GASTRIC CARCINOMA RAJESH R 2002 BATCH EPIDEMIOLOGY 2
GASTRIC CARCINOMA RAJESH. R 2002 BATCH

EPIDEMIOLOGY 2 nd most common cause of death due to Ca in world l incidence high in japan; 70/1 lakh l male : female – 2: 1 l incidence increases with age l Japan has adopted a screening programme based on endoscopy l

Pathology l Gastric Adenocarcinoma (~ 95%) subdivided- Papillary, tubular, mucinous, signet ring l l l Squamous Cell Carcinoma Adenoacanthoma Carcinoid Gastrointestinal stromal tumors (GISTs) Lymphoma

GASTRIC ADENOCARCINOMA Risk factors l l l * diet (high in dry salted, smoked or preserved foods and low in fruits and vegetables); * bacteria (including Helicobacter pylori infection); * advanced age; * male gender; * atrophic gastritis and intestinal metaplasia; * pernicious anemia; * cigarette smoking; * Menetrier's disease (giant hypertrophic gastritis), and * gastric adenomatous polyps and familial polyposis
GASTRIC ADENOCARCINOMA Pathology Topography l antrum and prepylorus ~ 30% l cardia and fundus ~ 35% l lesser curvature ~ 20% l greater curvature ~ 3 - 5% l entire stomach ~ 5 - 10%
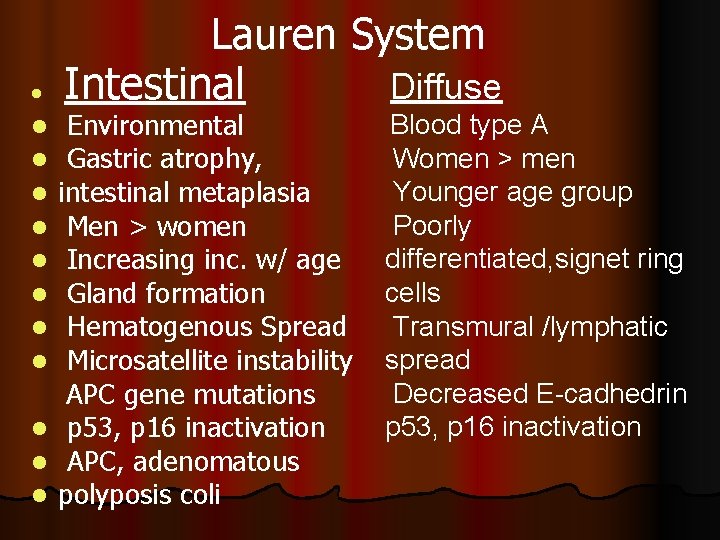
l Lauren System Diffuse Intestinal Environmental Gastric atrophy, intestinal metaplasia Men > women Increasing inc. w/ age Gland formation Hematogenous Spread Microsatellite instability APC gene mutations l p 53, p 16 inactivation l APC, adenomatous l polyposis coli l l l l Blood type A Women > men Younger age group Poorly differentiated, signet ring cells Transmural /lymphatic spread Decreased E-cadhedrin p 53, p 16 inactivation
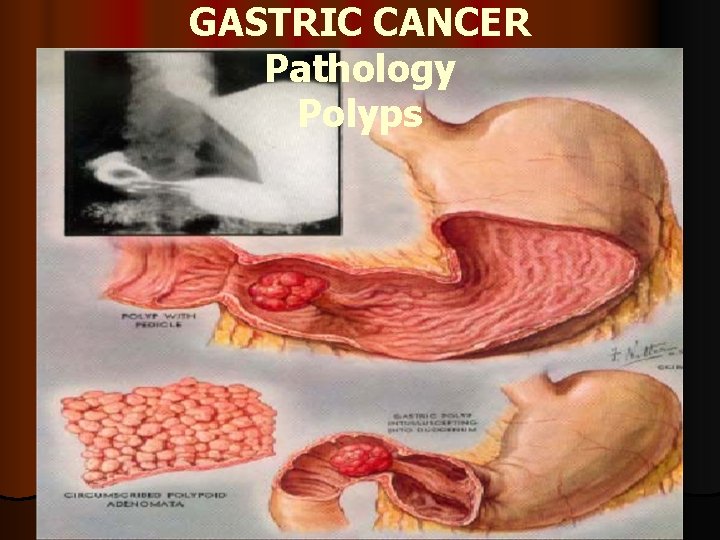
GASTRIC CANCER Pathology Polyps
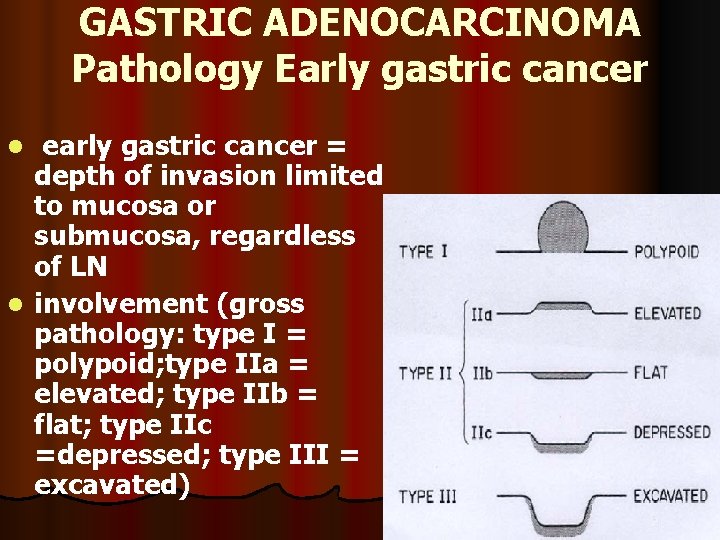
GASTRIC ADENOCARCINOMA Pathology Early gastric cancer early gastric cancer = depth of invasion limited to mucosa or submucosa, regardless of LN l involvement (gross pathology: type I = polypoid; type IIa = elevated; type IIb = flat; type IIc =depressed; type III = excavated) l

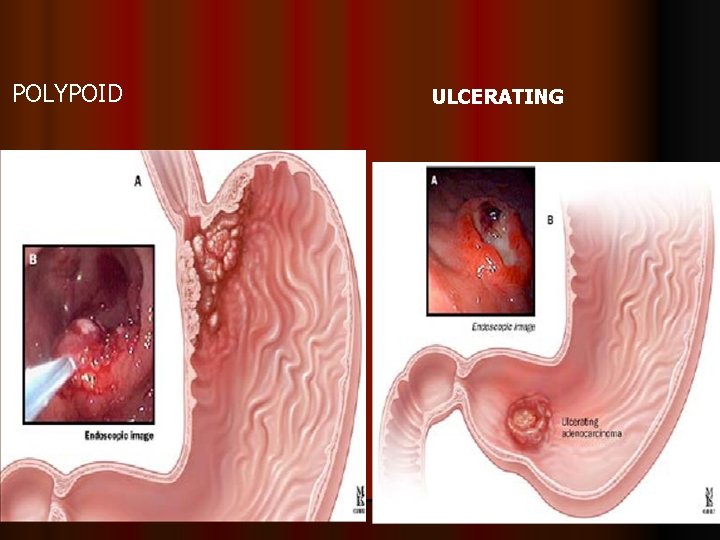
ADVANCED GASTRIC CARCINOMA l advanced gastric cancer = disease penetrates muscular layer, usually associated with contiguous or distant spread [gross pathology l (Borrmann classification): I = polypoid; II =fungating; III = ulcerating - infiltrating; IV =infiltrating]
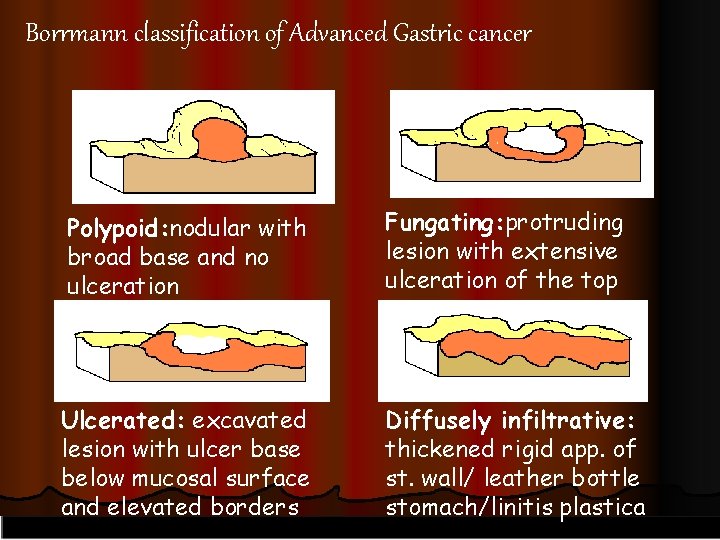
Borrmann classification of Advanced Gastric cancer Polypoid: nodular with broad base and no ulceration Fungating: protruding lesion with extensive ulceration of the top Ulcerated: excavated lesion with ulcer base below mucosal surface and elevated borders Diffusely infiltrative: thickened rigid app. of st. wall/ leather bottle stomach/linitis plastica
POLYPOID ULCERATING
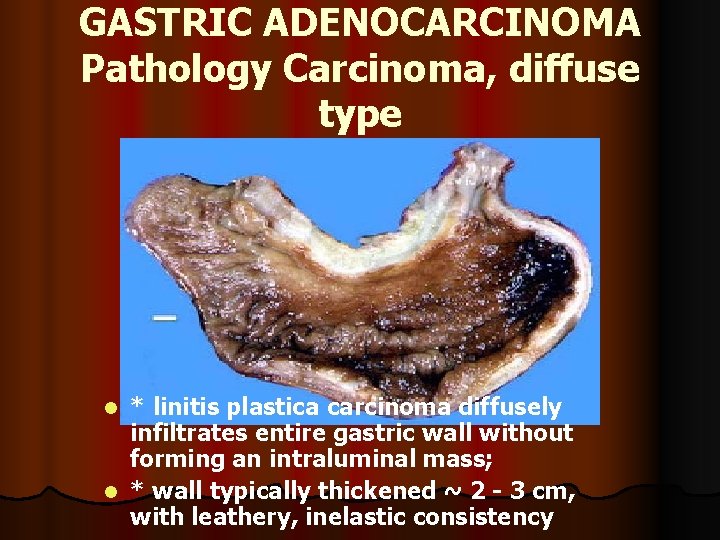
GASTRIC ADENOCARCINOMA Pathology Carcinoma, diffuse type * linitis plastica carcinoma diffusely infiltrates entire gastric wall without forming an intraluminal mass; l * wall typically thickened ~ 2 - 3 cm, with leathery, inelastic consistency l

GASTRIC ADENOCARCINOMA Clinical features * usually no symptoms when superficial and surgically curable; l * with more extensive tumors → insidious upper abdominal discomfort (from a vague, postprandial fullness to severe, steady pain), anorexia (often with slight nausea) and weight loss; l * no early physical sign (finding of a palpable abdominal mass generally indicates long – standing growth and regional extension) l

GASTRIC ADENOCARCINOMA Clinical features……. . l* iron - deficiency anemia l occult blood in stool l unusual clinical features: l - migratory thrombophlebitis; l - microangiopathic hemolytic anemia, l - acanthosis nigricans l Blumer’s shelf, Sr. Joseph’s nodules, Troisier’s sign, Krukenberg tumour

GASTRIC ADENOCARCINOMA Dissemination l * by direct extension through gastric wall to perigastric tissues (occasionally adhering to adjacent organs such as pancreas, colon or liver- via lymphatics to intraabdominal (frequent) and supraclavicular lymph nodes (Troisier’ sign); l - by seeding of peritoneal surfaces [metastatic nodules to ovary (Krukenberg's tumor), periumbilical region ("Sister Mary Joseph node") or peritoneal cul-desac (Blumer's shelf)]; l - malignant ascites; l - hematogenous spread (more frequently to liver)
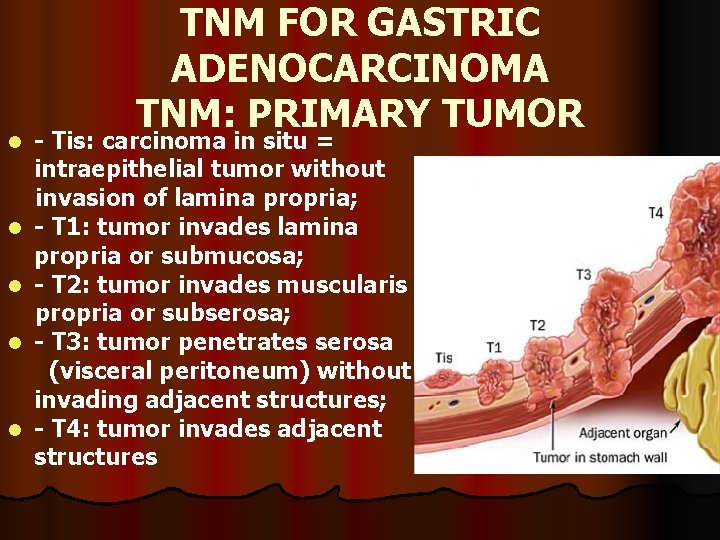
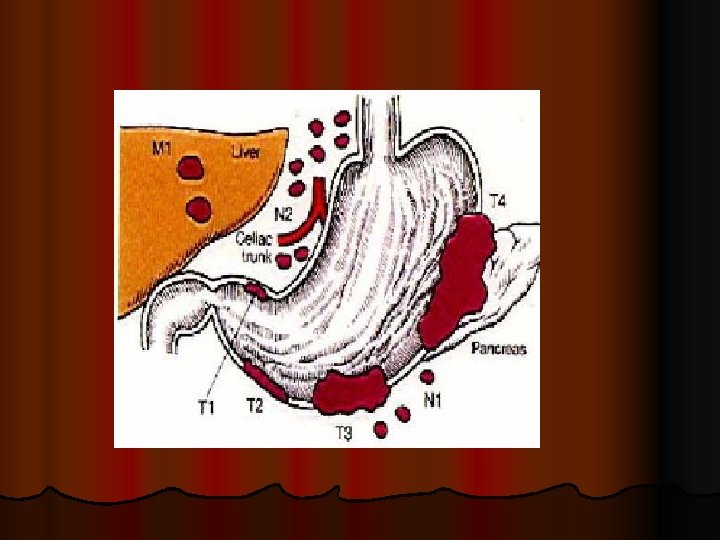
l l l TNM FOR GASTRIC ADENOCARCINOMA TNM: PRIMARY TUMOR - Tis: carcinoma in situ = intraepithelial tumor without invasion of lamina propria; - T 1: tumor invades lamina propria or submucosa; - T 2: tumor invades muscularis propria or subserosa; - T 3: tumor penetrates serosa (visceral peritoneum) without invading adjacent structures; - T 4: tumor invades adjacent structures
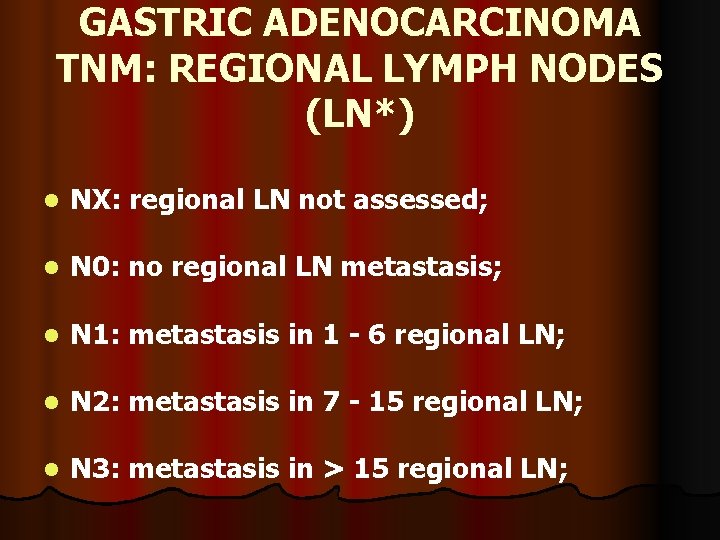
GASTRIC ADENOCARCINOMA TNM: REGIONAL LYMPH NODES (LN*) l NX: regional LN not assessed; l N 0: no regional LN metastasis; l N 1: metastasis in 1 - 6 regional LN; l N 2: metastasis in 7 - 15 regional LN; l N 3: metastasis in > 15 regional LN;
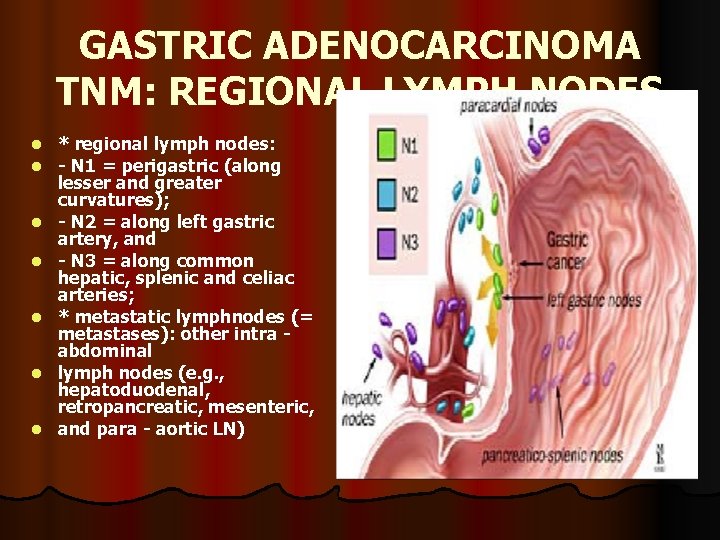
GASTRIC ADENOCARCINOMA TNM: REGIONAL LYMPH NODES l l l l * regional lymph nodes: - N 1 = perigastric (along lesser and greater curvatures); - N 2 = along left gastric artery, and - N 3 = along common hepatic, splenic and celiac arteries; * metastatic lymphnodes (= metastases): other intra abdominal lymph nodes (e. g. , hepatoduodenal, retropancreatic, mesenteric, and para - aortic LN)

GASTRIC ADENOCARCINOMA TNM: DISTANT METASTASIS (M) l MX: distant metastasis cannot be assessed; l M 0: no distant metastasis l M 1: distant metastasis
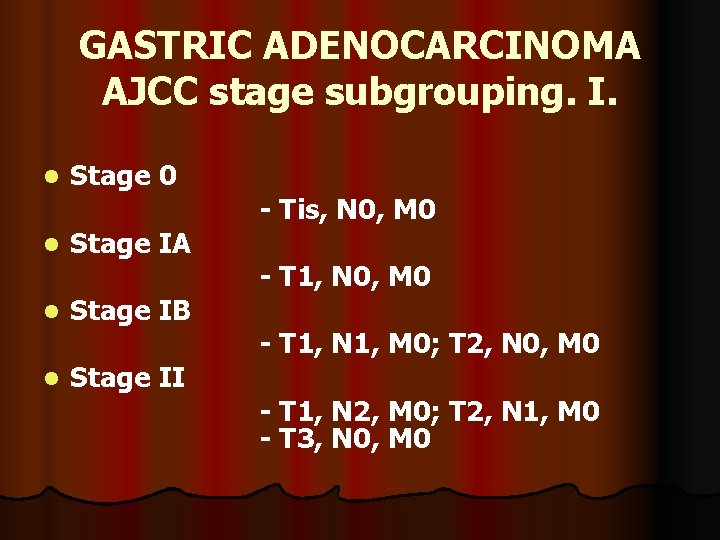
GASTRIC ADENOCARCINOMA AJCC stage subgrouping. I. l l Stage 0 Stage IA Stage IB Stage II - Tis, N 0, M 0 - T 1, N 1, M 0; T 2, N 0, M 0 - T 1, N 2, M 0; T 2, N 1, M 0 - T 3, N 0, M 0
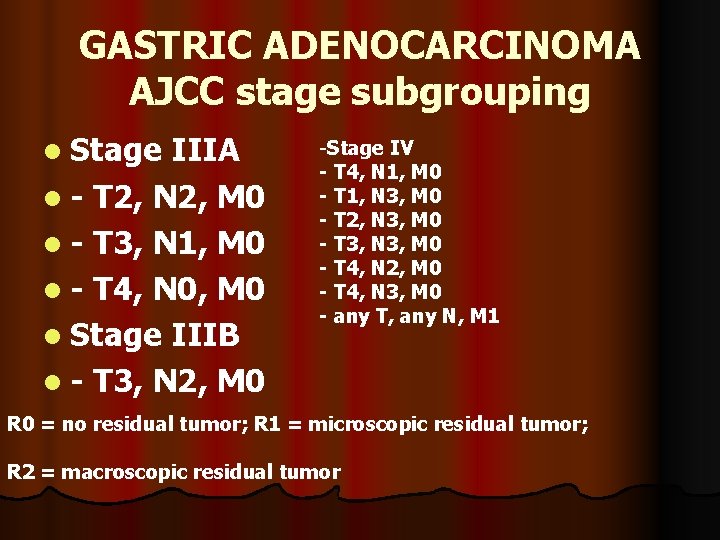
GASTRIC ADENOCARCINOMA AJCC stage subgrouping l Stage IIIA l - T 2, N 2, M 0 l - T 3, N 1, M 0 l - T 4, N 0, M 0 l Stage IIIB l - T 3, N 2, M 0 -Stage IV - T 4, N 1, M 0 - T 1, N 3, M 0 - T 2, N 3, M 0 - T 3, N 3, M 0 - T 4, N 2, M 0 - T 4, N 3, M 0 - any T, any N, M 1 R 0 = no residual tumor; R 1 = microscopic residual tumor; R 2 = macroscopic residual tumor
Routine Investigations l Hb – anaemia l ESR – elevated l STOOL – occult blood l LFT - ? Any liver involvement l CXR - ? Pul. Involvement l Biopsy of the palpable LN

TUMOUR MARKERS l CEA l CA 19 -9 l CA 125 l AFP
GASTRIC ADENOCARCINOMA Diagnosis * double - contrast radiographic examination (detecting small lesions by improving mucosal details) = (old) simplest diagnostic procedure for evaluation of pts with epigastric complaints; l - stomach distended during radiographic examinations (decreased distensibility may be the only indication of diffuse infiltrative carcinoma) l
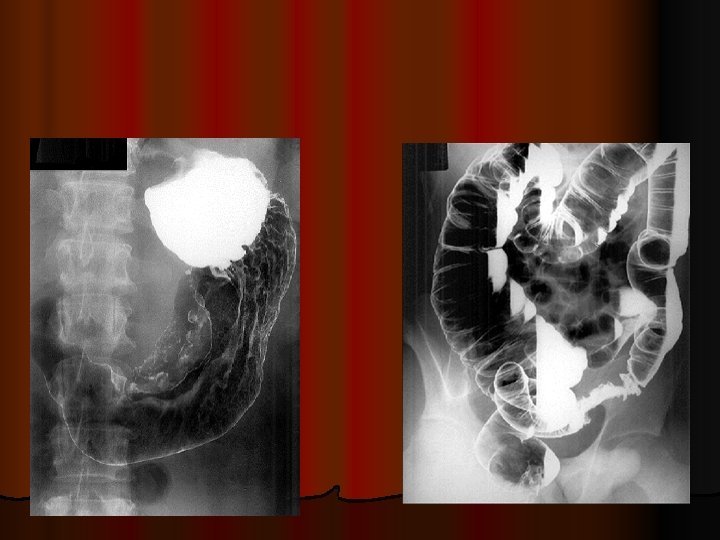
INVESTIGATIONS ENDOSCOPY l Observer dependent l Flexible fibre-optic endoscopy – most definitive l Advantage- - tissue diagnosis (biopsy & exfoliative cytology) l Only gastric biopsy differentiates malignant from benign ulcer

GASTRIC ADENOCARCINOMA Gastroscopy l * gastroscopic biopsy and brush cytologymandatory for pts with a gastric ulcer; to identify malignant gastric ulcers before they penetrate into surrounding tissues (rate of cure of lesions limited to mucosa or submucosa > 80%); l as deep as possible, due to submucosal location of lymphoid tumors (GC difficult to distinguish clinically or radiographically from gastric lymphomas) and gastrointestinal stromal tumors (GISTs)
ENDOSCOPIC ULTRASOUND.

INVESTIGATIONS LAPAROSCOPY l Valuable as an initial operative assessment to exclude extensive local disease l Detects small intraperitoneal & liver mets not detected by CT l Combined with Endoscopic USG – most accurate to select patients for potentially curative resection
Other methods 1. USG- to visualize gastric 2. Doppler- to estimate 3. Manometry – by distention and peristalsis flow thru pylorus measuring intragastric pressure, differentiation of mechanical from myopathic and neurological causes of obstruction. An esophageal manometer can also be connected. Electrogastrograp hy 5. MRI- measure frequency 4. , amplitude and velocity of electrogastrgraphy

TREATMENT
; SURGERY

GASTRIC ADENOCARCINOMA Treatment Surgery * surgical removal of complete tumor + adjacent lymph nodes = only chance for cure; l possible in < 30% of pts; l In general; subtotal gastrectomy = treatment of choice for pts with distal GC; l total or near - total gastrectomy for more proximal tumors l

BILLROTH I II l for cancers at distal stomach (connecting with duodenum) → partial gastrectomy, with sparing the valve (cardiac sphincter) between oesophagus and stomach;

BILLROTH I l partial gastectomy; for very small tumors in lower part of stomach (near pylorus) connecting with duodenum, with sparing valve (cardiac sphincter) between esophagus and stomach
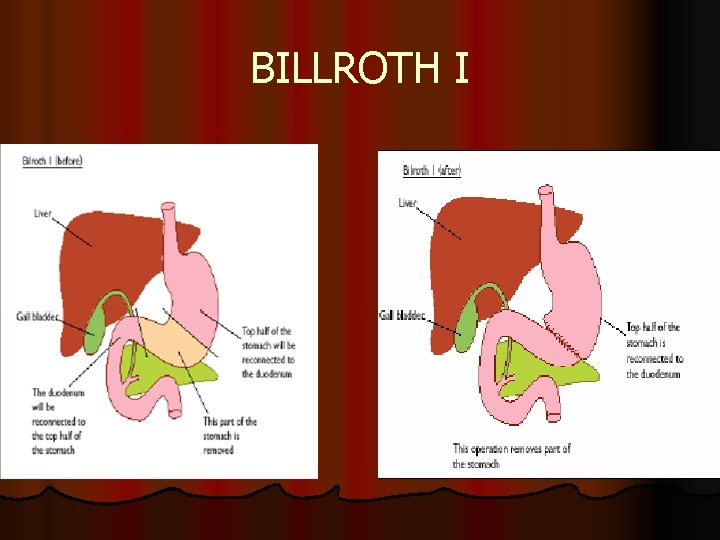
BILLROTH I
BILLROTH II l partial gastectomy (Billroth II), for major tumors in lower part of stomach (near pylorus) connecting with small bowel, still with sparing valve (cardiac sphincter) between oesophagus and stomach
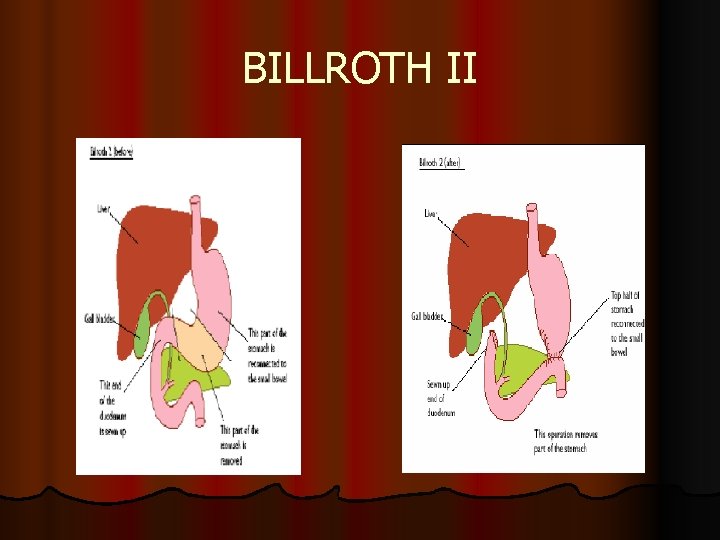
BILLROTH II
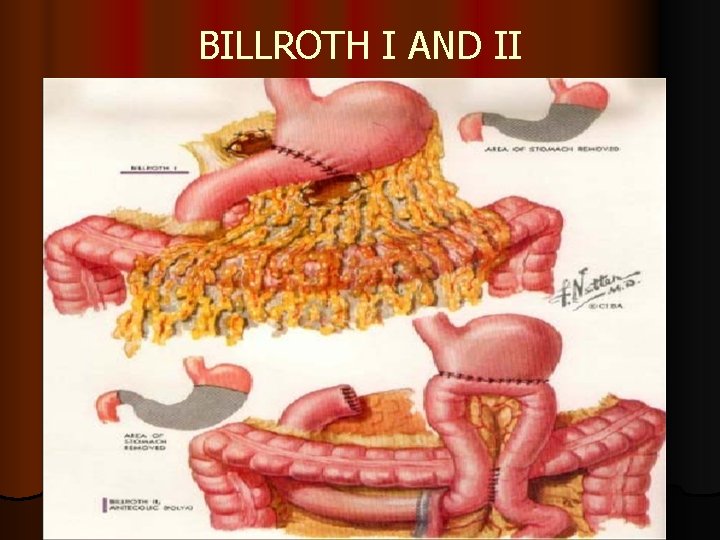
BILLROTH I AND II
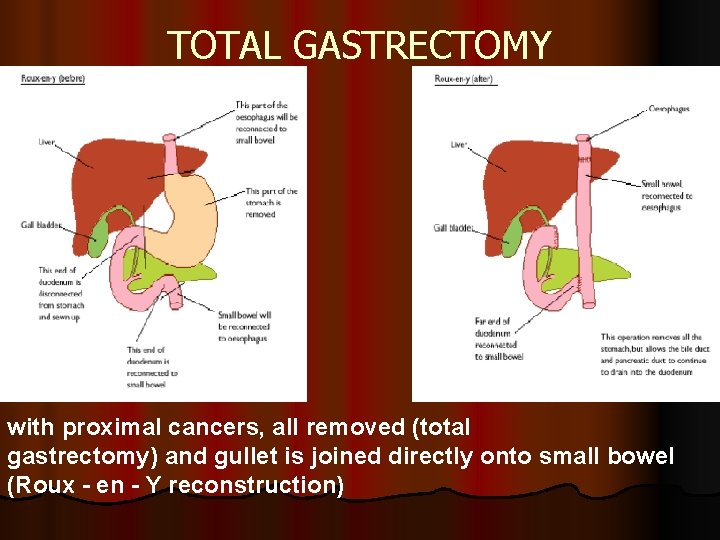
TOTAL GASTRECTOMY with proximal cancers, all removed (total gastrectomy) and gullet is joined directly onto small bowel (Roux - en - Y reconstruction)

R and D dissections Ø Lymph node dissection is denoted using the D descriptor Ø D 0 ---all N 1 nodes have not been completely removed D 1 --- all N 1 nodes removed D 2 --- all N 1 and N 2 nodes are removed D 3 --- all N 3 nodes removed Ø Ø Ø Till about five years back, R was used to denote the degree of resection R 1 – Complete removal of perigastric nodes R 2 – Includes resection of stomach, perigastric and lymph nodes along the named arteries of stomach (Partial N 2) Left gastric Common hepatic R 3 – R 2 + coeliac nodes (complete N 2) R 4 – R 3 + paraaortic nodes
GASTRIC ADENOCARCINOMA Prognosis after surgery l adversely influenced by degree of tumor penetration into stomach wall, regional lymphnode involvement, vascular invasion and abnormal DNA content (i. e. , aneuploidy, occurring in most pts); for < 30% of pts able to undergo a complete resection of GC, 5 - yr survival is 20% for distal tumors and < 10% for proximal tumors l (recurrences occurs for ≥ 8 yrs after surgery)

RADIOTHERAPY GC is a radioresistant tumor (= achieving adequate control of primary tumor requires doses of external beam radiation exceeding tolerance of surrounding structures, such as bowel mucosa and spinal cord); l - palliation of pain = major role of radiation therapy in pts with GC l

CHEMOTHERAPY l combinations of cytotoxic drugs to pts with advanced GC reduces > 50% measurable tumor mass in 30 - 50% of cases ("partial response"), with significant clinical benefit; l - drug combinations generally include 5 – FU (variously with doxorubicin, mitomycin - C, cisplatin, high doses of methotrexate, irinotecan and oxaliplatin);

ADJUVANT CHEMOTHERAPY prophylactic (i. e. , adjuvant) chemotherapy following complete resection of GC (as a mean of eradicating clinically undetectable micrometastases and improving potential for cure possibly unsuccessful: l → adjuvant treatment l preoperative ("neoadjuvant")] chemotherapy l still investigational l

ADJUVANT RADIO –CHEMOTHERAPY l radiation therapy alone after a complete resection does not prolong survival; l however, survival is prolonged when 5 -fluorouracil (5 - FU) is given in combination with radiation therapy (5 – FU) functions as a radiosensitizer.
Palliation l 20 – 30% of gastric cancer presents w/ stage IV disease l Relief of symptoms w/ minimal morbidity l Surgical palliation l Percutaneous, endoscopic, radiotherapuetic techniques l Nonoperative tx l Laser recanalization, endoscopic dilatation
Recurrence After gastrectomy quite high 40 – 80 % l Most occur w/in first 3 years l Anastomosis, gastric bed and regional nodes l Peritoneal dissemination – 54% l

POST OPERATIVE COMPLICATIONS l Leakage of oesophago-jejunostomy l Fistula l Leakage from duodenalstump l Biliary peritonitis l Sec. haemorrhage l loss of parietal cells – Vit. B 12 deficiency

THANK YOU
- Slides: 51