Gases Kinetic Molecular Theory The kinetic molecular theory




- Slides: 13

Gases

Kinetic Molecular Theory The kinetic molecular theory is used to explain the behavior of gases. • All matter is made up of particles called atoms. • The atoms are in constant motion. • Collisions between the particles are elastic (no energy is gained or lost due to the collision).

Properties used to describe gases. The following four properties are used to describe gases: • Temperature (o. C or K)-how fast the particles making up the gas are moving. • Volume (L)-the amount of space the gas occupies (usually the size of the container) • Amount of sample (moles)-# of particles in the sample • Pressure (atm, k. Pa, mm Hg, torrs)

Pressure • Pressure is the force that is exerted on a surface. • P = F/A • Units for pressure are N/m 2 • One pascal (Pa) = 1 N/m 2

Gases and Pressure • Gases exert pressure due to the collisions between the molecules and the walls of the container. • The atmosphere exerts a pressure of 14. 7 lbs/in 2. • Gravity acts as the atmosphere’s container.

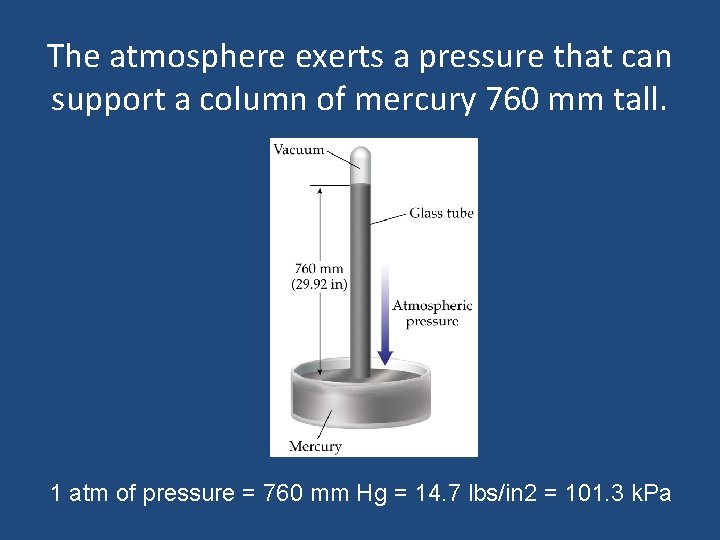
The atmosphere exerts a pressure that can support a column of mercury 760 mm tall. 1 atm of pressure = 760 mm Hg = 14. 7 lbs/in 2 = 101. 3 k. Pa

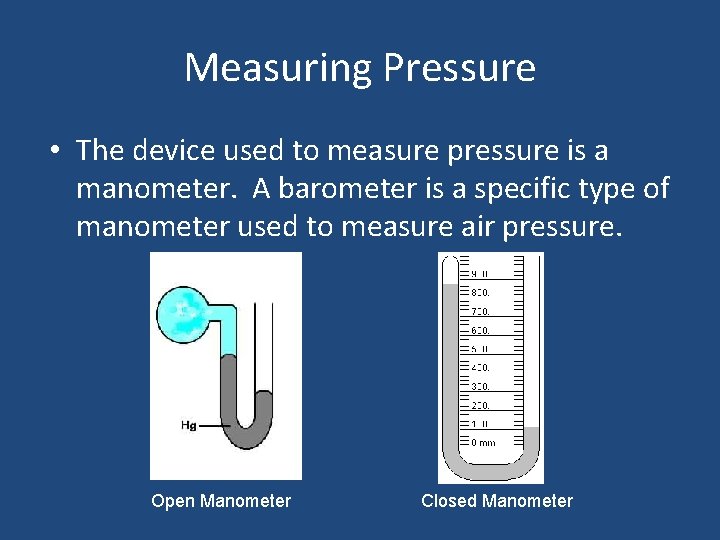
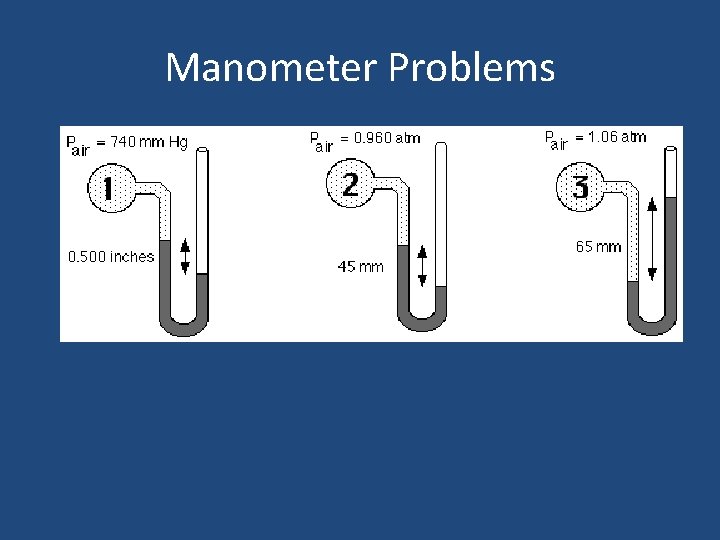
Measuring Pressure • The device used to measure pressure is a manometer. A barometer is a specific type of manometer used to measure air pressure. Open Manometer Closed Manometer

Manometer Problems

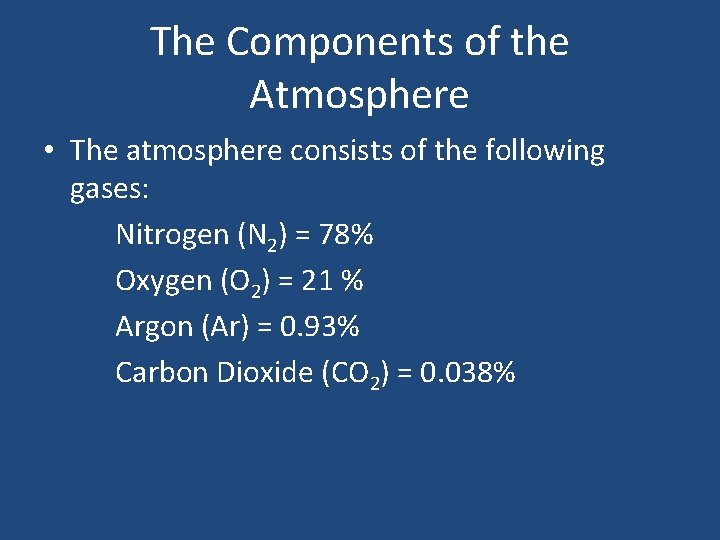
The Components of the Atmosphere • The atmosphere consists of the following gases: Nitrogen (N 2) = 78% Oxygen (O 2) = 21 % Argon (Ar) = 0. 93% Carbon Dioxide (CO 2) = 0. 038%

Gas Laws

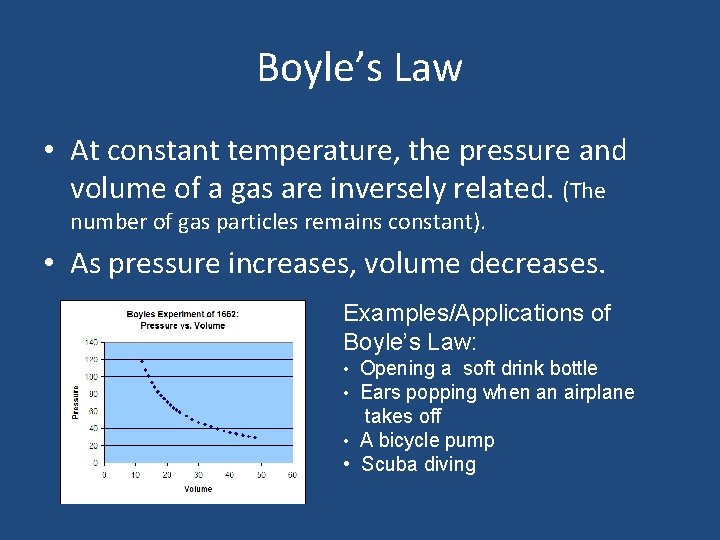
Boyle’s Law • At constant temperature, the pressure and volume of a gas are inversely related. (The number of gas particles remains constant). • As pressure increases, volume decreases. Examples/Applications of Boyle’s Law: • Opening a soft drink bottle • Ears popping when an airplane takes off • A bicycle pump • Scuba diving

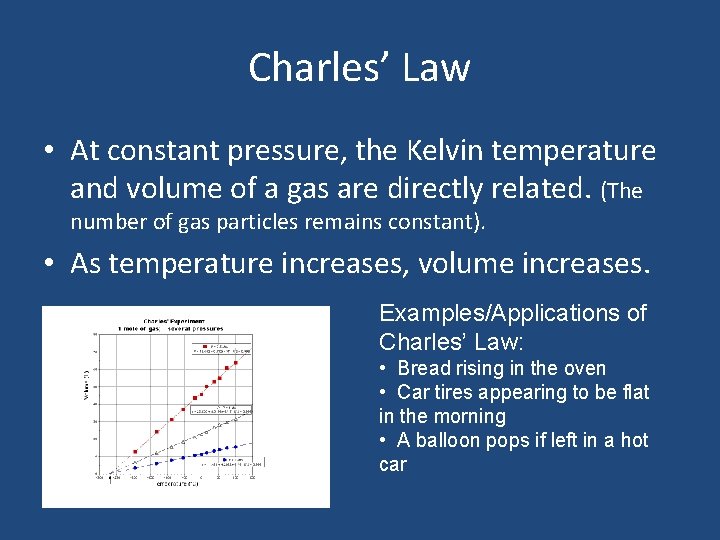
Charles’ Law • At constant pressure, the Kelvin temperature and volume of a gas are directly related. (The number of gas particles remains constant). • As temperature increases, volume increases. Examples/Applications of Charles’ Law: • Bread rising in the oven • Car tires appearing to be flat in the morning • A balloon pops if left in a hot car

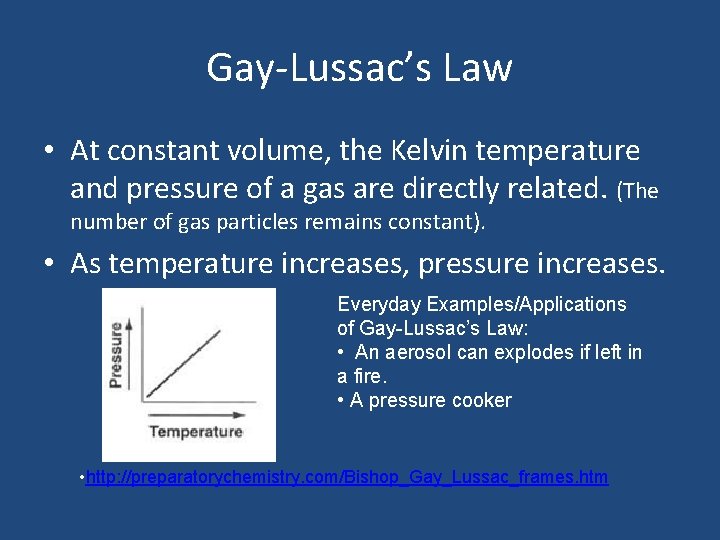
Gay-Lussac’s Law • At constant volume, the Kelvin temperature and pressure of a gas are directly related. (The number of gas particles remains constant). • As temperature increases, pressure increases. Everyday Examples/Applications of Gay-Lussac’s Law: • An aerosol can explodes if left in a fire. • A pressure cooker • http: //preparatorychemistry. com/Bishop_Gay_Lussac_frames. htm