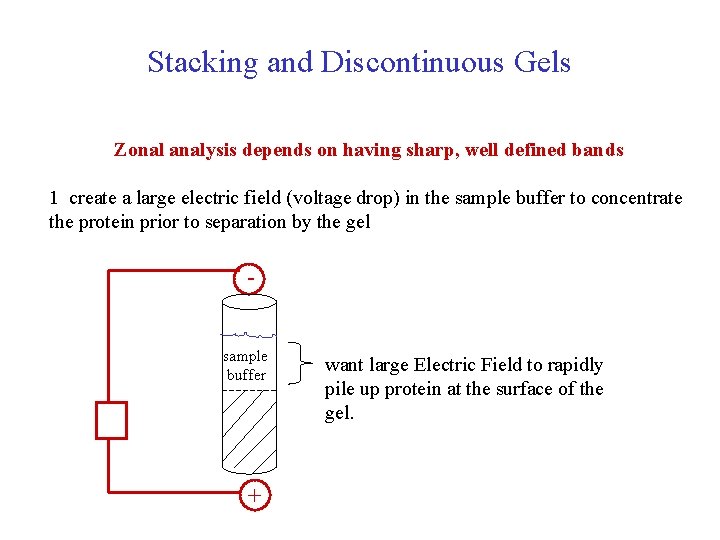
Stacking of the Sample in Electrophoresis is a zonal method, in which the sample is layered on top of the separating retarding matrix and the mobility is measured by observing the migrating band of material. Hence, it is important to have a narrow band of material to optimize the resolution of the technique. Stacking is used to produce a very sharp band of material prior to entering the separating gel. The procedure is shown below.

Stacking and Discontinuous Gels Zonal analysis depends on having sharp, well defined bands 1 create a large electric field (voltage drop) in the sample buffer to concentrate the protein prior to separation by the gel sample buffer + want large Electric Field to rapidly pile up protein at the surface of the gel.

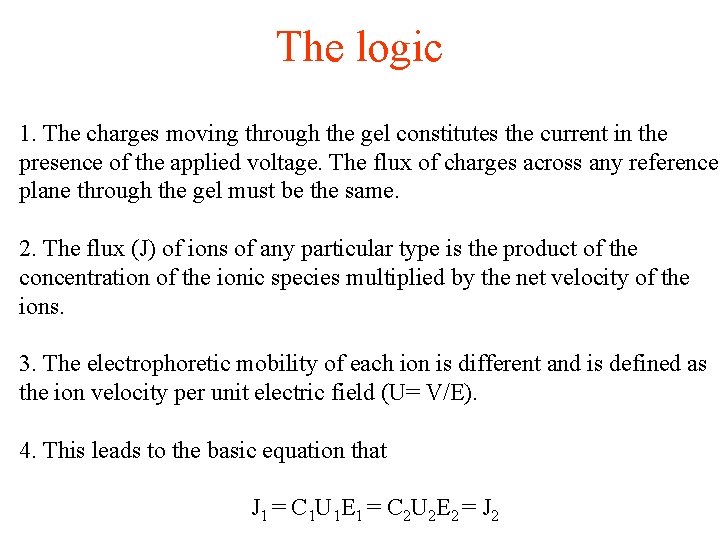
The logic 1. The charges moving through the gel constitutes the current in the presence of the applied voltage. The flux of charges across any reference plane through the gel must be the same. 2. The flux (J) of ions of any particular type is the product of the concentration of the ionic species multiplied by the net velocity of the ions. 3. The electrophoretic mobility of each ion is different and is defined as the ion velocity per unit electric field (U= V/E). 4. This leads to the basic equation that J 1 = C 1 U 1 E 1 = C 2 U 2 E 2 = J 2

This means that at places in the gel where either the concentration or the mobility of the major charge carrier is low, the result is a high electric field. This just means that where the resistance to current flow is high, there will be a large voltage drop (meaning a large electric field). In the Laemmli buffer system, used routinely for SDS PAGE systems, there is a discontinuous buffer (hence, disc-gel electrophoresis). Glycine is the major buffer in the sample, and the p. H is such that the average charge is low on the glycine, reducing its electrophoretic mobility (U 1 in the next slide). Chloride is the major ionic carrier of charge in the gel itself. As a result of the electric resistance due to the low mobility of glycine, the electric field across this part of the system is very high (E 1). This is where the protein is loaded, so the SDS-protein complex is subject to this very large field, which causes the complexes to move rapidly down, where all the proteins pile up on top of the separating acrylamide matrix. This forms a very narrow band, which then enters the gel, and separation takes place. After this initial formation of the narrow band, during the rest of the process, the band broadens by diffusion. This is illustrated in the next slide. The alternative way to achieve the same high resistance to current flow in the region where the sample is first loaded onto the gel, is by simply lowering the ion concentration in this region. Hence, the same ion can be in both the separating gel and in the sample, but in the sample the buffer is diluted 10 -fold.

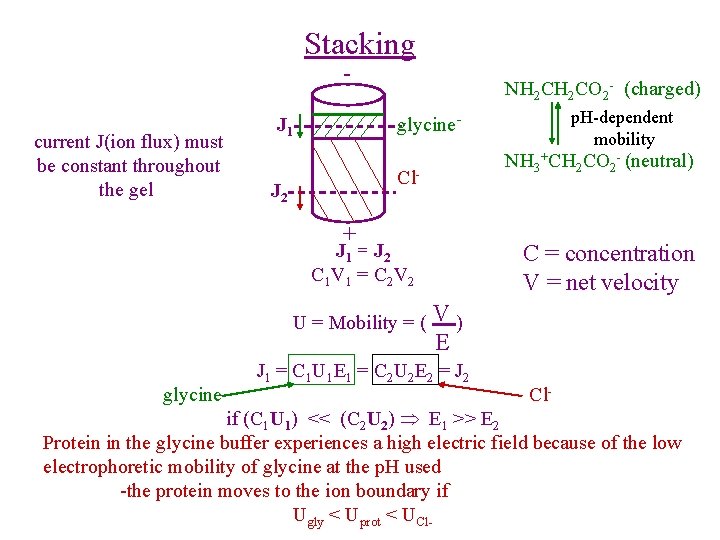
Stacking - current J(ion flux) must be constant throughout the gel NH 2 CO 2 - (charged) NH 3+CH 2 CO 2 - (neutral) Cl- J 2 + J 1 = J 2 C 1 V 1 = C 2 V 2 U = Mobility = ( glycine p. H-dependent mobility glycine- J 1 C = concentration V = net velocity V) E J 1 = C 1 U 1 E 1 = C 2 U 2 E 2 = J 2 Cl- if (C 1 U 1) << (C 2 U 2) E 1 >> E 2 Protein in the glycine buffer experiences a high electric field because of the low electrophoretic mobility of glycine at the p. H used -the protein moves to the ion boundary if Ugly < Uprot < UCl-

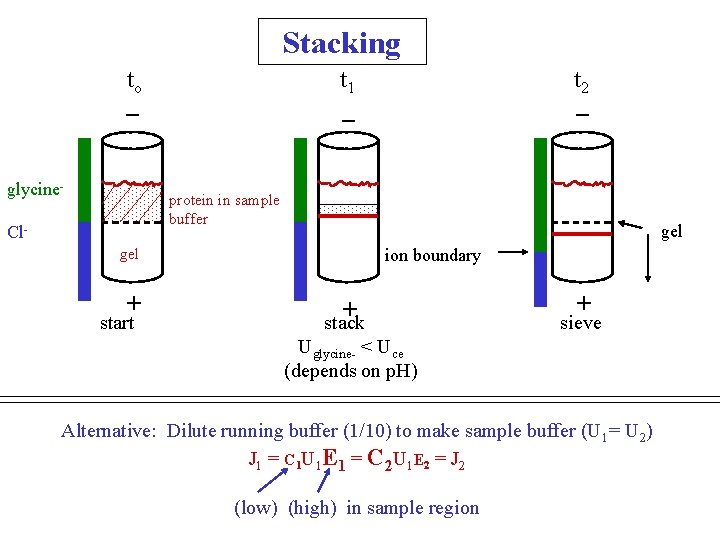
Stacking to _ glycine- t 1 _ t 2 _ protein in sample buffer Cl- gel + start ion boundary + stack + sieve Uglycine- < Uce (depends on p. H) Alternative: Dilute running buffer (1/10) to make sample buffer (U 1= U 2) J 1 = C 1 U 1 E 1 = C 2 U 1 E 2 = J 2 (low) (high) in sample region

Two variants of gel electrophoresis 1. Gel mobility-shift assay for protein-DNA interactions 2. Pulsed field gel electrophoresis for separating very large DNA (chromosomes)

Electrophoresis of Protein-DNA complexes An interesting application of electrophoresis technology is the gel mobility shift assay for DNA-protein complexes. This is summarized in the next slide. The main idea is quite simple. A protein complex with DNA is formed in the sample. The mobility of this complex is different (less than) that of the DNA alone. As a result, the complex is visualized as a separate band in the gel after it is run and then visualized for the presence of the DNA. Small fragments of DNA are used in these assays.

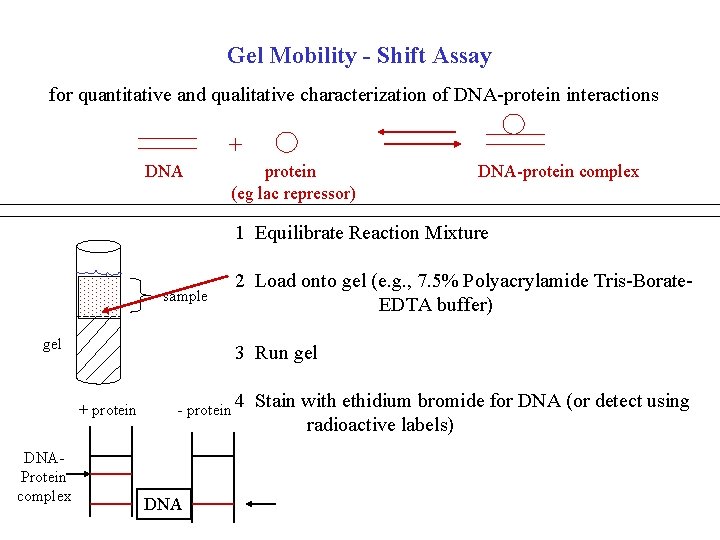
Gel Mobility - Shift Assay for quantitative and qualitative characterization of DNA-protein interactions + DNA protein (eg lac repressor) DNA-protein complex 1 Equilibrate Reaction Mixture sample gel 3 Run gel + protein DNAProtein complex 2 Load onto gel (e. g. , 7. 5% Polyacrylamide Tris-Borate. EDTA buffer) - protein DNA 4 Stain with ethidium bromide for DNA (or detect using radioactive labels)

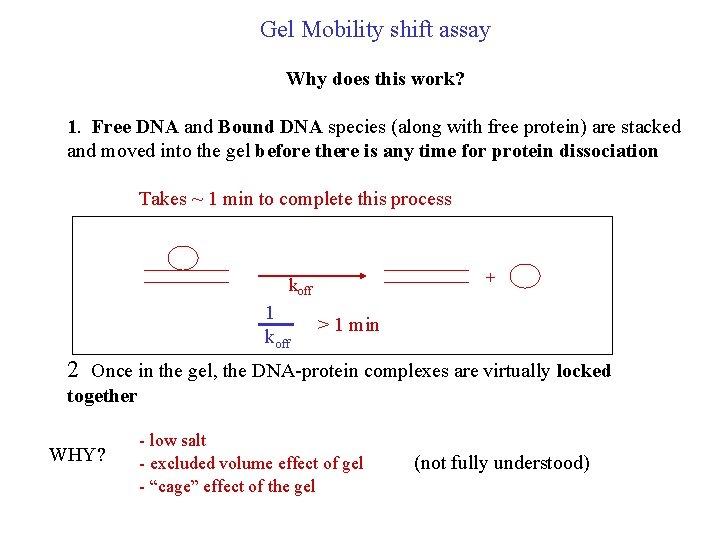
Gel Mobility shift assay Why does this work? 1. Free DNA and Bound DNA species (along with free protein) are stacked and moved into the gel before there is any time for protein dissociation Takes ~ 1 min to complete this process + koff 1 koff > 1 min 2 Once in the gel, the DNA-protein complexes are virtually locked together WHY? - low salt - excluded volume effect of gel - “cage” effect of the gel (not fully understood)

The “cage effect” of the gel is such that when the protein and DNA dissociate they are trapped by the gel and cannot diffuse apart. Hence, the entropic advantage to dissociation is lost, so the effective dissociation constant shifts to greatly favor the complex staying together. The mobility shift assay can be used qualitatively to detect the presence of complexes, or it can be used quantitatively to determine the affinity constant in solution between the protein and DNA. This is because the assay provides information about the amount of DNA that is free in solution and the amount which is bound to the protein in the solution prior to getting trapped in the gel. As shown below, enough parameters can be measured to get a binding isotherm and a dissociation constant. Because of the ability to detect very low concentrations of DNA, very high affinity binding can be measured.

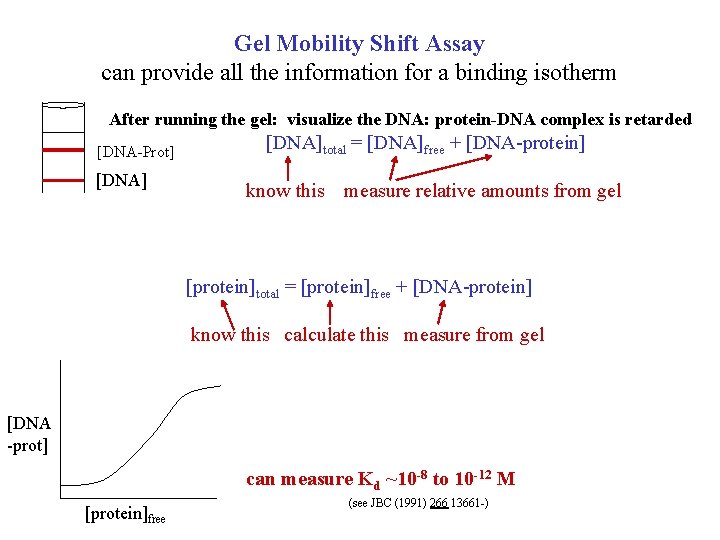
Gel Mobility Shift Assay can provide all the information for a binding isotherm After running the gel: visualize the DNA: protein-DNA complex is retarded [DNA-Prot] [DNA]total = [DNA]free + [DNA-protein] know this measure relative amounts from gel [protein]total = [protein]free + [DNA-protein] know this calculate this measure from gel [DNA -prot] can measure Kd ~10 -8 to 10 -12 M [protein]free (see JBC (1991) 266 13661 -)

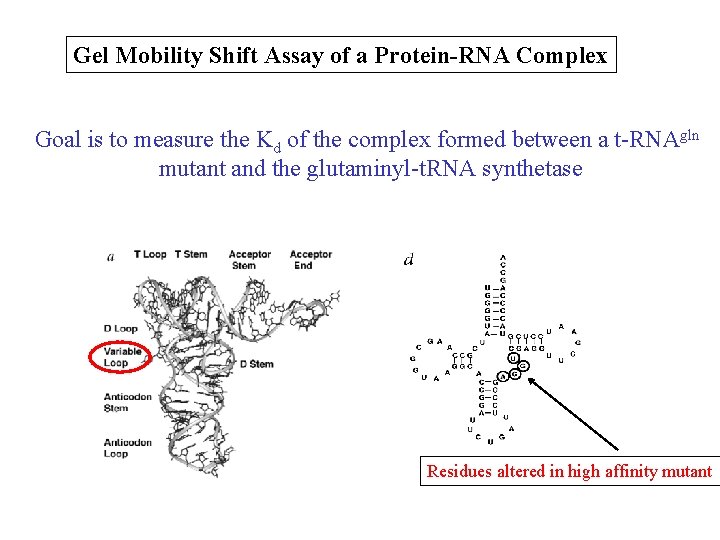
Gel Mobility Shift Assay of a Protein-RNA Complex Goal is to measure the Kd of the complex formed between a t-RNAgln mutant and the glutaminyl-t. RNA synthetase Residues altered in high affinity mutant

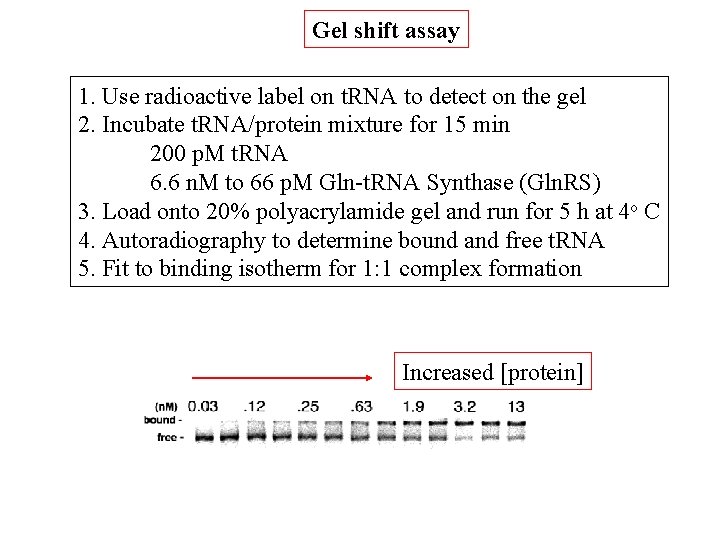
Gel shift assay 1. Use radioactive label on t. RNA to detect on the gel 2. Incubate t. RNA/protein mixture for 15 min 200 p. M t. RNA 6. 6 n. M to 66 p. M Gln-t. RNA Synthase (Gln. RS) 3. Load onto 20% polyacrylamide gel and run for 5 h at 4 o C 4. Autoradiography to determine bound and free t. RNA 5. Fit to binding isotherm for 1: 1 complex formation Increased [protein]

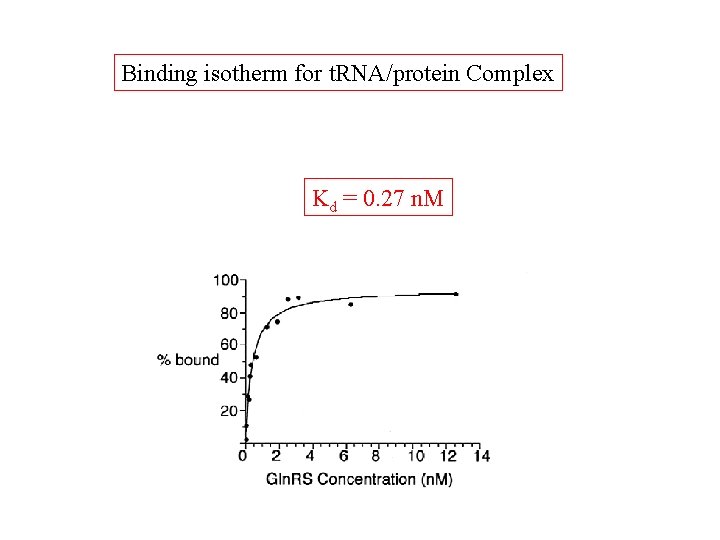
Binding isotherm for t. RNA/protein Complex Kd = 0. 27 n. M

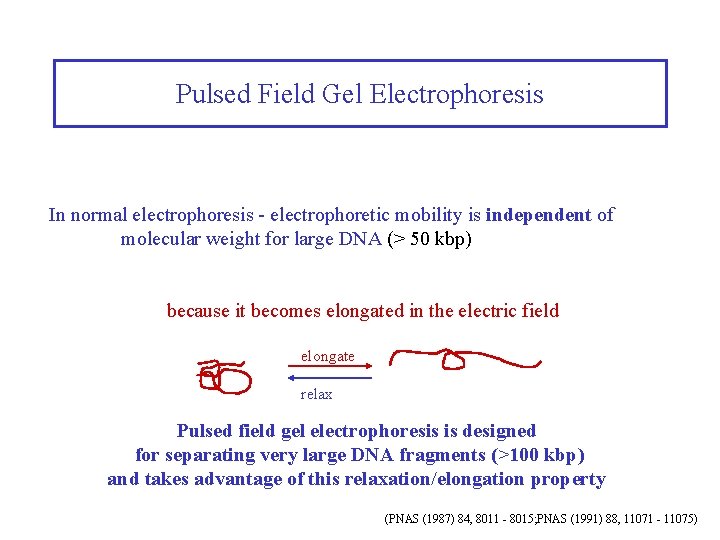
Pulsed Field Gel Electrophoresis In normal electrophoresis - electrophoretic mobility is independent of molecular weight for large DNA (> 50 kbp) because it becomes elongated in the electric field elongate relax Pulsed field gel electrophoresis is designed for separating very large DNA fragments (>100 kbp) and takes advantage of this relaxation/elongation property (PNAS (1987) 84, 8011 - 8015; PNAS (1991) 88, 11071 - 11075)

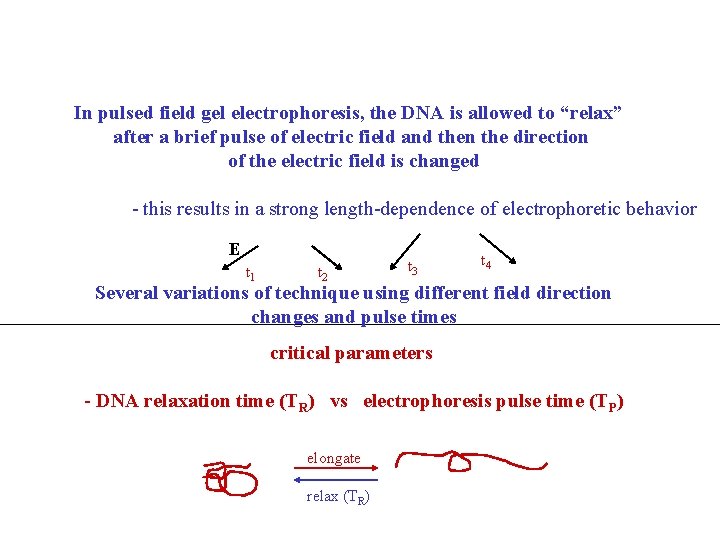
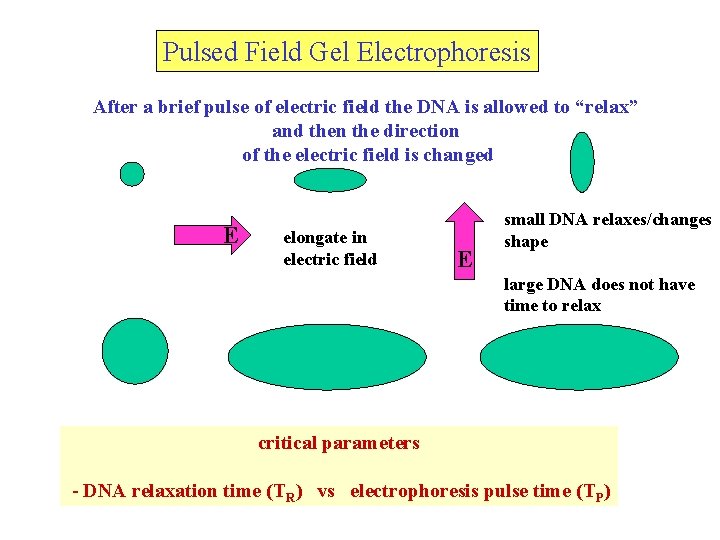
In pulsed field gel electrophoresis, the DNA is allowed to “relax” after a brief pulse of electric field and then the direction of the electric field is changed - this results in a strong length-dependence of electrophoretic behavior E t 1 t 2 t 3 t 4 Several variations of technique using different field direction changes and pulse times critical parameters - DNA relaxation time (TR) vs electrophoresis pulse time (TP) elongate relax (TR)

Field Inversion Gel Electrophoresis (FIGE). Another interesting and useful application of electrophoresis technology is field inversion gel electrophoresis (FIGE), which is used to separate very large DNA. The usual agarose electrophoresis is limited in the ability to separate DNA of sizes larger than 50 kbp (50, 000 basepairs). This is because the DNA becomes elongated in the electric field and the gel no longer separates. FIGE takes advantage of this elongation produced by the electric field to separate DNA fragments larger than 100 kbp. The main idea is to change the direction and of the electric field at a certain pulse rate (TP, pulse time). The large DNA is elongated by the field in the initial direction, and this elongation makes it much easier for the DNA to move in this direction, and very difficult for it to penetrate the gel in the perpendicular direction. The amount of time it takes the DNA to relax back to a spherical shape from the elongation is dependent on size.

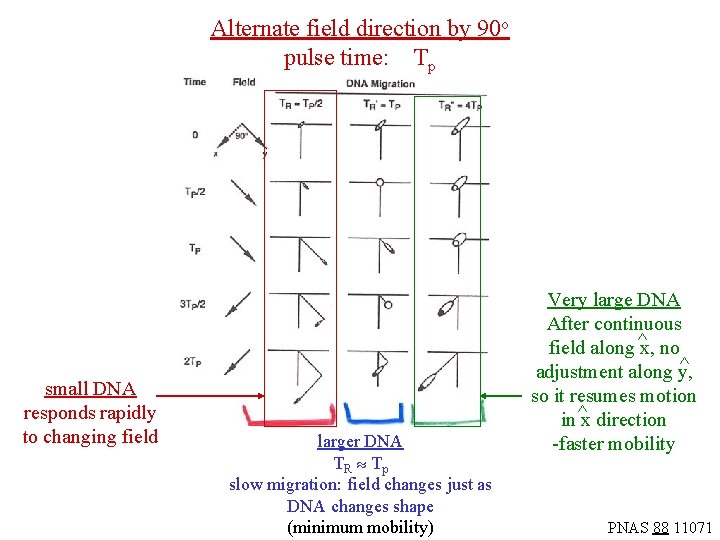
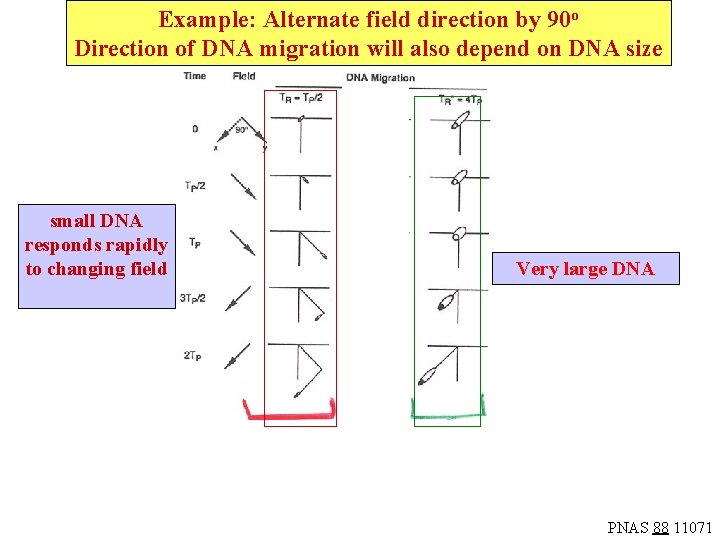
If the electric field direction is changed before the DNA has had a chance to relax, then the DNA will not be able to respond to the altered direction and move in the new direction since it is elongated in the “wrong” way. Smaller DNA will relax more rapidly, and will move along with the altered field direction. Large DNA will not respond. Hence, the path of migration in the gel will vary with DNA size. The critical parameters are the pulse time (TP) for the electric field and the relaxation time for the DNA elongation (TR). The next slide shows three conditions, where the relaxation time is much shorter than, equal to, or much larger than the pulse time. The mobility of the DNA actually is minimized when the pulse time is about equal to the DNA relaxation time, as pictured.

Alternate field direction by 90 o pulse time: Tp small DNA responds rapidly to changing field larger DNA TR Tp slow migration: field changes just as DNA changes shape (minimum mobility) Very large DNA After continuous field along ^x, no ^ adjustment along y, so it resumes motion in ^x direction -faster mobility PNAS 88 11071

Pulsed Field Gel Electrophoresis In normal electrophoresis - electrophoretic mobility is independent of molecular weight for large DNA (> 50 kbp) Pulsed field gel electrophoresis is a collection of techniques to increase the resolution of DNA fragments to extend to very large DNA fragments (>>100 kbp) based on the size-dependent relaxation properties of DNA elongates along the direction of the electric field (E) DNA relaxes when the field is turned off DNA shape changes when field direction is changed DNA mobility depends on the shape/determined by previous pulse sequence E E elongate relax (PNAS (1987) 84, 8011 - 8015; PNAS (1991) 88, 11071 - 11075)

Pulsed Field Gel Electrophoresis After a brief pulse of electric field the DNA is allowed to “relax” and then the direction of the electric field is changed E elongate in electric field E small DNA relaxes/changes shape large DNA does not have time to relax critical parameters - DNA relaxation time (TR) vs electrophoresis pulse time (TP)

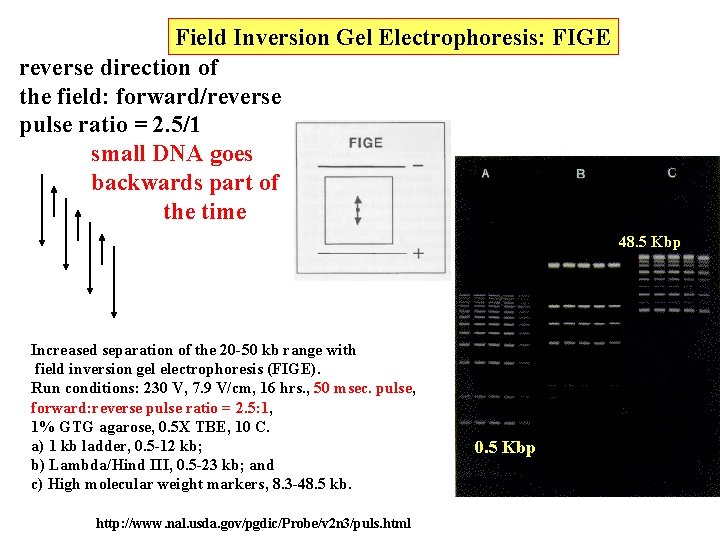
Field Inversion Gel Electrophoresis: FIGE reverse direction of the field: forward/reverse pulse ratio = 2. 5/1 small DNA goes backwards part of the time 48. 5 Kbp Increased separation of the 20 -50 kb range with field inversion gel electrophoresis (FIGE). Run conditions: 230 V, 7. 9 V/cm, 16 hrs. , 50 msec. pulse, forward: reverse pulse ratio = 2. 5: 1, 1% GTG agarose, 0. 5 X TBE, 10 C. a) 1 kb ladder, 0. 5 -12 kb; b) Lambda/Hind III, 0. 5 -23 kb; and c) High molecular weight markers, 8. 3 -48. 5 kb. http: //www. nal. usda. gov/pgdic/Probe/v 2 n 3/puls. html 0. 5 Kbp

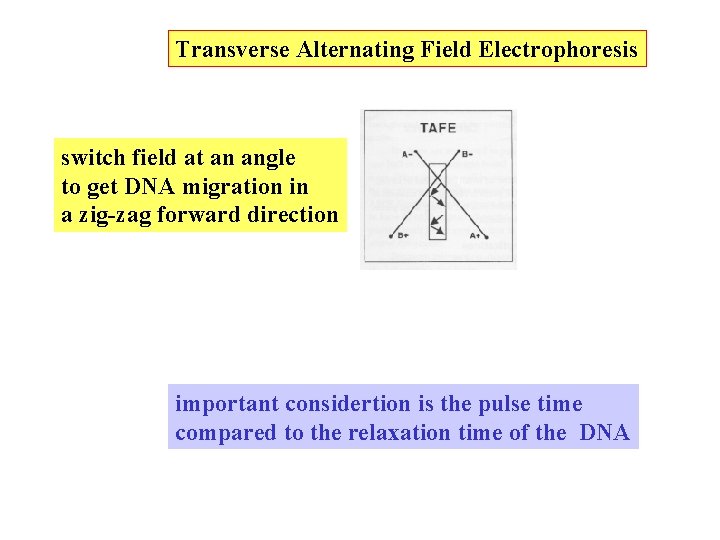
Transverse Alternating Field Electrophoresis switch field at an angle to get DNA migration in a zig-zag forward direction important considertion is the pulse time compared to the relaxation time of the DNA

Example: Alternate field direction by 90 o Direction of DNA migration will also depend on DNA size small DNA responds rapidly to changing field Very large DNA PNAS 88 11071
 What is void volume in gel filtration
What is void volume in gel filtration Advantages of gel filtration chromatography
Advantages of gel filtration chromatography Gel filtration chromatography definition
Gel filtration chromatography definition The inlet pressure in a constant rate filtration
The inlet pressure in a constant rate filtration Gel filtration application
Gel filtration application Gel filtration calibration kit
Gel filtration calibration kit Kav chromatography
Kav chromatography Gel filtration
Gel filtration Kav chromatography
Kav chromatography Agarose vs acrylamide gel
Agarose vs acrylamide gel Gel kim
Gel kim Agarose gel
Agarose gel Gel permeation chromatography instrument
Gel permeation chromatography instrument Zinc oxide + nitric acid → zinc nitrate + water
Zinc oxide + nitric acid → zinc nitrate + water Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Covalent bond
Covalent bond Impingement filtration
Impingement filtration Filtration membrane
Filtration membrane Metabolic waste
Metabolic waste Ul 900 testing
Ul 900 testing Fteps
Fteps Nafa guide to air filtration
Nafa guide to air filtration Columbus industries
Columbus industries Nafa guide to air filtration
Nafa guide to air filtration Filtration introduction
Filtration introduction Factors effecting gfr
Factors effecting gfr Barrière de filtration glomérulaire
Barrière de filtration glomérulaire Mixtures separated by distillation
Mixtures separated by distillation Vibrating membrane filtration
Vibrating membrane filtration Why is separating mixtures important
Why is separating mixtures important National air filter association
National air filter association Closely packed cells
Closely packed cells Pyrogen
Pyrogen Advantages of filtration
Advantages of filtration Impingement in filtration
Impingement in filtration Abnormal and normal constituents of urine
Abnormal and normal constituents of urine Mechanical separation of mixtures
Mechanical separation of mixtures Filtering solids from liquids
Filtering solids from liquids Precoat filtration
Precoat filtration Centrifugal filtration definition
Centrifugal filtration definition Objective of filtration
Objective of filtration National air filter
National air filter Obiggs filter
Obiggs filter Membrane filtration
Membrane filtration Filtration introduction
Filtration introduction Prismaflex crrt troubleshooting
Prismaflex crrt troubleshooting Mongo seeds will dissolve in one cup of water yes or no
Mongo seeds will dissolve in one cup of water yes or no Pixabay
Pixabay Filtration of blood
Filtration of blood Separation of mixtures evaporation
Separation of mixtures evaporation Glomerular filtration
Glomerular filtration Filtration coefficient formula
Filtration coefficient formula Nafa guide to air filtration
Nafa guide to air filtration Oncotic vs hydrostatic pressure
Oncotic vs hydrostatic pressure Blood filtration
Blood filtration Glomerular filtration
Glomerular filtration Sparation
Sparation Figure 15-3 the urinary system
Figure 15-3 the urinary system Fluting filter paper
Fluting filter paper Wholesome water
Wholesome water General filter
General filter What is filtration
What is filtration Filter plates for solvent filtration
Filter plates for solvent filtration Filtration in chemical engineering
Filtration in chemical engineering