Gases Describing Gases Review of Kinetic Theory Particles

Gases Describing Gases

Review of Kinetic Theory Particles in an ideal gas… 1. gases are hard, small, spherical particles 2. don’t attract or repel each other. 3. are in constant, random, straight-line motion. 4. indefinite shape and volume. 5. have “perfectly” elastic
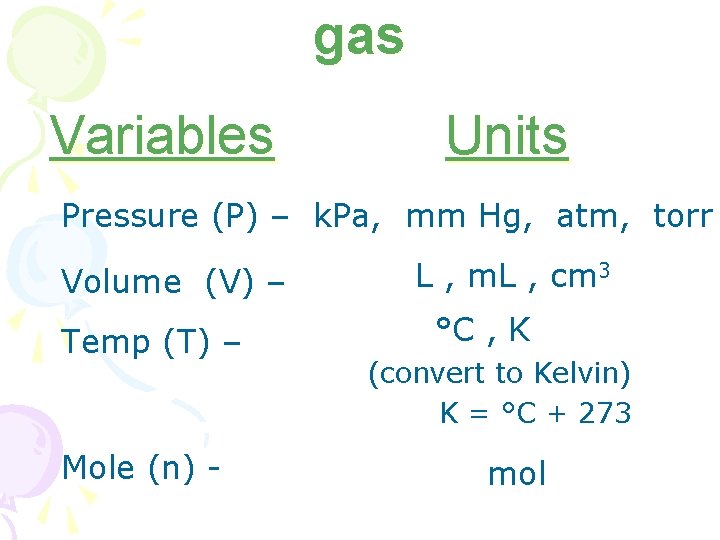
gas Variables Units Pressure (P) – k. Pa, mm Hg, atm, torr Volume (V) – Temp (T) – Mole (n) - L , m. L , cm 3 °C , K (convert to Kelvin) K = °C + 273 mol
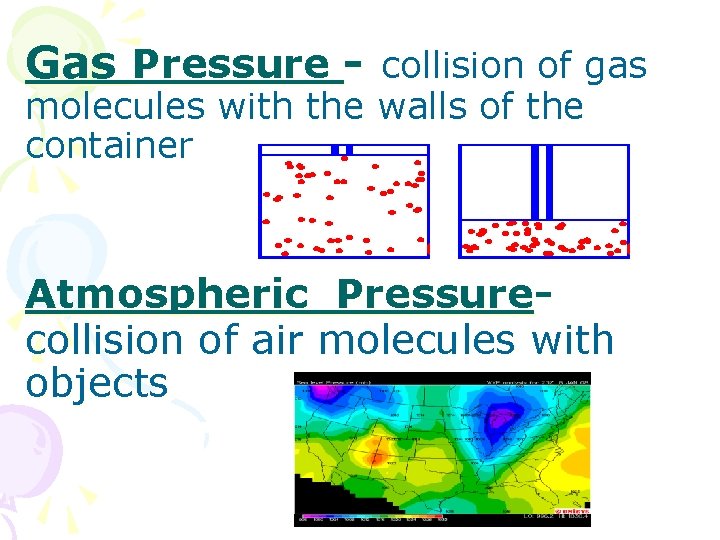
Gas Pressure - collision of gas molecules with the walls of the container Atmospheric Pressurecollision of air molecules with objects
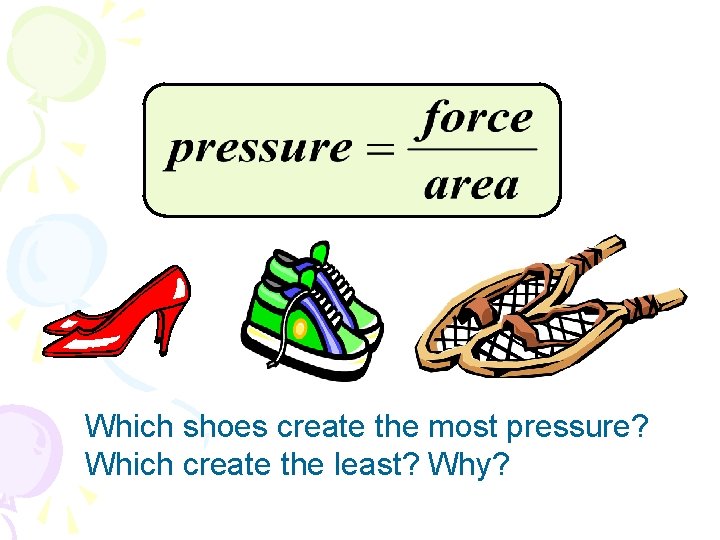
Which shoes create the most pressure? Which create the least? Why?
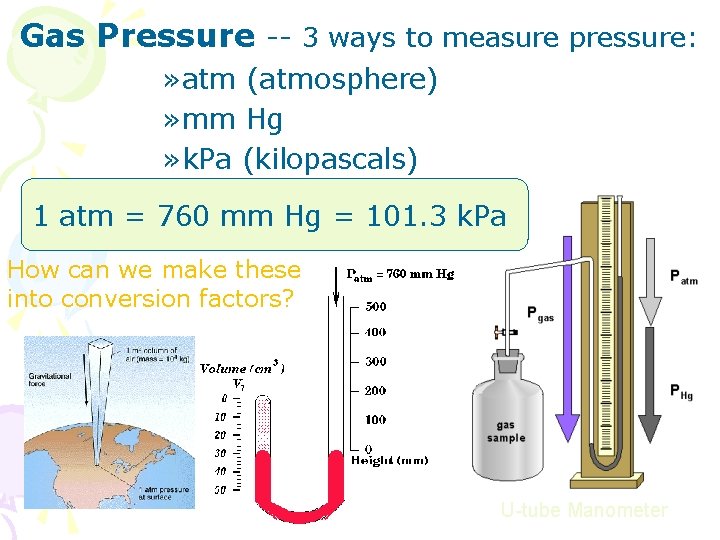
Gas Pressure -- 3 ways to measure pressure: » atm (atmosphere) » mm Hg » k. Pa (kilopascals) 1 atm = 760 mm Hg = 101. 3 k. Pa How can we make these into conversion factors? U-tube Manometer
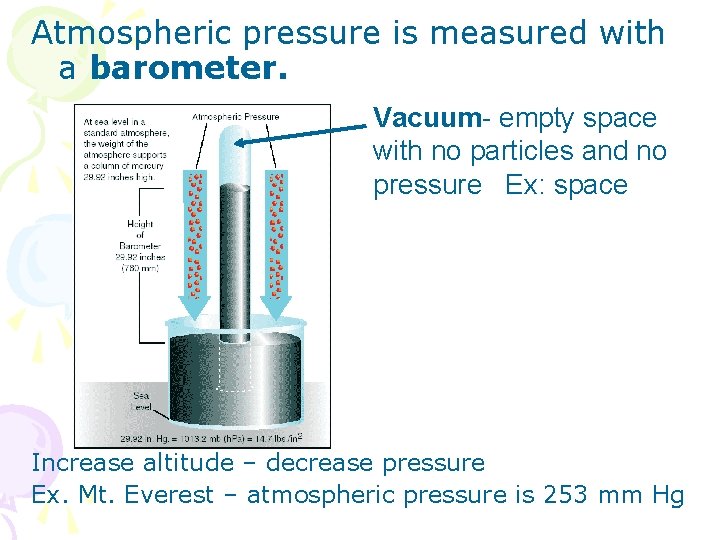
Atmospheric pressure is measured with a barometer. Vacuum- empty space with no particles and no pressure Ex: space Increase altitude – decrease pressure Ex. Mt. Everest – atmospheric pressure is 253 mm Hg
Gases Kelvin Temperature Scale is directly proportional to the average kinetic energy, so Temperature is a description of the movement of particles (not how hot or cold it is)
STP Standard Temperature and Pressure Standard pressure – 1 atm, 760 mm Hg, or 101. 3 k. Pa Standard temp. – 0° Celsius or 273 Kelvin
- Slides: 9