GASPHASE MOLECULAR STRUCTURE OF NOPINONE AND ITS WATER





- Slides: 18

GAS-PHASE MOLECULAR STRUCTURE OF NOPINONE AND ITS WATER COMPLEXES STUDIED BY MICROWAVE FOURIER TRANSFORM SPECTROSCOPY AND QUANTUM CHEMICAL CALCULATIONS Elias M. NEEMAN, J. -R Aviles Moreno, Therese R. HUET Laboratoire Ph. LAM, UMR 8523 CNRS - Université Lille 1, Villeneuve d’Ascq, France. http: //www-phlam. univ-lille 1. fr Elias. Neeman@univ-lille 1. fr ISMS - 71 st Meeting - June 20 -24, 2016 - Champaign-Urbana, Illinois 1

Outline Introduction FTMW spectrometer in Lille Theoretical methodology Results: • Nopinone-Water complexes Conclusions and perspectives ISMS - 71 st Meeting - June 20 -24, 2016 - Champaign-Urbana, Illinois 2

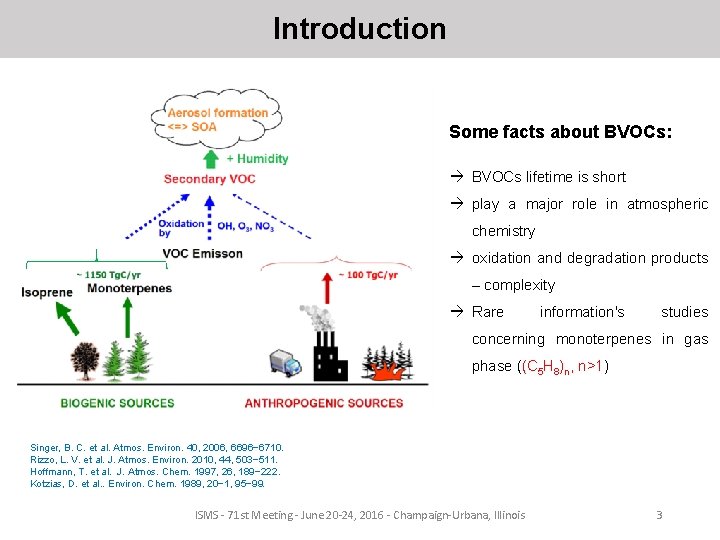
Introduction Some facts about BVOCs: à BVOCs lifetime is short à play a major role in atmospheric chemistry à oxidation and degradation products – complexity à Rare information's studies concerning monoterpenes in gas phase ((C 5 H 8)n, n>1) Singer, B. C. et al. Atmos. Environ. 40, 2006, 6696− 6710. Rizzo, L. V. et al. J. Atmos. Environ. 2010, 44, 503− 511. Hoffmann, T. et al. J. Atmos. Chem. 1997, 26, 189− 222. Kotzias, D. et al. . Environ. Chem. 1989, 20− 1, 95− 99. ISMS - 71 st Meeting - June 20 -24, 2016 - Champaign-Urbana, Illinois 3

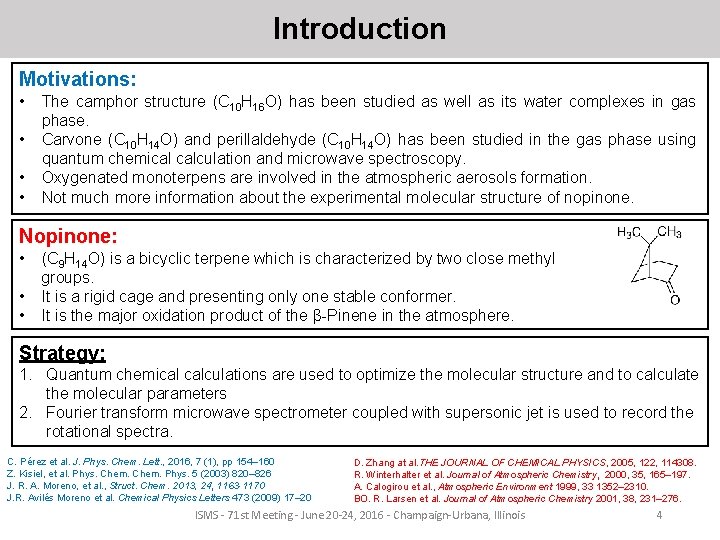
Introduction Motivations: • • The camphor structure (C 10 H 16 O) has been studied as well as its water complexes in gas phase. Carvone (C 10 H 14 O) and perillaldehyde (C 10 H 14 O) has been studied in the gas phase using quantum chemical calculation and microwave spectroscopy. Oxygenated monoterpens are involved in the atmospheric aerosols formation. Not much more information about the experimental molecular structure of nopinone. Nopinone: • • • (C 9 H 14 O) is a bicyclic terpene which is characterized by two close methyl groups. It is a rigid cage and presenting only one stable conformer. It is the major oxidation product of the β-Pinene in the atmosphere. Strategy: 1. Quantum chemical calculations are used to optimize the molecular structure and to calculate the molecular parameters 2. Fourier transform microwave spectrometer coupled with supersonic jet is used to record the rotational spectra. C. Pérez et al. J. Phys. Chem. Lett. , 2016, 7 (1), pp 154– 160 Z. Kisiel, et al. Phys. Chem. Phys. 5 (2003) 820– 826 J. R. A. Moreno, et al. , Struct. Chem. 2013, 24, 1163 -1170 J. R. Avilés Moreno et al. Chemical Physics Letters 473 (2009) 17– 20 D. Zhang at al. THE JOURNAL OF CHEMICAL PHYSICS, 2005, 122, 114308. R. Winterhalter et al. Journal of Atmospheric Chemistry, 2000, 35, 165– 197. A. Calogirou et al. , Atmospheric Environment 1999, 33 1352– 2310. BO. R. Larsen et al. Journal of Atmospheric Chemistry 2001, 38, 231– 276. ISMS - 71 st Meeting - June 20 -24, 2016 - Champaign-Urbana, Illinois 4

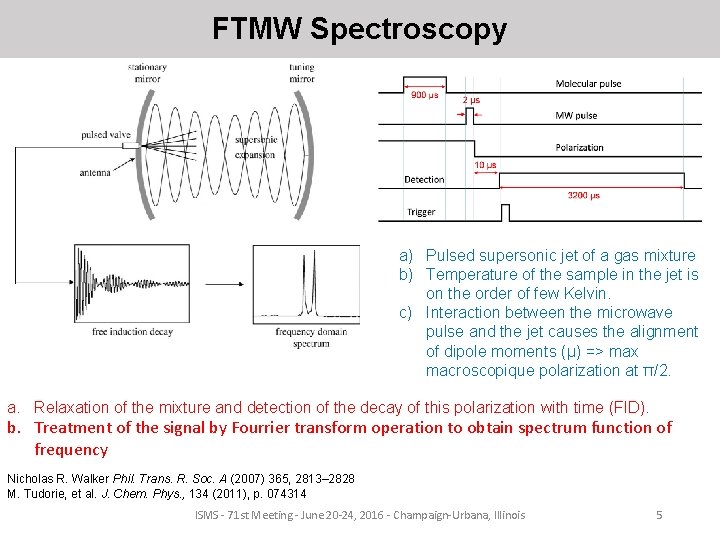
FTMW Spectroscopy a) Pulsed supersonic jet of a gas mixture b) Temperature of the sample in the jet is on the order of few Kelvin. c) Interaction between the microwave pulse and the jet causes the alignment of dipole moments (μ) => max macroscopique polarization at π/2. a. Relaxation of the mixture and detection of the decay of this polarization with time (FID). b. Treatment of the signal by Fourrier transform operation to obtain spectrum function of frequency Nicholas R. Walker Phil. Trans. R. Soc. A (2007) 365, 2813– 2828 M. Tudorie, et al. J. Chem. Phys. , 134 (2011), p. 074314 ISMS - 71 st Meeting - June 20 -24, 2016 - Champaign-Urbana, Illinois 5

Nopinone ISMS - 71 st Meeting - June 20 -24, 2016 Champaign-Urbana, Illinois 6

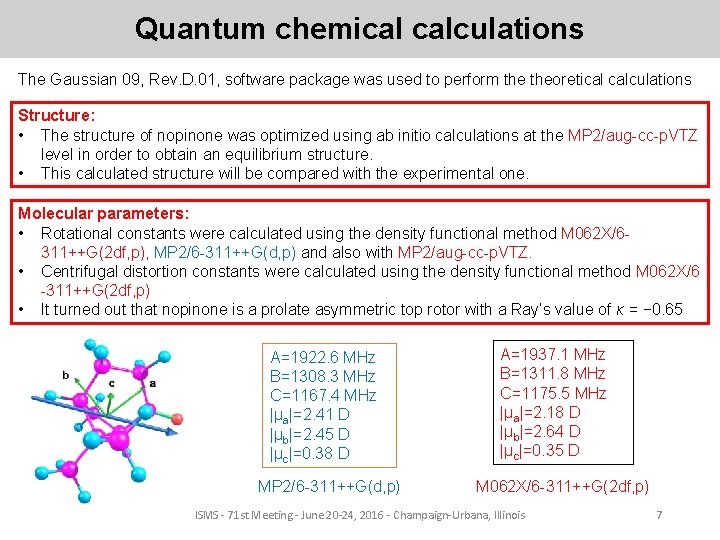
Quantum chemical calculations The Gaussian 09, Rev. D. 01, software package was used to perform theoretical calculations Structure: • The structure of nopinone was optimized using ab initio calculations at the MP 2/aug-cc-p. VTZ level in order to obtain an equilibrium structure. • This calculated structure will be compared with the experimental one. Molecular parameters: • Rotational constants were calculated using the density functional method M 062 X/6311++G(2 df, p), MP 2/6 -311++G(d, p) and also with MP 2/aug-cc-p. VTZ. • Centrifugal distortion constants were calculated using the density functional method M 062 X/6 -311++G(2 df, p) • It turned out that nopinone is a prolate asymmetric top rotor with a Ray’s value of κ = − 0. 65 A=1922. 6 MHz B=1308. 3 MHz C=1167. 4 MHz |μa|=2. 41 D |μb|=2. 45 D |μc|=0. 38 D MP 2/6 -311++G(d, p) A=1937. 1 MHz B=1311. 8 MHz C=1175. 5 MHz |μa|=2. 18 D |μb|=2. 64 D |μc|=0. 35 D M 062 X/6 -311++G(2 df, p) ISMS - 71 st Meeting - June 20 -24, 2016 - Champaign-Urbana, Illinois 7

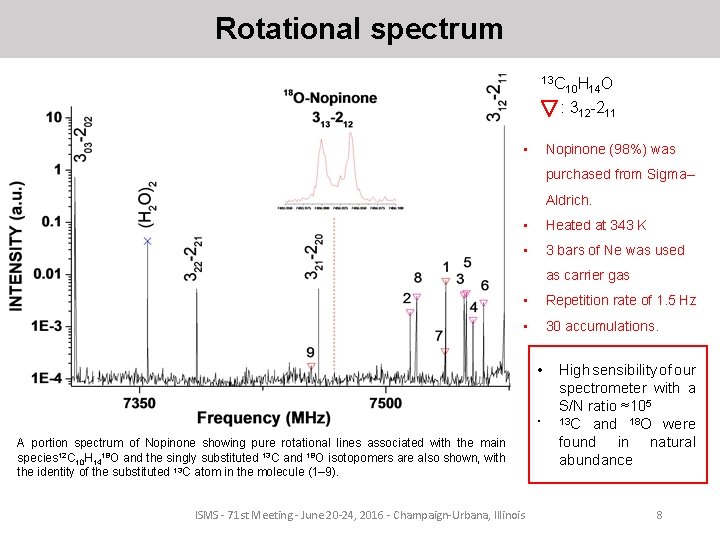
Rotational spectrum 13 C 10 H 14 O : 312 -211 • Nopinone (98%) was purchased from Sigma– Aldrich. • Heated at 343 K • 3 bars of Ne was used as carrier gas • Repetition rate of 1. 5 Hz • 30 accumulations. • • A portion spectrum of Nopinone showing pure rotational lines associated with the main species 12 C 10 H 1418 O and the singly substituted 13 C and 18 O isotopomers are also shown, with the identity of the substituted 13 C atom in the molecule (1– 9). ISMS - 71 st Meeting - June 20 -24, 2016 - Champaign-Urbana, Illinois High sensibility of our spectrometer with a S/N ratio ≈105 13 C and 18 O were found in natural abundance 8

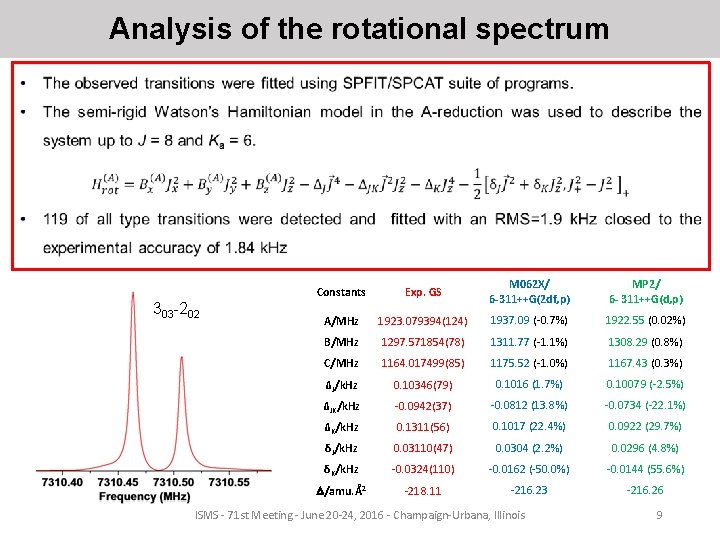
Analysis of the rotational spectrum 303 -202 Constants Exp. GS M 062 X/ 6 -311++G(2 df, p) MP 2/ 6 - 311++G(d, p) A/MHz 1923. 079394(124) 1937. 09 (-0. 7%) 1922. 55 (0. 02%) B/MHz 1297. 571854(78) 1311. 77 (-1. 1%) 1308. 29 (0. 8%) C/MHz 1164. 017499(85) 1175. 52 (-1. 0%) 1167. 43 (0. 3%) ΔJ/k. Hz 0. 10346(79) 0. 1016 (1. 7%) 0. 10079 (-2. 5%) ΔJK/k. Hz -0. 0942(37) -0. 0812 (13. 8%) -0. 0734 (-22. 1%) ΔK/k. Hz 0. 1311(56) 0. 1017 (22. 4%) 0. 0922 (29. 7%) δJ/k. Hz 0. 03110(47) 0. 0304 (2. 2%) 0. 0296 (4. 8%) δK/k. Hz -0. 0324(110) -0. 0162 (-50. 0%) -0. 0144 (55. 6%) /amu. Å2 -218. 11 -216. 23 -216. 26 ISMS - 71 st Meeting - June 20 -24, 2016 - Champaign-Urbana, Illinois 9

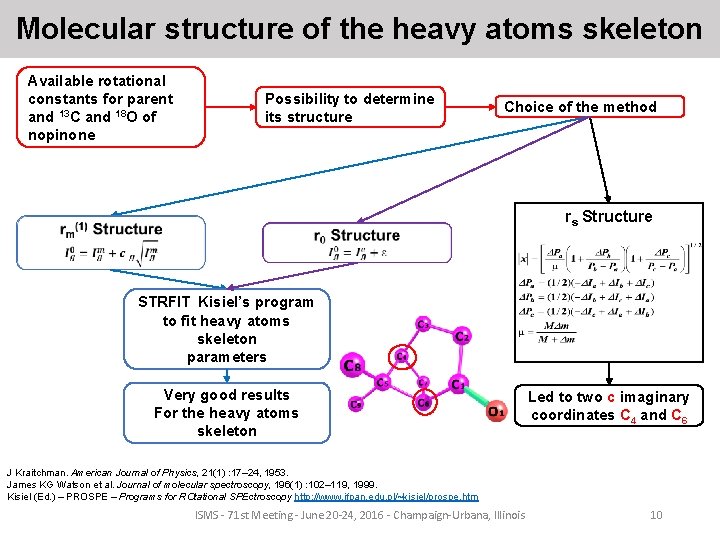
Molecular structure of the heavy atoms skeleton Available rotational constants for parent and 13 C and 18 O of nopinone Possibility to determine its structure Choice of the method rs Structure STRFIT Kisiel’s program to fit heavy atoms skeleton parameters Very good results For the heavy atoms skeleton Led to two c imaginary coordinates C 4 and C 6 J Kraitchman. American Journal of Physics, 21(1) : 17– 24, 1953. James KG Watson et al. Journal of molecular spectroscopy, 196(1) : 102– 119, 1999. Kisiel (Ed. ) – PROSPE – Programs for ROtational SPEctroscopy http: //www. ifpan. edu. pl/~kisiel/prospe. htm ISMS - 71 st Meeting - June 20 -24, 2016 - Champaign-Urbana, Illinois 10

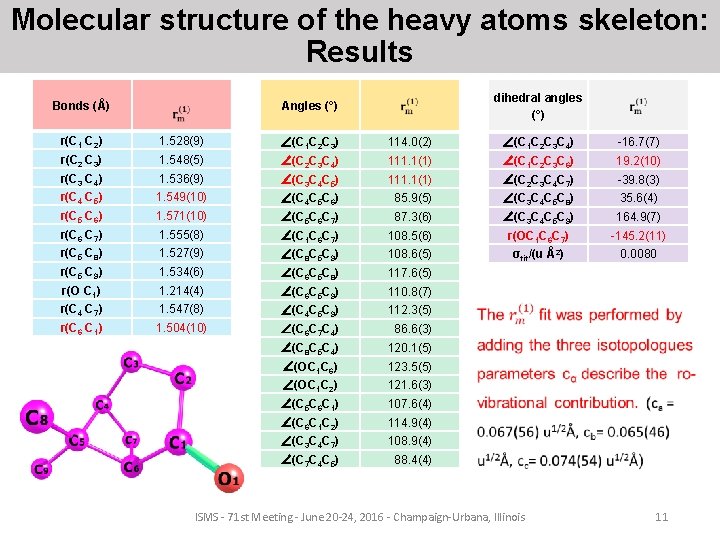
Molecular structure of the heavy atoms skeleton: Results Bonds (Å) dihedral angles (°) Angles (°) r(C 1 C 2) 1. 528(9) ∠(C 1 C 2 C 3) 114. 0(2) ∠(C 1 C 2 C 3 C 4) -16. 7(7) r(C 2 C 3) 1. 548(5) ∠(C 2 C 3 C 4) 111. 1(1) ∠(C 1 C 2 C 3 C 6) 19. 2(10) r(C 3 C 4) 1. 536(9) ∠(C 3 C 4 C 5) 111. 1(1) ∠(C 2 C 3 C 4 C 7) -39. 8(3) r(C 4 C 5) 1. 549(10) ∠(C 4 C 5 C 6) 85. 9(5) ∠(C 3 C 4 C 5 C 8) 35. 6(4) r(C 5 C 6) 1. 571(10) ∠(C 5 C 6 C 7) 87. 3(6) ∠(C 3 C 4 C 5 C 9) 164. 9(7) r(C 6 C 7) 1. 555(8) ∠(C 1 C 6 C 7) 108. 5(6) r(OC 1 C 6 C 7) -145. 2(11) r(C 5 C 8) 1. 527(9) ∠(C 8 C 5 C 9) 108. 6(5) σfit/(u Å2) 0. 0080 r(C 5 C 9) 1. 534(6) ∠(C 6 C 5 C 8) 117. 6(5) r(O C 1) 1. 214(4) ∠(C 6 C 5 C 9) 110. 8(7) r(C 4 C 7) 1. 547(8) ∠(C 4 C 5 C 9) 112. 3(5) r(C 6 C 1) 1. 504(10) ∠(C 6 C 7 C 4) 86. 6(3) ∠(C 8 C 5 C 4) 120. 1(5) ∠(OC 1 C 6) 123. 5(5) ∠(OC 1 C 2) 121. 6(3) ∠(C 5 C 6 C 1) 107. 6(4) ∠(C 6 C 1 C 2) 114. 9(4) ∠(C 3 C 4 C 7) 108. 9(4) ∠(C 7 C 4 C 5) 88. 4(4) ISMS - 71 st Meeting - June 20 -24, 2016 - Champaign-Urbana, Illinois 11

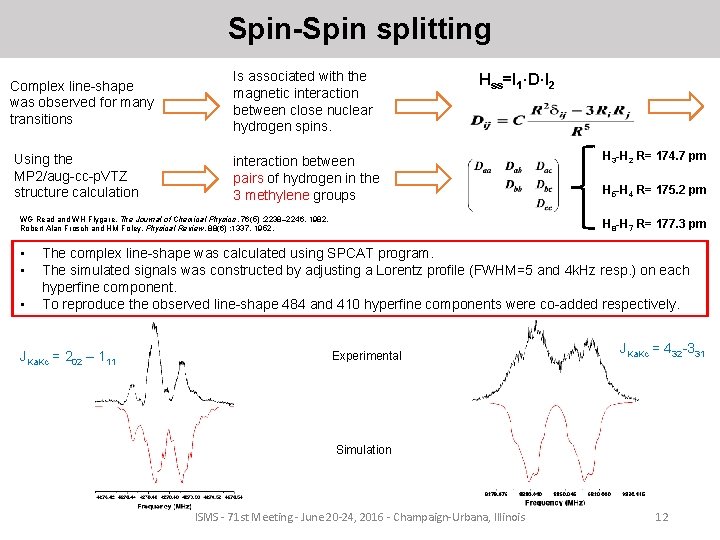
Spin-Spin splitting Complex line-shape was observed for many transitions Using the MP 2/aug-cc-p. VTZ structure calculation Is associated with the magnetic interaction between close nuclear hydrogen spins. Hss=I 1·D·I 2 interaction between pairs of hydrogen in the 3 methylene groups WG Read and WH Flygare, The Journal of Chemical Physics, 76(5) : 2238– 2246, 1982. Robert Alan Frosch and HM Foley. Physical Review, 88(6) : 1337, 1952. • • • H 3 -H 2 R= 174. 7 pm H 5 -H 4 R= 175. 2 pm H 8 -H 7 R= 177. 3 pm The complex line-shape was calculated using SPCAT program. The simulated signals was constructed by adjusting a Lorentz profile (FWHM=5 and 4 k. Hz resp. ) on each hyperfine component. To reproduce the observed line-shape 484 and 410 hyperfine components were co-added respectively. JKa. Kc = 202 – 111 Experimental JKa. Kc = 432 -331 Simulation ISMS - 71 st Meeting - June 20 -24, 2016 - Champaign-Urbana, Illinois 12

Nopinone-water complexes ISMS - 71 st Meeting - June 20 -24, 2016 - Champaign-Urbana, Illinois 13

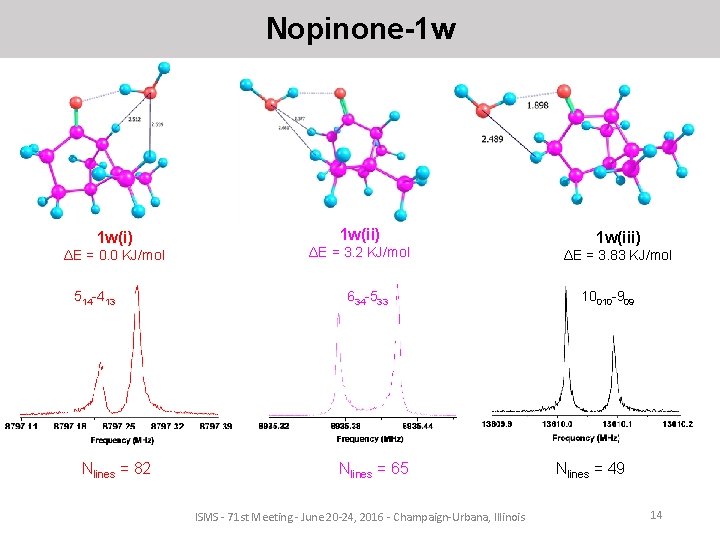
Nopinone-1 w 1 w(i) ΔE = 0. 0 KJ/mol 514 -413 Nlines = 82 1 w(ii) ΔE = 3. 2 KJ/mol 634 -533 Nlines = 65 ISMS - 71 st Meeting - June 20 -24, 2016 - Champaign-Urbana, Illinois 1 w(iii) ΔE = 3. 83 KJ/mol 10010 -909 Nlines = 49 14

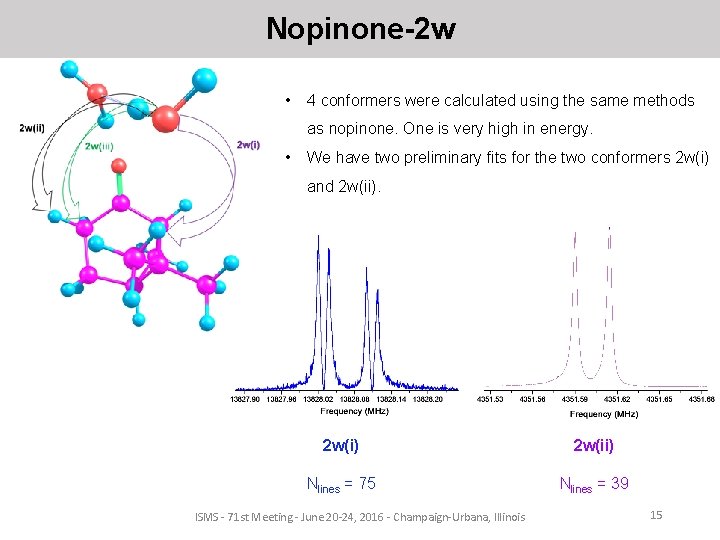
Nopinone-2 w • 4 conformers were calculated using the same methods as nopinone. One is very high in energy. • We have two preliminary fits for the two conformers 2 w(i) and 2 w(ii). 2 w(i) 2 w(ii) Nlines = 75 Nlines = 39 ISMS - 71 st Meeting - June 20 -24, 2016 - Champaign-Urbana, Illinois 15

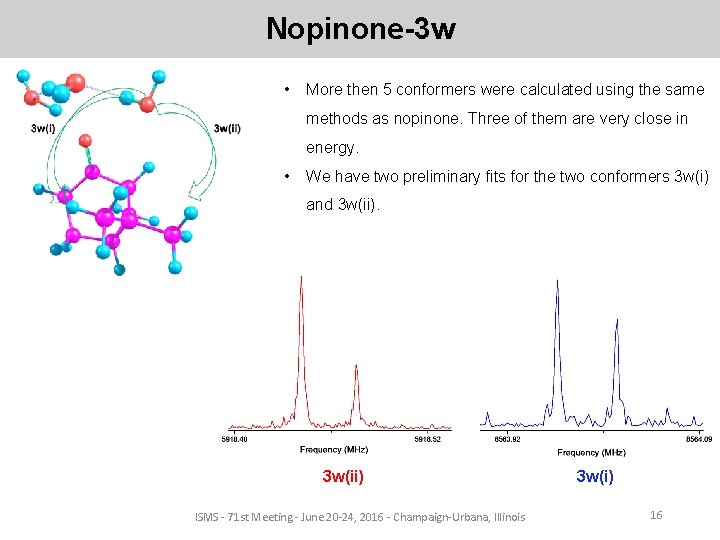
Nopinone-3 w • More then 5 conformers were calculated using the same methods as nopinone. Three of them are very close in energy. • We have two preliminary fits for the two conformers 3 w(i) and 3 w(ii) ISMS - 71 st Meeting - June 20 -24, 2016 - Champaign-Urbana, Illinois 3 w(i) 16

Conclusion • Microwave spectroscopy combined with quantum chemistry calculations are powerful tools for studying isolated and wet molecules in the gas phase. • We have obtained molecular parameters of the nopinone and of all its isotopemers 13 C and 18 O. • We have determine using different methods the Nopinone structure. • We have evidenced the spin-spin structure associated with the hydrogens atoms of the 3 CH 2 groups. • We have studied nopinone-water complexes: 3 monohydrates are identified, we also have two preliminary fits for 2 dihydrates and 2 trihydrates of nopinone. ISMS - 71 st Meeting - June 20 -24, 2016 - Champaign-Urbana, Illinois 17

The present work was funded by the French ANR Labex Ca. PPA through the PIA under contract ANR-11 -LABX-0005 -01, by the Regional Council Nord-Pas-de-Calais-Picardie and by the European Funds for Regional Economic Development (FEDER). THANK YOU FOR YOUR ATTENTION