Gases Particles in a Solid Liquid and Gas











- Slides: 39

Gases

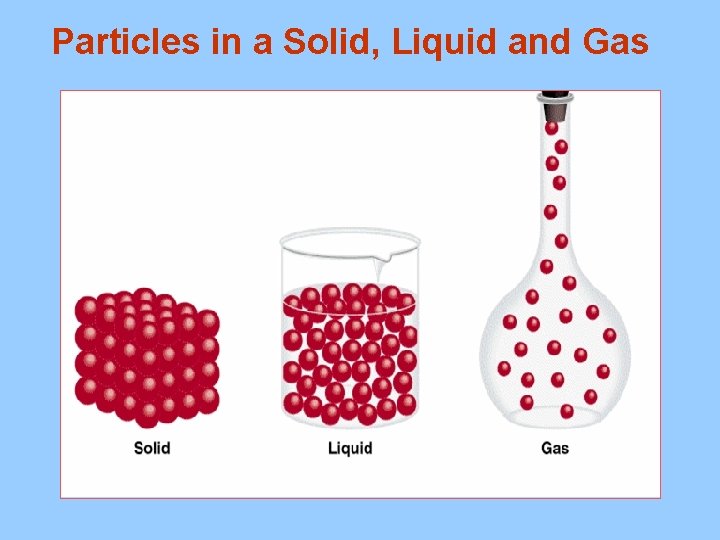
Particles in a Solid, Liquid and Gas

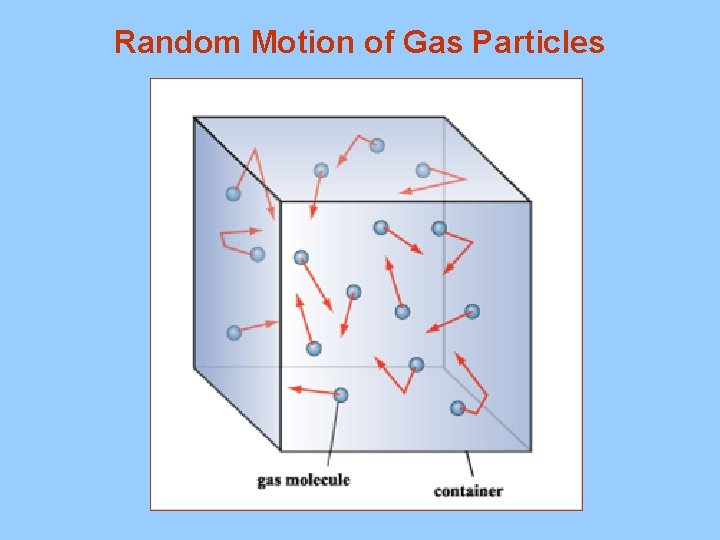
Random Motion of Gas Particles

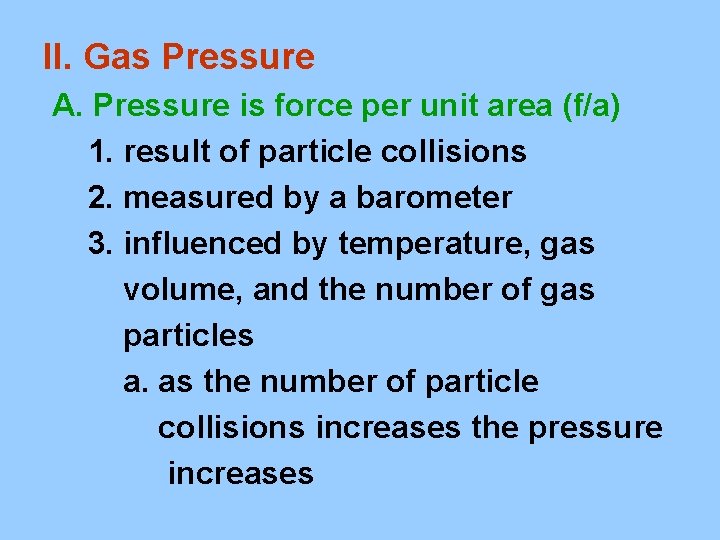
II. Gas Pressure A. Pressure is force per unit area (f/a) 1. result of particle collisions 2. measured by a barometer 3. influenced by temperature, gas volume, and the number of gas particles a. as the number of particle collisions increases the pressure increases

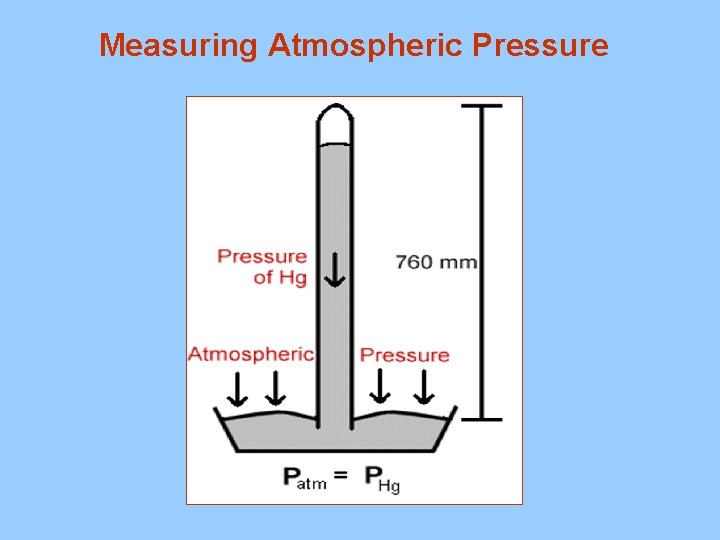
Pressure at Sea Level 14. 7 psi = 1. 0 atm = 760 mm of Hg = 750 Torr = 101. 3 k. Pa = 1, 013 mbars

I. Kinetic Theory A. Assumptions 1. gas particles do not attract each other 2. gas particles are very small 3. particles are very far apart 4. constant, random motion 5. elastic collisions 6. kinetic energy varies with temperature

B. Properties of Gases 1. low density (grams/liter) 2. can expand can be compressed 3. can diffuse and effuse a. rate related to molar mass b. diffusion is the movement of particles from an area of greater concentration to an area of lesser concentration c. effusion is the movement of gas particles through a small opening

Measuring Atmospheric Pressure

Aneroid Baramoter Mercury Barometer

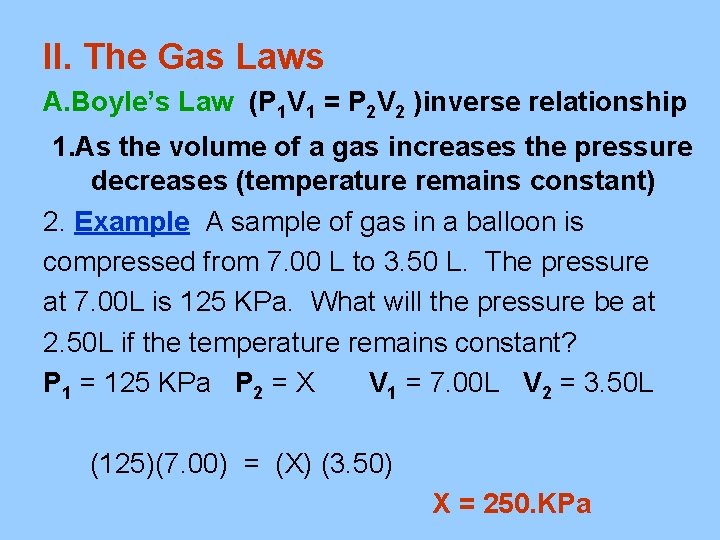
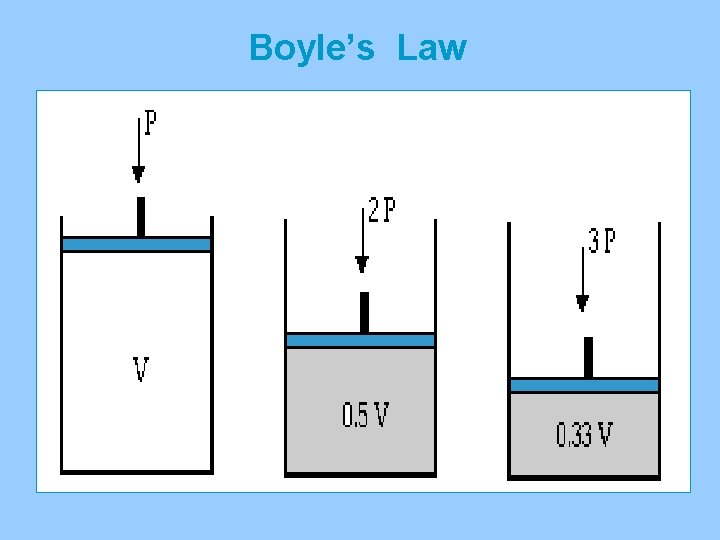
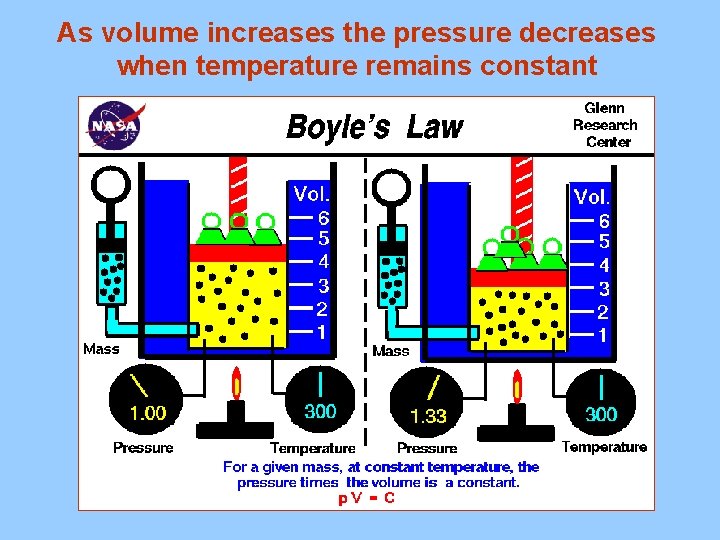
II. The Gas Laws A. Boyle’s Law (P 1 V 1 = P 2 V 2 )inverse relationship 1. As the volume of a gas increases the pressure decreases (temperature remains constant) 2. Example A sample of gas in a balloon is compressed from 7. 00 L to 3. 50 L. The pressure at 7. 00 L is 125 KPa. What will the pressure be at 2. 50 L if the temperature remains constant? P 1 = 125 KPa P 2 = X V 1 = 7. 00 L V 2 = 3. 50 L (125)(7. 00) = (X) (3. 50) X = 250. KPa

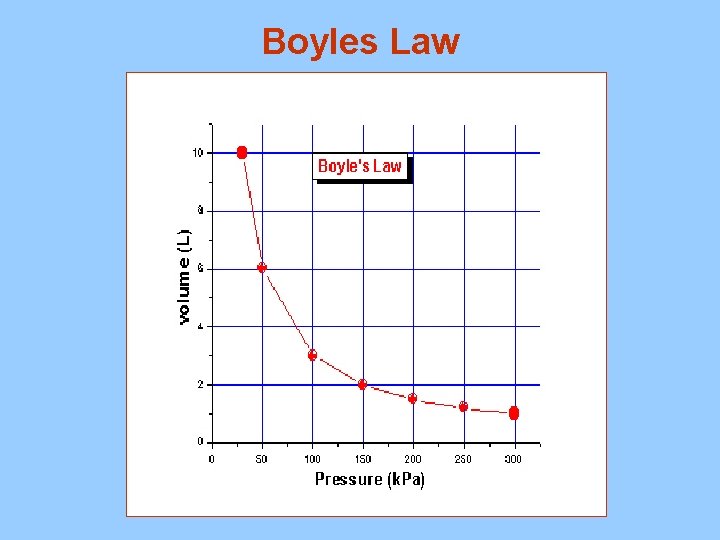
Boyle’s Law

As volume increases the pressure decreases when temperature remains constant

Boyles Law

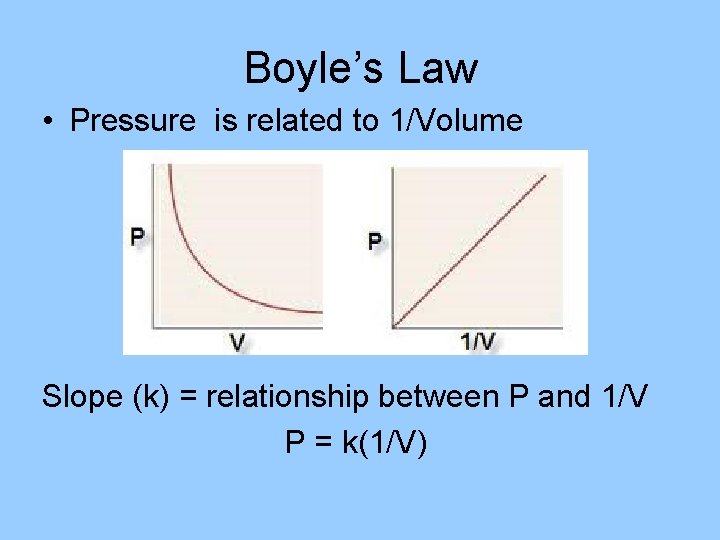
Boyle’s Law • Pressure is related to 1/Volume Slope (k) = relationship between P and 1/V P = k(1/V)

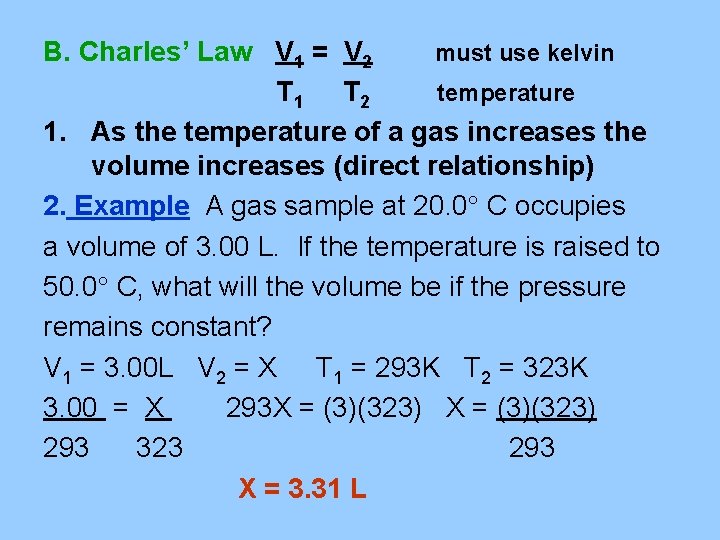
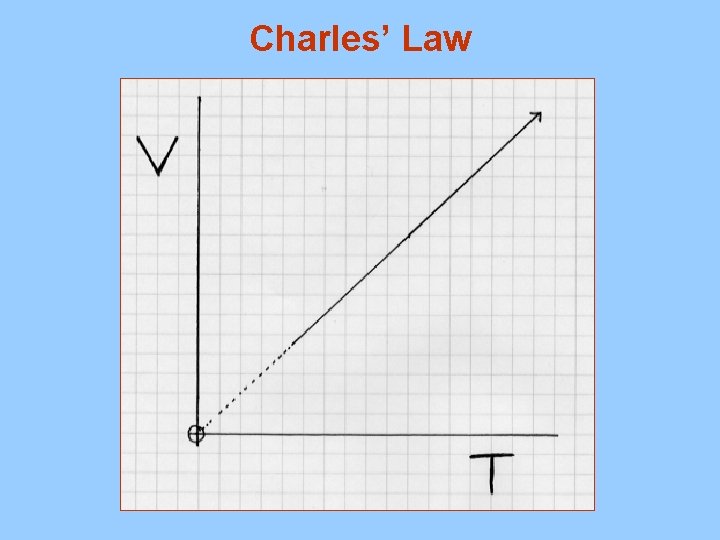
B. Charles’ Law V 1 = V 2 must use kelvin T 1 T 2 temperature 1. As the temperature of a gas increases the volume increases (direct relationship) 2. Example A gas sample at 20. 0 C occupies a volume of 3. 00 L. If the temperature is raised to 50. 0 C, what will the volume be if the pressure remains constant? V 1 = 3. 00 L V 2 = X T 1 = 293 K T 2 = 323 K 3. 00 = X 293 X = (3)(323) 293 323 293 X = 3. 31 L

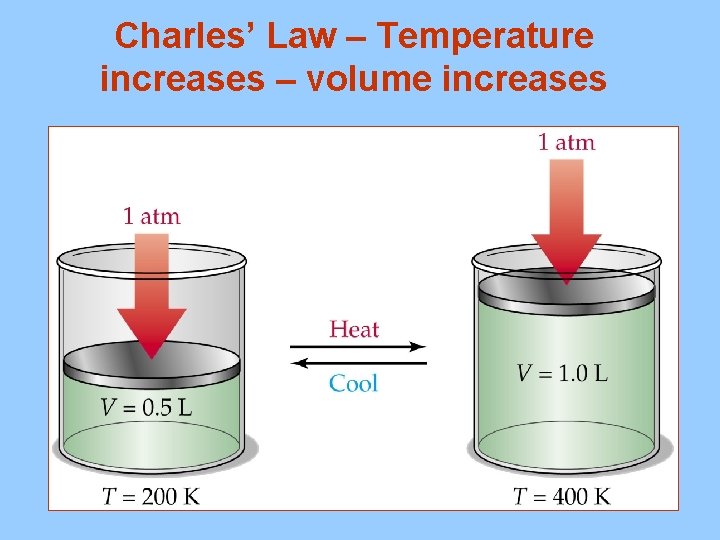
Charles’ Law – Temperature increases – volume increases

Charles’ Law

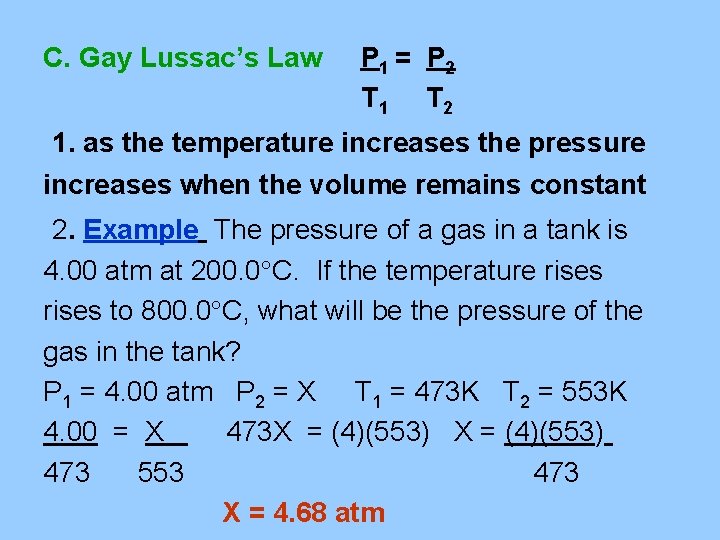
C. Gay Lussac’s Law P 1 = P 2 T 1 T 2 1. as the temperature increases the pressure increases when the volume remains constant 2. Example The pressure of a gas in a tank is 4. 00 atm at 200. 0 C. If the temperature rises to 800. 0 C, what will be the pressure of the gas in the tank? P 1 = 4. 00 atm P 2 = X T 1 = 473 K T 2 = 553 K 4. 00 = X 473 X = (4)(553) 473 553 473 X = 4. 68 atm

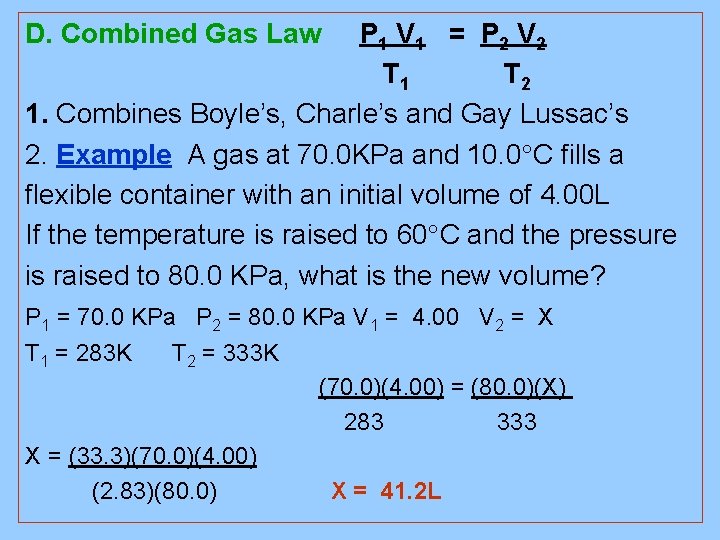
D. Combined Gas Law P 1 V 1 = P 2 V 2 T 1 T 2 1. Combines Boyle’s, Charle’s and Gay Lussac’s 2. Example A gas at 70. 0 KPa and 10. 0 C fills a flexible container with an initial volume of 4. 00 L If the temperature is raised to 60 C and the pressure is raised to 80. 0 KPa, what is the new volume? P 1 = 70. 0 KPa P 2 = 80. 0 KPa V 1 = 4. 00 V 2 = X T 1 = 283 K T 2 = 333 K (70. 0)(4. 00) = (80. 0)(X) 283 333 X = (33. 3)(70. 0)(4. 00) (2. 83)(80. 0) X = 41. 2 L

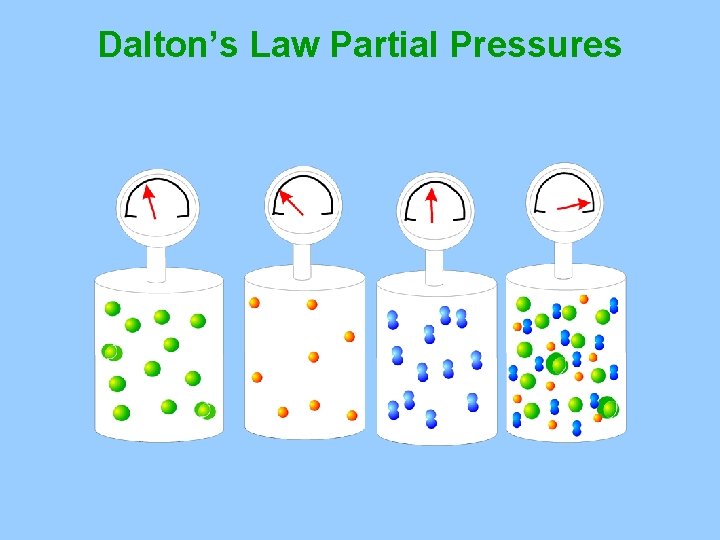
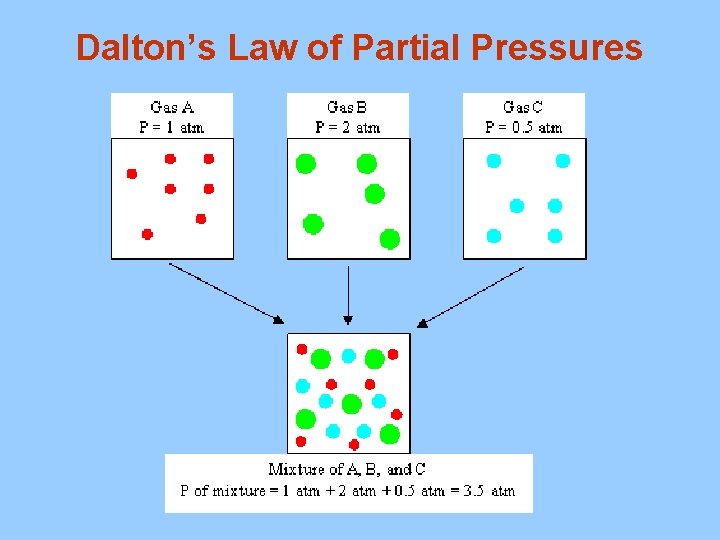
E. Dalton’s Law of Partial Pressures Ptotal = P 1 + P 2 + P 3 +. . . Pn The total pressure of a mixture of gases is equal to the sum of the pressures of all the gases in the mixture 1. Example Find the total pressure for a mixture that contains four gases with partial pressures of 5. 00 k. Pa, 4. 56 k. Pa, 3. 02 k. Pa and 1. 20 k. Pa.

Dalton’s Law Partial Pressures

Dalton’s Law of Partial Pressures

2. Suppose two gases in a container have a total pressure of 1. 20 atm. What is the pressure of gas B if the partial pressure of gas A is 0. 75 atm? 3. What is the partial pressure of hydrogen gas in a mixture of hydrogen and helium if the total pressure is 600. 0 mm. Hg and the partial pressure of helium is 439 mm. Hg?

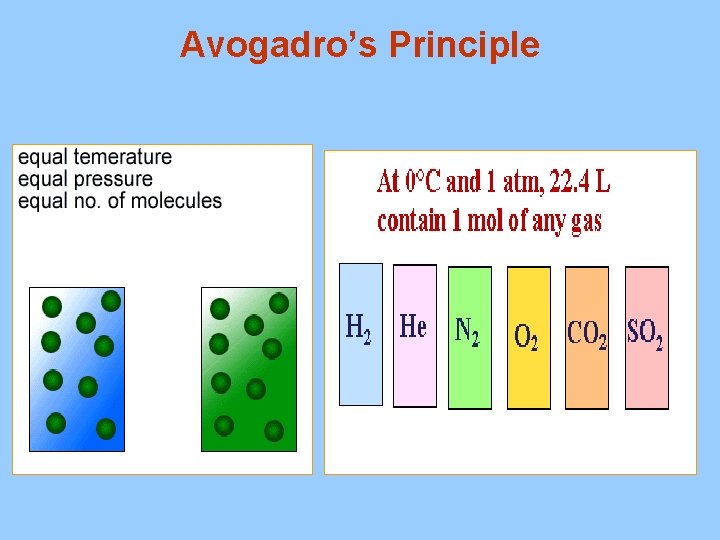
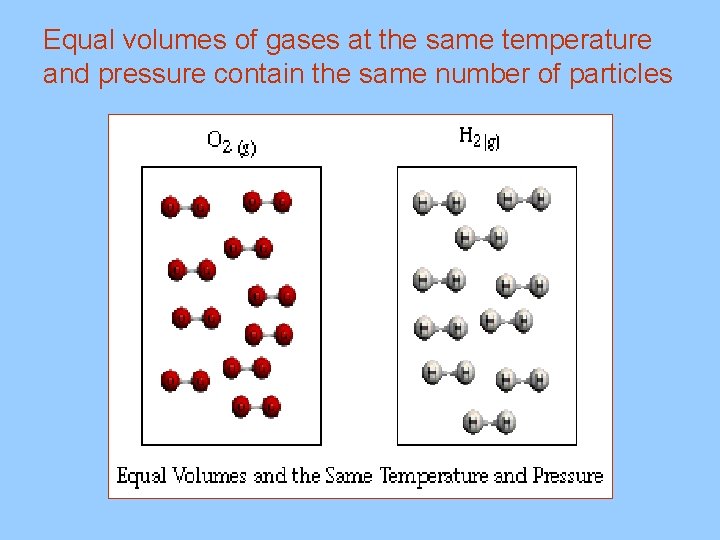
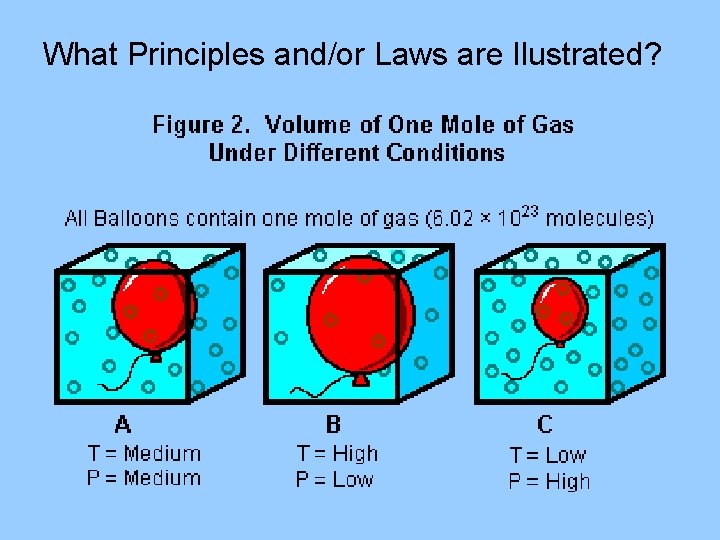
III. Avogadro’s Principle A. Equal volumes of gases at the same temperature and pressure have the same number of particles B. Molar Volume (22. 4 L at STP) 1. volume of one mole of gas particles at STP(standard temperature and pressure) 0 C and 1. 00 atm (760 mm Hg) * 1 mole of any gas at STP = 22. 4 L 2. conversion factors 1 mol 22. 4 L 1 mol

Avogadro’s Principle

Equal volumes of gases at the same temperature and pressure contain the same number of particles

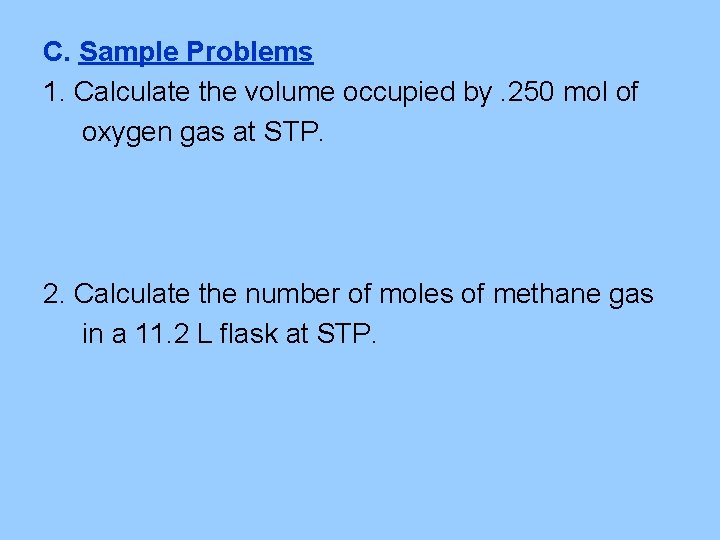
C. Sample Problems 1. Calculate the volume occupied by. 250 mol of oxygen gas at STP. 2. Calculate the number of moles of methane gas in a 11. 2 L flask at STP.

3. Calculate the volume of 88. 0 g of CO 2 at STP. 4. How many grams of He are found in a 5. 60 L balloon at STP?

5. Calculate the density of H 2 at STP. D = molar mass molar volume 6. Calculate the molar mass of a gas that has a density of 3. 2 g/L.

IV. Ideal vs Real Gases A. Ideal compared to Real Gases 1. ideal gas a) particles do not have volume b) there are no intermolecular attractions c) all particle collisions are elastic d) obey all kinetic theory assumptions

2. real gases behave like ideal gases except when a) pressure is very high b) temperatures are low c) molecules are very large d) spaces between particles is small (small volume)

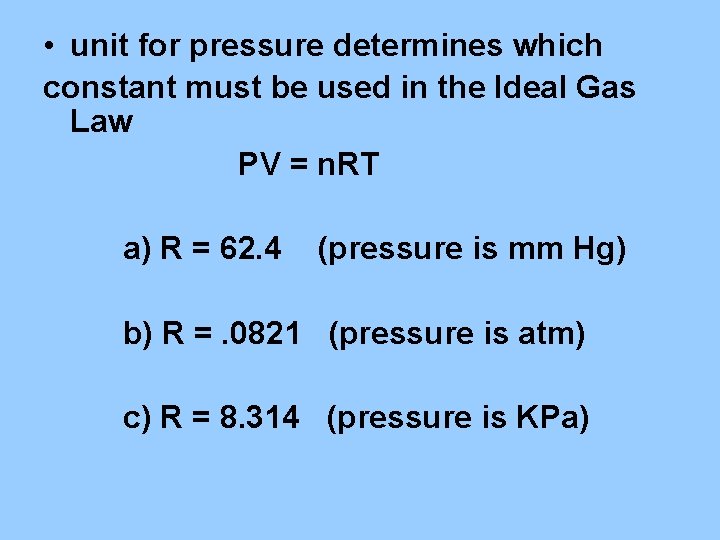
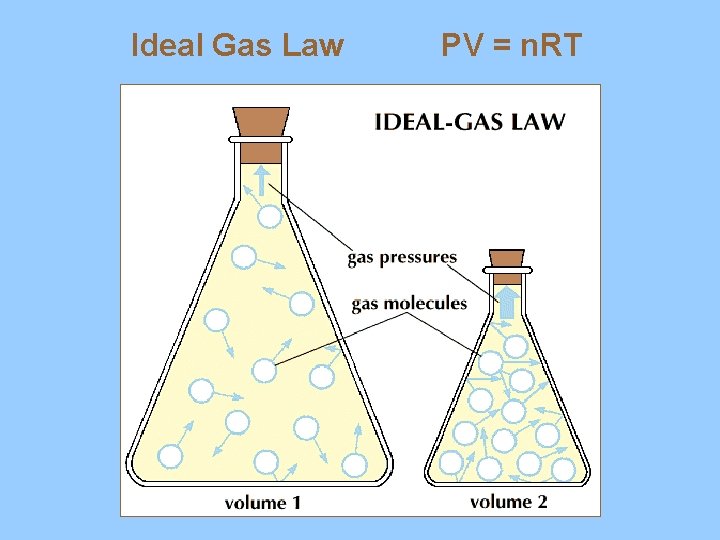
B. Ideal Gas Law - PV = n. RT 1. pressure ( atm, mm Hg, KPa) 2. volume (liters) 3. temperature (kelvin) 4. number of moles (n) 5. R = constant (L) (pressure unit*) (mol) (K)

• unit for pressure determines which constant must be used in the Ideal Gas Law PV = n. RT a) R = 62. 4 (pressure is mm Hg) b) R =. 0821 (pressure is atm) c) R = 8. 314 (pressure is KPa)

Ideal Gas Law PV = n. RT

What Principles and/or Laws are Ilustrated?

C. Application Problems (PV = n. RT) 1. How many moles of O 2 are in a 2. 00 L container at 2. 00 atm pressure and 200 K? 2. Calculate the volume occupied by 2. 00 mol of N 2 at 300 K and. 800 atm pressure.

3. What is the pressure in mm Hg of. 200 moles of gas in a 5. 00 L container at 27 C? 4. Calculate the number of grams of oxygen in a 4. 00 L sample of gas at 1. 00 atm and 27 C.

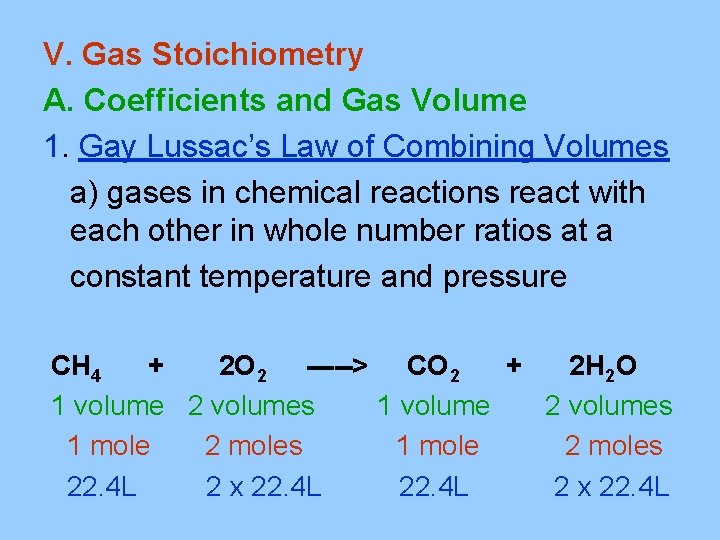
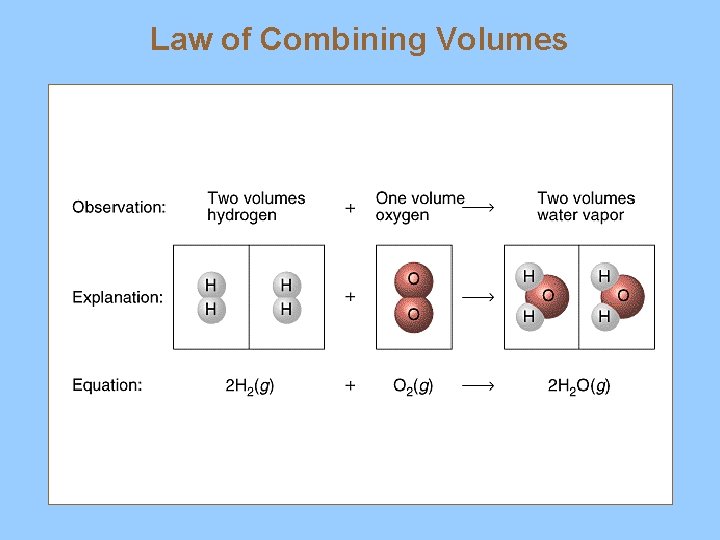
V. Gas Stoichiometry A. Coefficients and Gas Volume 1. Gay Lussac’s Law of Combining Volumes a) gases in chemical reactions react with each other in whole number ratios at a constant temperature and pressure CH 4 + 2 O 2 -----> CO 2 + 2 H 2 O 1 volume 2 volumes 1 mole 2 moles 22. 4 L 2 x 22. 4 L

Law of Combining Volumes