Gases Characteristics of Gases n Gases are fluids







- Slides: 11

Gases

Characteristics of Gases n Gases are fluids – In other words, they can flow. n Gases have low density – Most of the volume occupied by gases are empty space. n Gases are highly compressible n Completely fills a container

n Pressure is defined as the force divided by area. n So, it is the force on an area. n That is why scientists derived the pascal to measure pressure. n A pascal is defined as one newton (the SI unit force) applied over an area of one square meter. n Pressure is caused when a gas collides with the walls of a container. n Collisions = force, container walls give us the area over which the force is applied

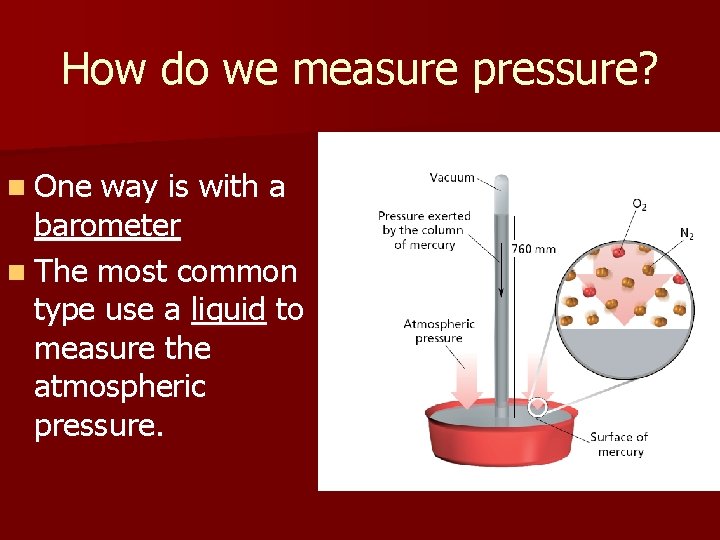
How do we measure pressure? n One way is with a barometer n The most common type use a liquid to measure the atmospheric pressure.

How is pressure recorded? n There are several different pressure units that can be used. Unit Abbreviation Equivalent number of pascals Atmosphere atm 1 atm = 101, 325 Pa Bar bar 1 bar = 100, 025 Millimeter of mercury mm Hg 1 mm Hg = 133. 322 Pascal Pa 1 Pounds per square inch psi 1 psi = 6. 89286 x 103 Pa Torr torr 1 torr = 133. 322

Example problems Convert each of the following pressures into the specified unit. n 72. 7 atm pascals n 12. 33 k. Pa millimeters of mercury (hint k. Pa means 1000 Pa) n 100. 0 k. Pa atmospheres n 44. 7 psi pascals

Answers n 7366327. 5 pascals n 92. 5 millimeters of mercury n 0. 987 atmospheres n 308196 pascals

Kinetic Molecular Theory n Book Definition - A theory that explains that the behavior of physical systems depends on the combined actions of the molecules constituting the system. n Another way to say this – the behavior of a system depends on the actions of the molecules that make it up.

Concepts of the Kinetic Molecular Theory n All matter is composed of particles. n All matter is in constant motion n Gases particles are in constant, rapid, and random motion n This motion represents the kinetic energy (KE = ½ mv 2) of the system. n Particles of a gas are very far apart relative to their size. n All collisions are perfectly elastic. In other words, there is no loss or gain in energy.

Temperature and Kinetic Energy n The average kinetic energy of random motion is proportional to the absolute temperature (temperature in Kelvin) n So, if the temperature changes; the random motion changes. n If temperature increases; random motion increases. n Note: this does not mean that all particles are moving at the same speed, but overall the rates increase the average.

Homework n Page 422: 8, 9, 10 n Page 446: 32, 34