Gases and Pressure Section 11 1 Vocabulary Pressure

Gases and Pressure Section 11. 1
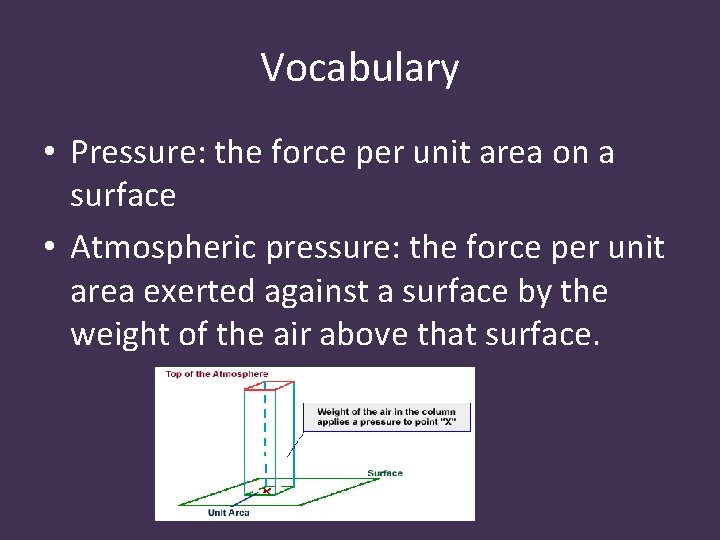
Vocabulary • Pressure: the force per unit area on a surface • Atmospheric pressure: the force per unit area exerted against a surface by the weight of the air above that surface.

Measuring Pressure • Barometer: device used to measure atmospheric pressure • First introduced by Evangelista Torricelli during the early 1600 s. • Discovered that atmospheric pressure supports a column of mercury about 760 mm above the surface of Hg in a dish
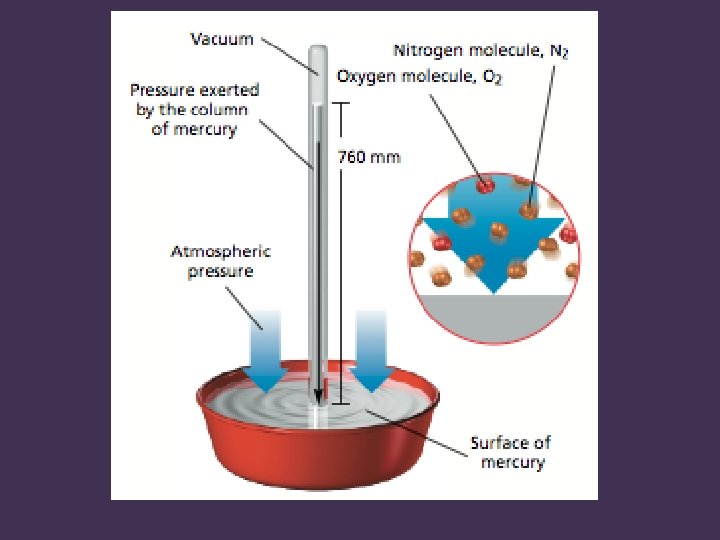

Units of Pressure • There are several units for pressure • mm Hg, also called torr in honor of Torricelli • Average atmospheric pressure at sea level at 0°C is 760 mm Hg, or 760 torr • Atmospheres: one atm is defined as being exactly equivalent to 760 mm Hg

More about Units • The SI unit of measurement for pressure is the pascal • One pascal (Pa) is the pressure exerted by a force of one newton acting on an area of one square meter • Usually expressed as k. Pa • 1 atm = 101. 325 k. Pa
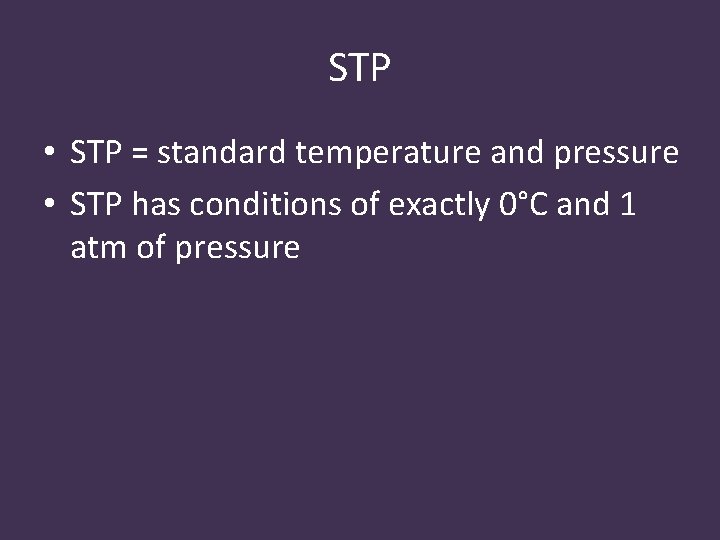
STP • STP = standard temperature and pressure • STP has conditions of exactly 0°C and 1 atm of pressure

Dalton’s Law of Partial Pressures • The pressure of each gas in a mixture is called the partial pressure • Dalton’s law states that the total pressure of a gas mixture is the sum of the partial pressures of the component gases • PT = P 1 + P 2 + P 3 + …

Problem 1 • A mixture of O 2, CO 2, and N 2 has a total pressure of 0. 97 atm. What is the partial pressure of O 2 if the partial pressure of CO 2 is 0. 70 atm and the partial pressure of N 2 is 0. 12 atm? • PT = Poxygen + Pcarbon dioxide + Pnitrogen • Poxygen = PT – (Pcarbon dioxide + Pnitrogen) • Poxygen = 0. 97 atm – (0. 70 atm + 0. 12 atm) • Poxygen = 0. 15 atm

Problem 2 • Find the total pressure for a mixture that contains four gases with partial pressure of 5. 00 k. Pa, 4. 56 k. Pa, 3. 02 k. Pa, and 1. 20 k. Pa • PT = P 1 + P 2 + P 3 + P 4 • PT = (5. 00 + 4. 56 + 3. 02 + 1. 20) k. Pa • PT = 13. 78 k. Pa

Water Displacement • Gas can be collected by water displacement, but some water vapor will be present • The total pressure will be the sum of the partial pressures of the gas collected and water vapor • The partial pressure of water vapor is found on a table (dependent on temperature)
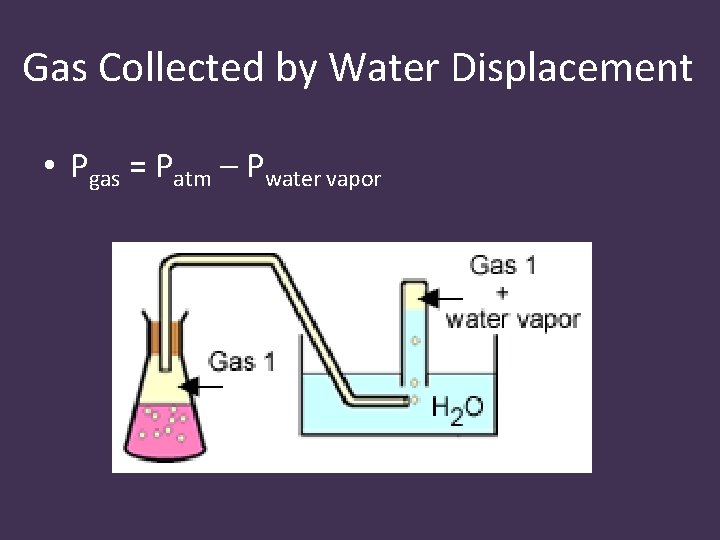
Gas Collected by Water Displacement • Pgas = Patm – Pwater vapor
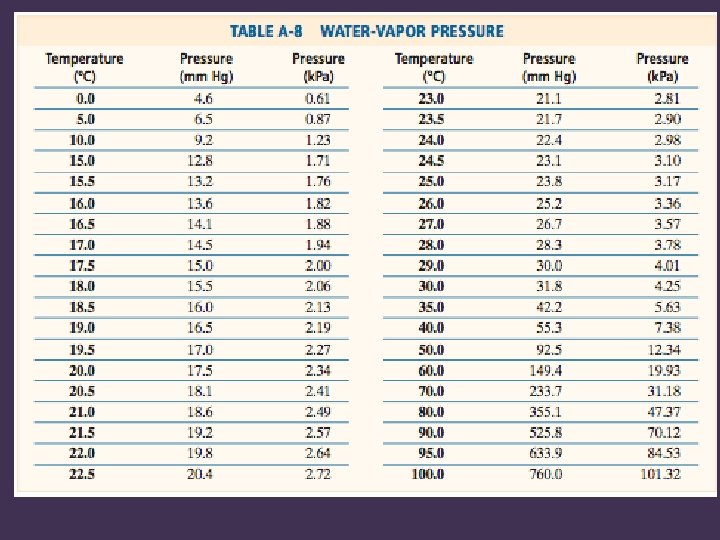

Problem 3 • O 2(g), from the decomposition of KCl. O 3, was collected by water displacement. The barometric (atmospheric) pressure and the temperature during the experiment were 97. 5 k. Pa and 20. 0° C. What was the partial pressure of the O 2 collected?

Solution • • • PT = Patm = 97. 5 k. Pa Pwater vapor = 2. 34 k. Pa Unknown = Poxygen gas = PT – Pwater vapor Poxygen gas = 97. 5 k. Pa – 2. 34 k. Pa Poxygen gas = 95. 2 k. Pa
- Slides: 15