Gas Turbine Combustion Systems 1 Recap Problem solutions
Gas Turbine Combustion Systems 1
• Recap • Problem solutions Ideal case: T 2 = 579 K, T 4 = 699 K, Wc = 280. 6 k. J/kg, Wt = 654 k. J/kg, BWR = 0. 43, =0. 48 Actual case: T 2’ = 624. 7 K, T 4’ = 579. 2 K, Wc = 326. 3 k. J/kg, Wt = 582. 1 k. J/kg, BWR = 0. 56, =0. 35 Recuperator case: T 5 = 741. 57 K, =0. 42 • Effect of Pr 2
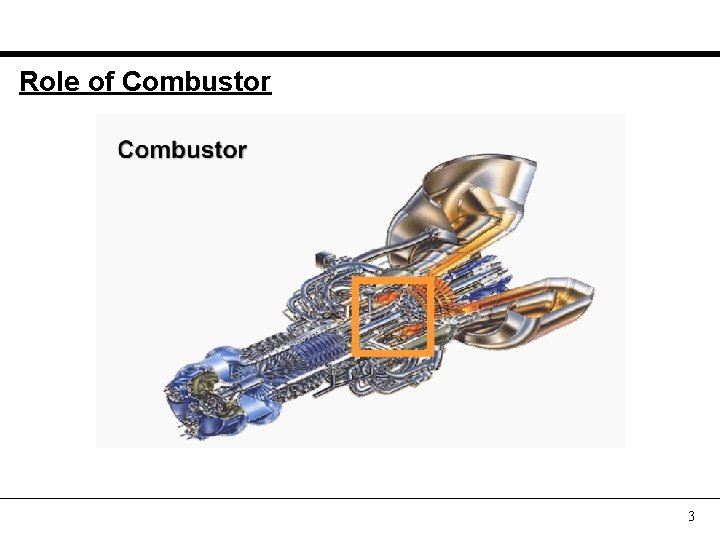
Role of Combustor 3
Main Job of the Combustor • Raise the temperature of the compressed air to the highest value the turbine can handle, with the smallest hot spots possible and within the prescribed radial profile. 4
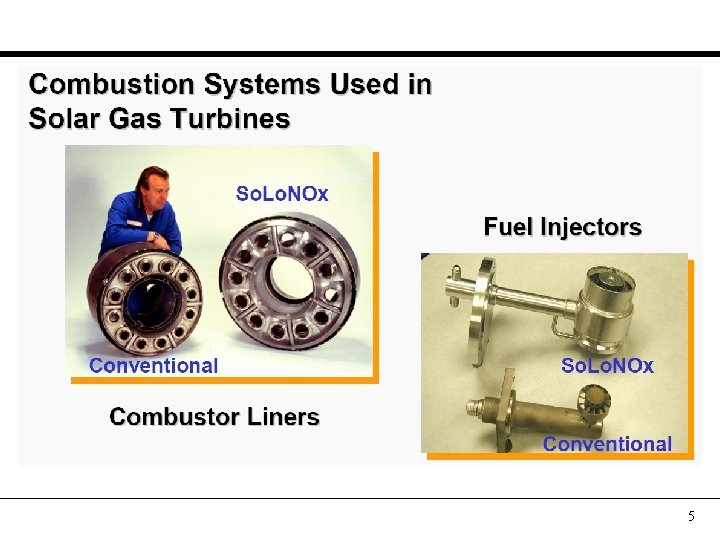
5
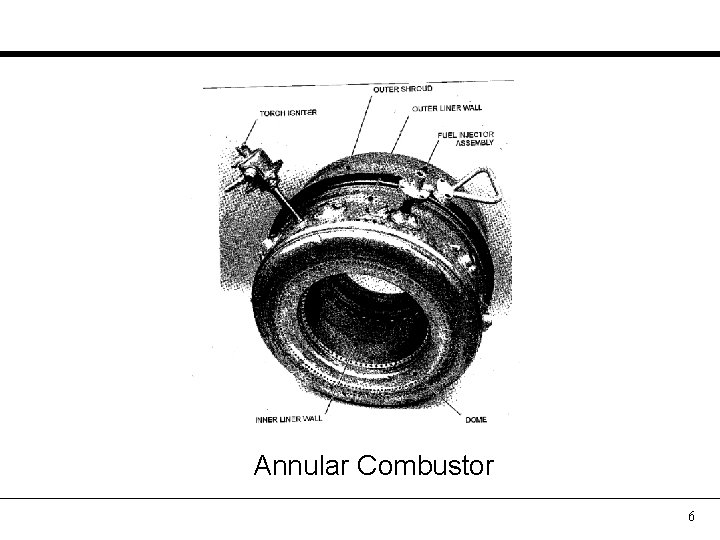
Annular Combustor 6
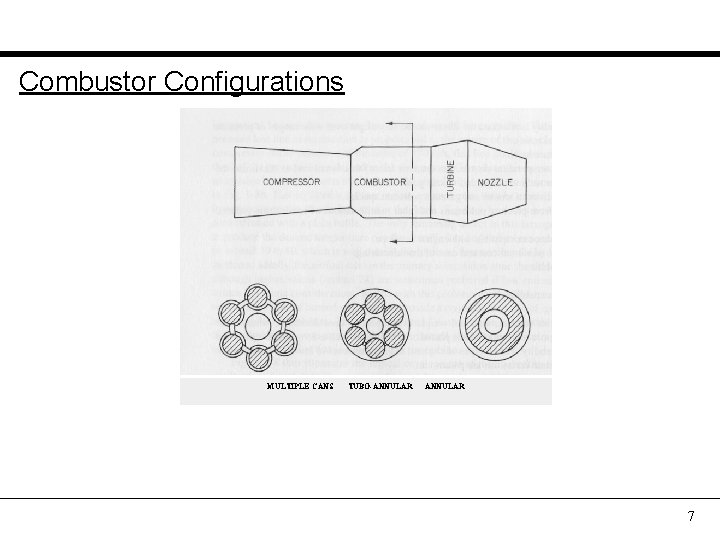
Combustor Configurations MULTIPLE CANS TUBO-ANNULAR 7
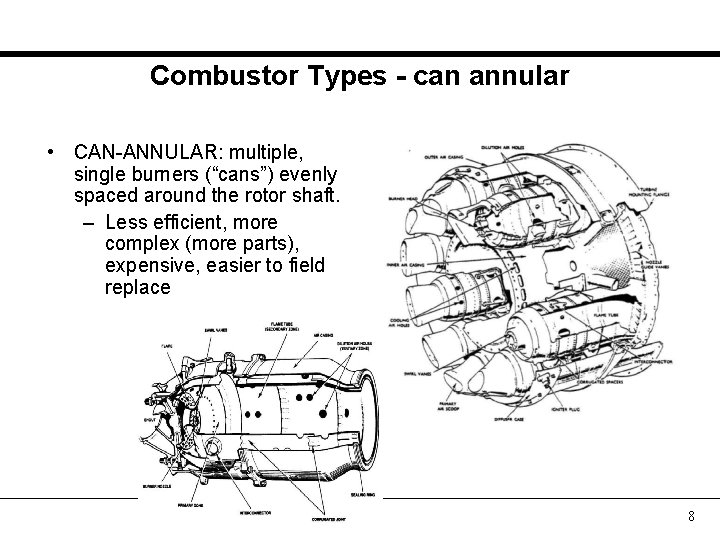
Combustor Types - can annular • CAN-ANNULAR: multiple, single burners (“cans”) evenly spaced around the rotor shaft. – Less efficient, more complex (more parts), expensive, easier to field replace 8
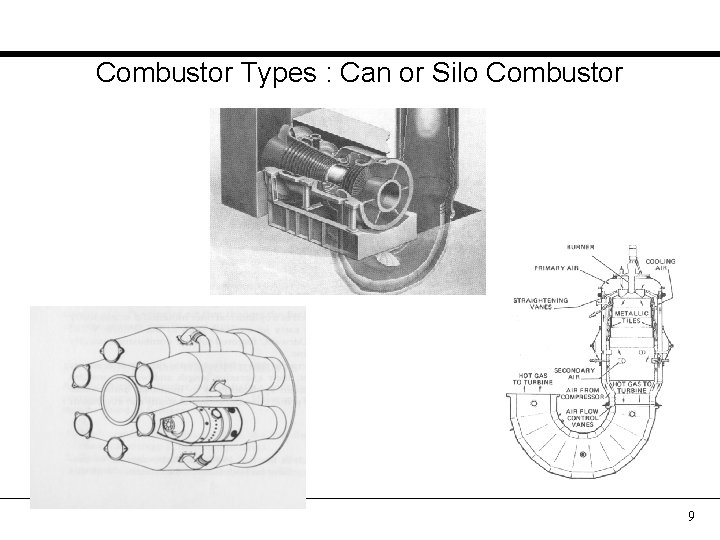
Combustor Types : Can or Silo Combustor 9
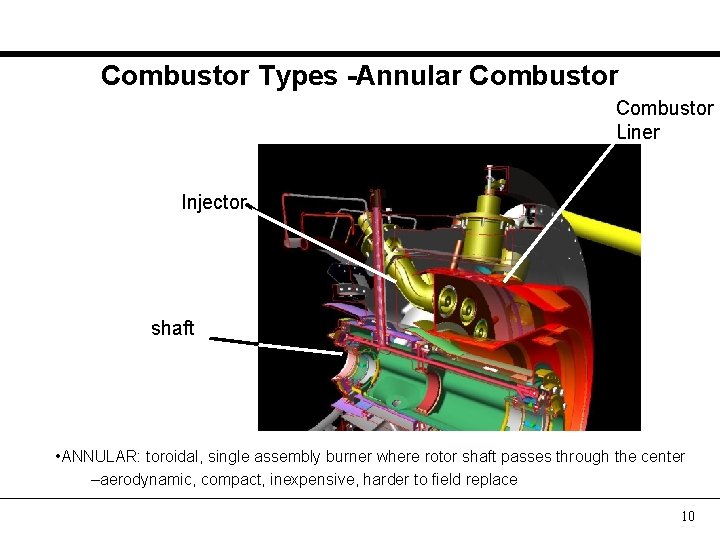
Combustor Types -Annular Combustor Liner Injector shaft • ANNULAR: toroidal, single assembly burner where rotor shaft passes through the center –aerodynamic, compact, inexpensive, harder to field replace 10
Factors for the selection of configuration 1. 2. 3. 4. 5. 6. 7. 8. Cooling challenges Pressure loss Temperature profile Development Cost Manufacturing cost Testing facility Maintainability in the field Achieving low NOx with catalytic methods 11
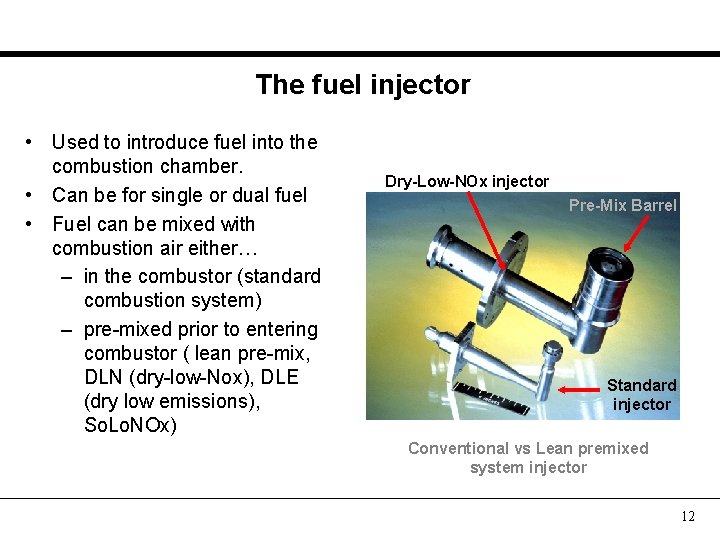
The fuel injector • Used to introduce fuel into the combustion chamber. • Can be for single or dual fuel • Fuel can be mixed with combustion air either… – in the combustor (standard combustion system) – pre-mixed prior to entering combustor ( lean pre-mix, DLN (dry-low-Nox), DLE (dry low emissions), So. Lo. NOx) Dry-Low-NOx injector Pre-Mix Barrel Standard injector Conventional vs Lean premixed system injector 12
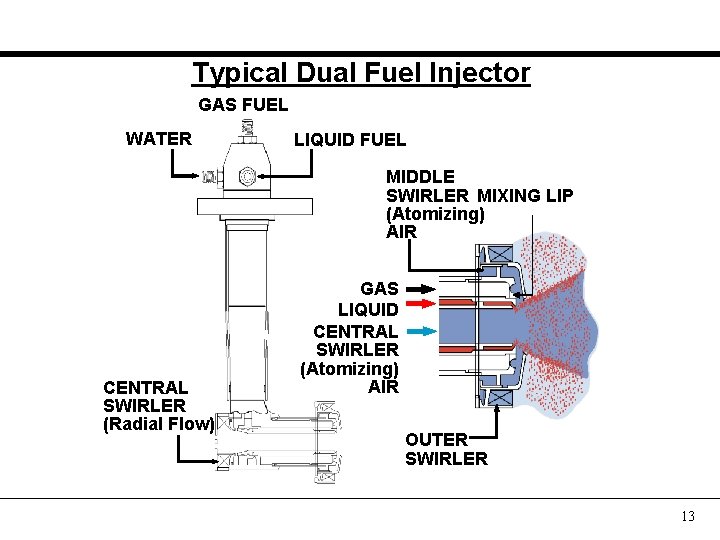
Typical Dual Fuel Injector GAS FUEL WATER LIQUID FUEL MIDDLE SWIRLER MIXING LIP (Atomizing) AIR CENTRAL SWIRLER (Radial Flow) GAS LIQUID CENTRAL SWIRLER (Atomizing) AIR OUTER SWIRLER 13
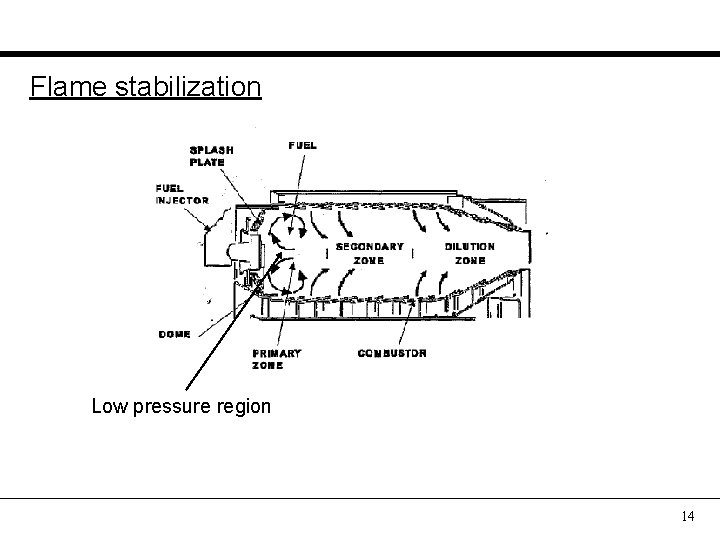
Flame stabilization Low pressure region 14
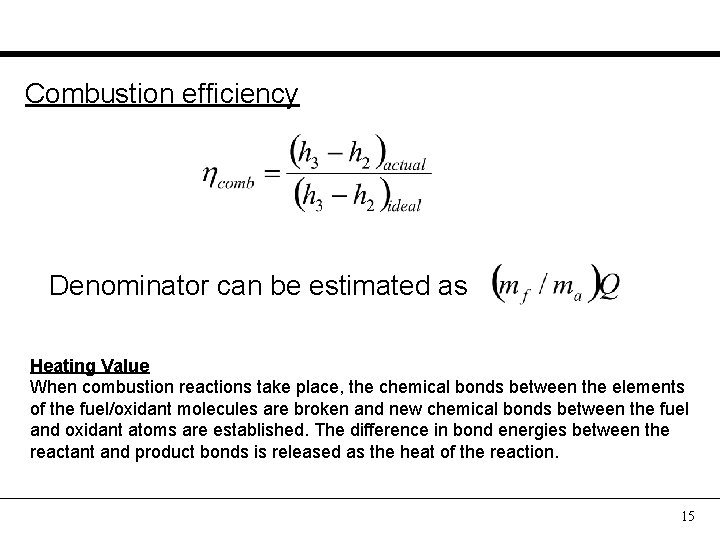
Combustion efficiency Denominator can be estimated as Heating Value When combustion reactions take place, the chemical bonds between the elements of the fuel/oxidant molecules are broken and new chemical bonds between the fuel and oxidant atoms are established. The difference in bond energies between the reactant and product bonds is released as the heat of the reaction. 15
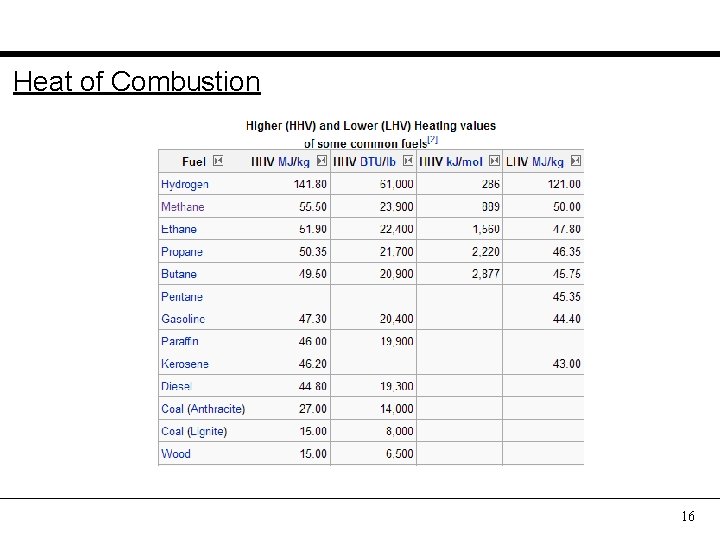
Heat of Combustion 16

Combustor Design Criteria • • • Reliable Ignition Proper fuel/air mixing w/ excess available air Sufficient transient chemical reaction time Good Combustion/flame Stability High Combustion Efficiency Low Smoke Satisfactory Emissions Levels Minimum Pressure Loss Satisfactory Exit Temperature Profile Low Oscillations Combustion of wide range of fuels Life 17
Caution • Systems not (always) adiabatic – Heat Loss • Gas Compositions Change • Gas Composition not known or incomplete 18

Why is Combustor Design so Challenging? Many different phenomena to consider: • • • Aerodynamics Hydrodynamics Chemical Reactions Heat Transfer Mechanical Durability 19
Some Combustion fundamentals • Equivalence ratio, Air/Fuel ratio • Diffusion flame and premixed flame • Laminar flame and turbulent flames • Flamespeed • Autoignition Delay times • Adiabtaic flame temperature • Heat of combustion • Heat of vaporization • Liquid fuel atomization • Flame stabilization • Flash back, blow out, flammability limits 20
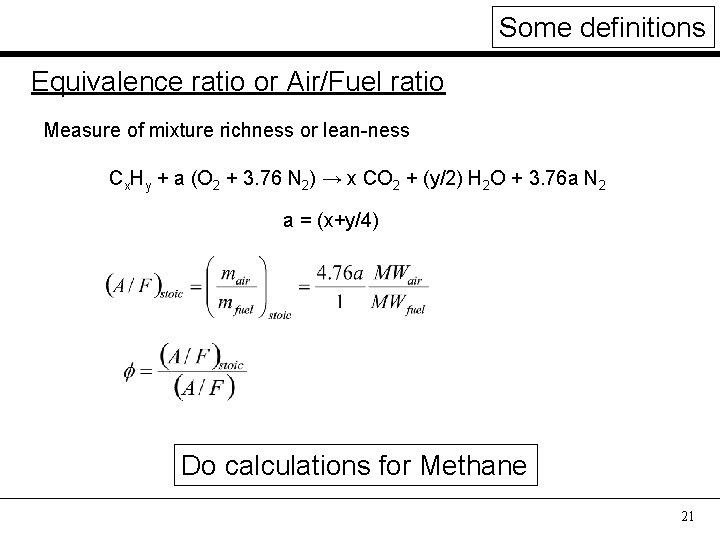
Some definitions Equivalence ratio or Air/Fuel ratio Measure of mixture richness or lean-ness Cx. Hy + a (O 2 + 3. 76 N 2) → x CO 2 + (y/2) H 2 O + 3. 76 a N 2 a = (x+y/4) Do calculations for Methane 21
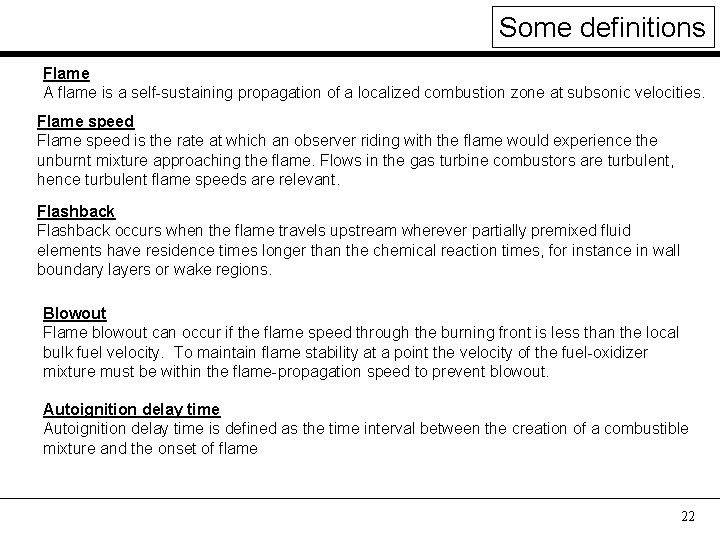
Some definitions Flame A flame is a self-sustaining propagation of a localized combustion zone at subsonic velocities. Flame speed is the rate at which an observer riding with the flame would experience the unburnt mixture approaching the flame. Flows in the gas turbine combustors are turbulent, hence turbulent flame speeds are relevant. Flashback occurs when the flame travels upstream wherever partially premixed fluid elements have residence times longer than the chemical reaction times, for instance in wall boundary layers or wake regions. Blowout Flame blowout can occur if the flame speed through the burning front is less than the local bulk fuel velocity. To maintain flame stability at a point the velocity of the fuel-oxidizer mixture must be within the flame-propagation speed to prevent blowout. Autoignition delay time is defined as the time interval between the creation of a combustible mixture and the onset of flame 22
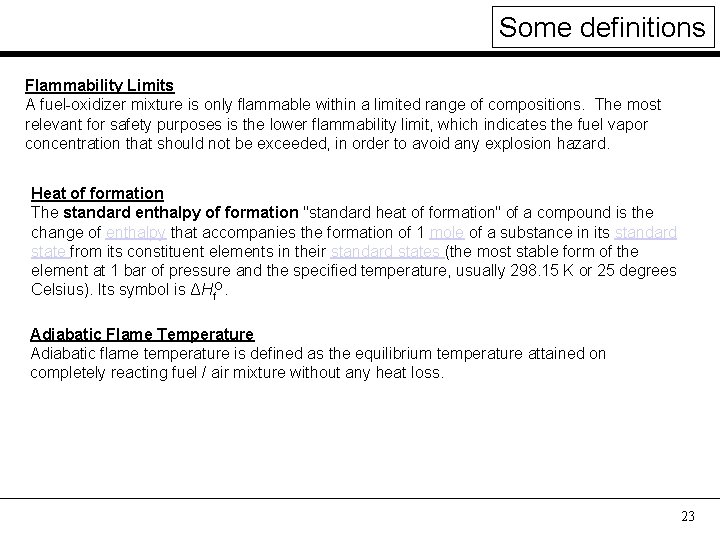
Some definitions Flammability Limits A fuel-oxidizer mixture is only flammable within a limited range of compositions. The most relevant for safety purposes is the lower flammability limit, which indicates the fuel vapor concentration that should not be exceeded, in order to avoid any explosion hazard. Heat of formation The standard enthalpy of formation "standard heat of formation" of a compound is the change of enthalpy that accompanies the formation of 1 mole of a substance in its standard state from its constituent elements in their standard states (the most stable form of the element at 1 bar of pressure and the specified temperature, usually 298. 15 K or 25 degrees Celsius). Its symbol is ΔHf. O. Adiabatic Flame Temperature Adiabatic flame temperature is defined as the equilibrium temperature attained on completely reacting fuel / air mixture without any heat loss. 23
Combustion Chemistry 24
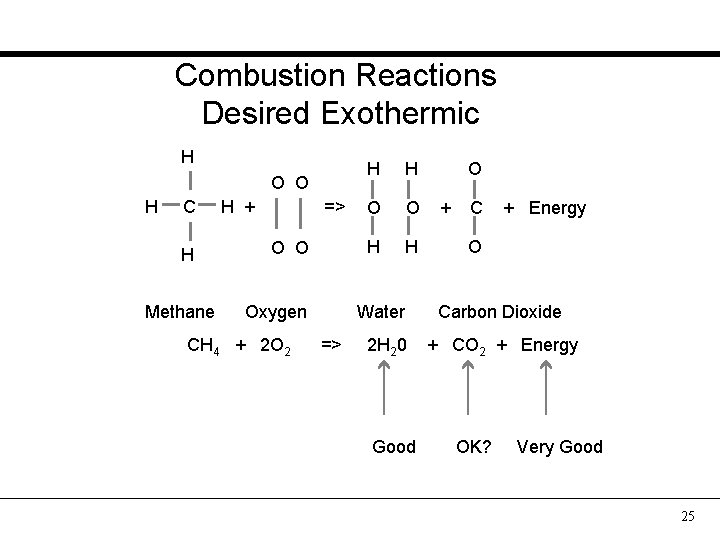
Combustion Reactions Desired Exothermic H O O H C H Methane H + => O O Oxygen CH 4 + 2 O 2 H H O O H H Water => 2 H 20 Good O + C + Energy O Carbon Dioxide + CO 2 + Energy OK? Very Good 25
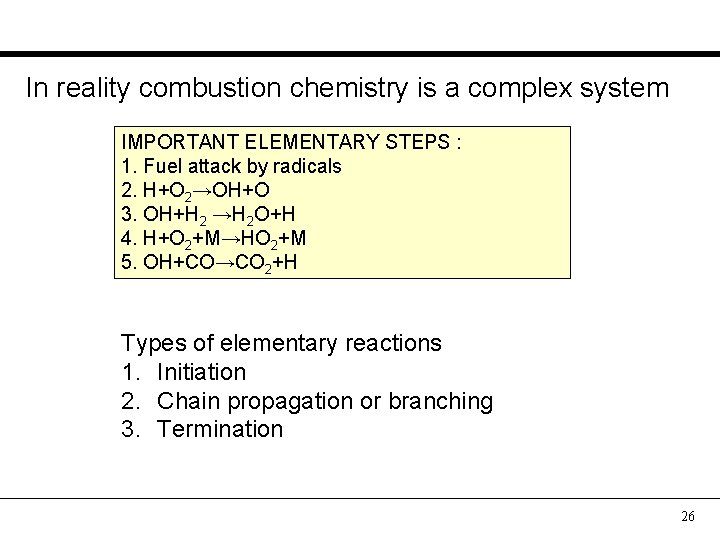
In reality combustion chemistry is a complex system IMPORTANT ELEMENTARY STEPS : 1. Fuel attack by radicals 2. H+O 2→OH+O 3. OH+H 2 →H 2 O+H 4. H+O 2+M→HO 2+M 5. OH+CO→CO 2+H Types of elementary reactions 1. Initiation 2. Chain propagation or branching 3. Termination 26
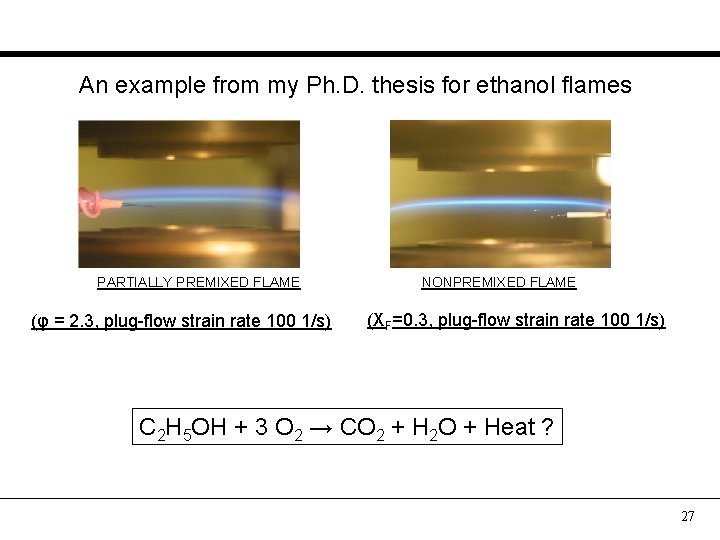
An example from my Ph. D. thesis for ethanol flames PARTIALLY PREMIXED FLAME (φ = 2. 3, plug-flow strain rate 100 1/s) NONPREMIXED FLAME (XF=0. 3, plug-flow strain rate 100 1/s) C 2 H 5 OH + 3 O 2 → CO 2 + H 2 O + Heat ? 27
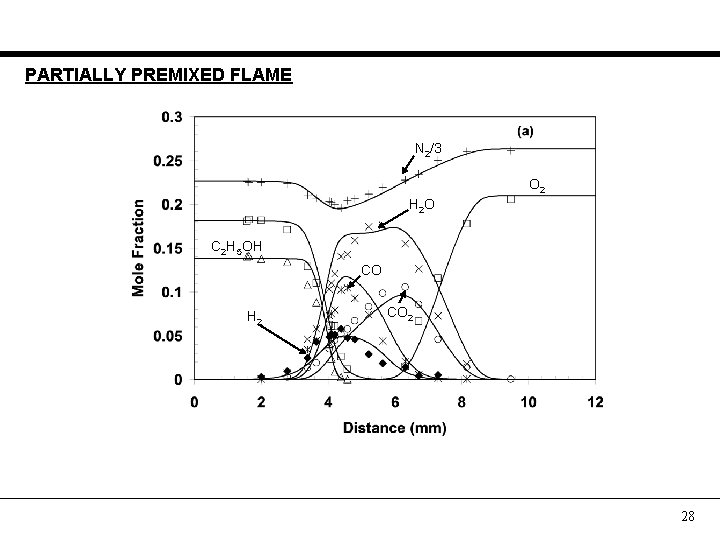
PARTIALLY PREMIXED FLAME N 2/3 O 2 H 2 O C 2 H 5 OH CO H 2 CO 2 28
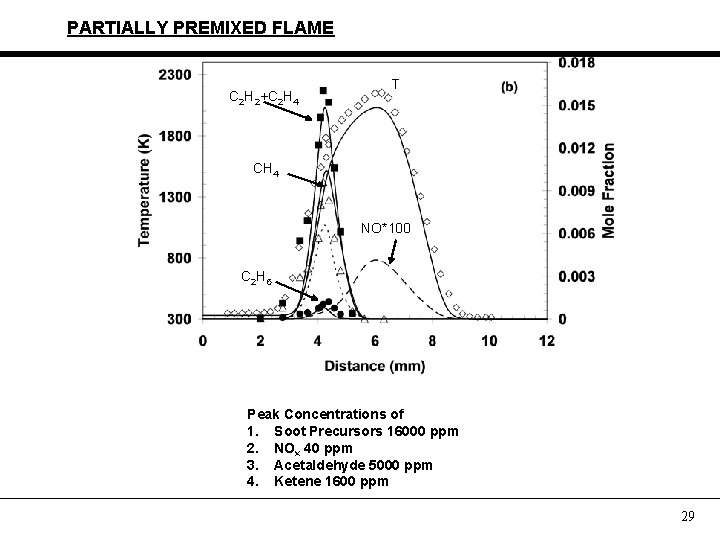
PARTIALLY PREMIXED FLAME C 2 H 2+C 2 H 4 T CH 4 NO*100 C 2 H 6 Peak Concentrations of 1. Soot Precursors 16000 ppm 2. NOx 40 ppm 3. Acetaldehyde 5000 ppm 4. Ketene 1600 ppm 29
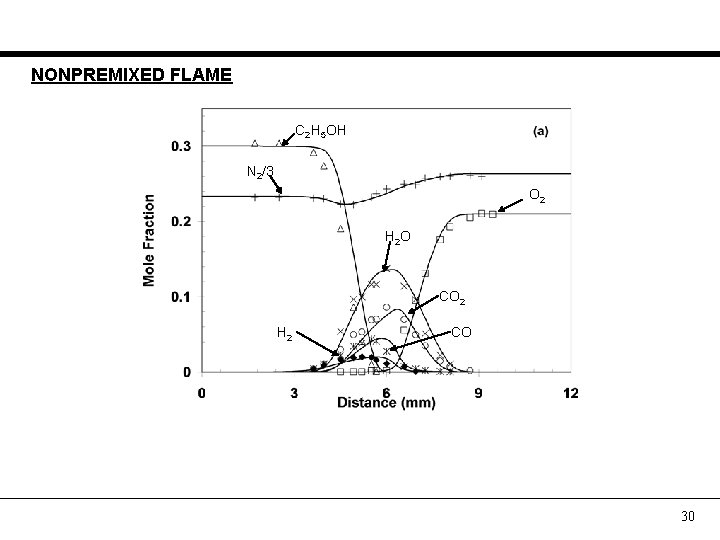
NONPREMIXED FLAME C 2 H 5 OH N 2/3 O 2 H 2 O CO 2 H 2 CO 30
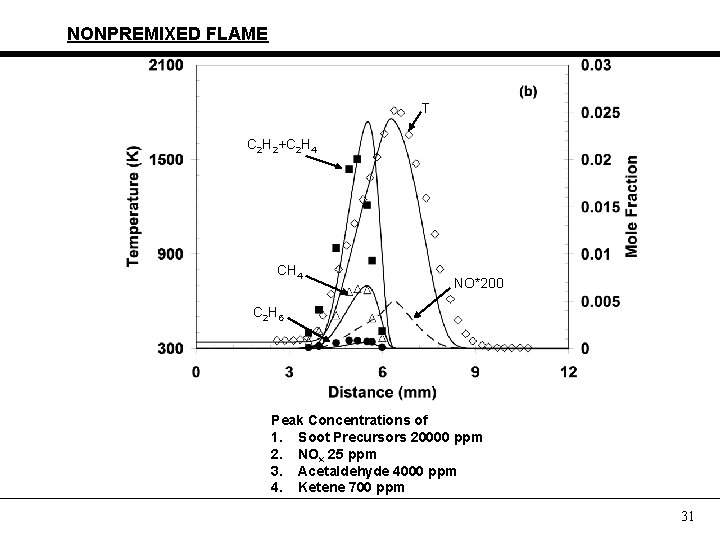
NONPREMIXED FLAME T C 2 H 2+C 2 H 4 CH 4 NO*200 C 2 H 6 Peak Concentrations of 1. Soot Precursors 20000 ppm 2. NOx 25 ppm 3. Acetaldehyde 4000 ppm 4. Ketene 700 ppm 31
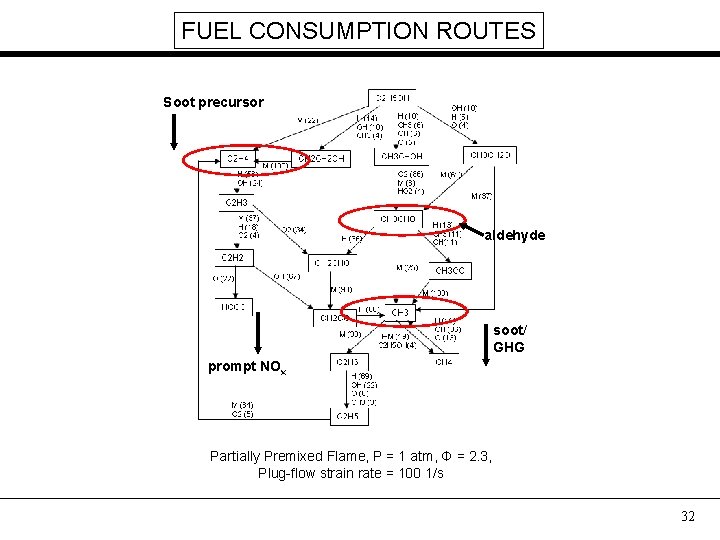
FUEL CONSUMPTION ROUTES Soot precursor aldehyde soot/ GHG prompt NOx Partially Premixed Flame, P = 1 atm, Φ = 2. 3, Plug-flow strain rate = 100 1/s 32
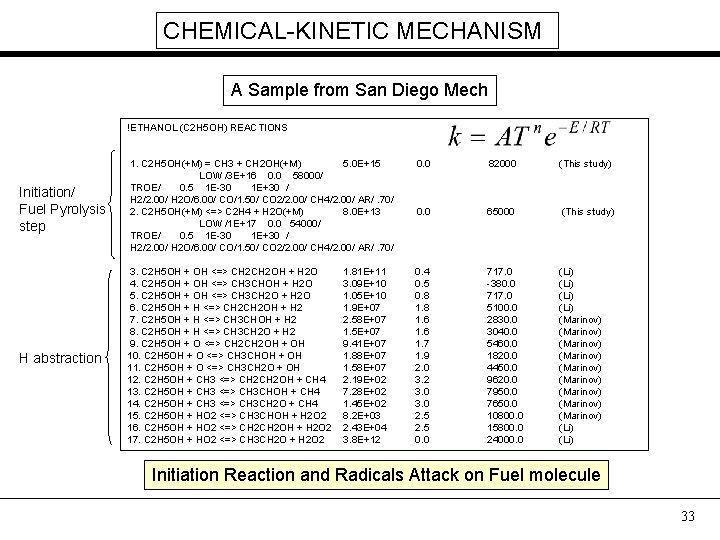
CHEMICAL-KINETIC MECHANISM A Sample from San Diego Mech !ETHANOL (C 2 H 5 OH) REACTIONS 0. 0 82000 (This study) Initiation/ Fuel Pyrolysis step 1. C 2 H 5 OH(+M) = CH 3 + CH 2 OH(+M) 5. 0 E+15 LOW /3 E+16 0. 0 58000/ TROE/ 0. 5 1 E-30 1 E+30 / H 2/2. 00/ H 2 O/6. 00/ CO/1. 50/ CO 2/2. 00/ CH 4/2. 00/ AR/. 70/ 2. C 2 H 5 OH(+M) <=> C 2 H 4 + H 2 O(+M) 8. 0 E+13 LOW /1 E+17 0. 0 54000/ TROE/ 0. 5 1 E-30 1 E+30 / H 2/2. 00/ H 2 O/6. 00/ CO/1. 50/ CO 2/2. 00/ CH 4/2. 00/ AR/. 70/ 0. 0 65000 (This study) H abstraction 3. C 2 H 5 OH + OH <=> CH 2 OH + H 2 O 4. C 2 H 5 OH + OH <=> CH 3 CHOH + H 2 O 5. C 2 H 5 OH + OH <=> CH 3 CH 2 O + H 2 O 6. C 2 H 5 OH + H <=> CH 2 OH + H 2 7. C 2 H 5 OH + H <=> CH 3 CHOH + H 2 8. C 2 H 5 OH + H <=> CH 3 CH 2 O + H 2 9. C 2 H 5 OH + O <=> CH 2 OH + OH 10. C 2 H 5 OH + O <=> CH 3 CHOH + OH 11. C 2 H 5 OH + O <=> CH 3 CH 2 O + OH 12. C 2 H 5 OH + CH 3 <=> CH 2 OH + CH 4 13. C 2 H 5 OH + CH 3 <=> CH 3 CHOH + CH 4 14. C 2 H 5 OH + CH 3 <=> CH 3 CH 2 O + CH 4 15. C 2 H 5 OH + HO 2 <=> CH 3 CHOH + H 2 O 2 16. C 2 H 5 OH + HO 2 <=> CH 2 OH + H 2 O 2 17. C 2 H 5 OH + HO 2 <=> CH 3 CH 2 O + H 2 O 2 0. 4 0. 5 0. 8 1. 6 1. 7 1. 9 2. 0 3. 2 3. 0 2. 5 0. 0 717. 0 -380. 0 717. 0 5100. 0 2830. 0 3040. 0 5460. 0 1820. 0 4450. 0 9620. 0 7950. 0 7650. 0 10800. 0 15800. 0 24000. 0 (Li) (Marinov) (Marinov) (Marinov) (Li) 1. 81 E+11 3. 09 E+10 1. 05 E+10 1. 9 E+07 2. 58 E+07 1. 5 E+07 9. 41 E+07 1. 88 E+07 1. 58 E+07 2. 19 E+02 7. 28 E+02 1. 45 E+02 8. 2 E+03 2. 43 E+04 3. 8 E+12 Initiation Reaction and Radicals Attack on Fuel molecule 33
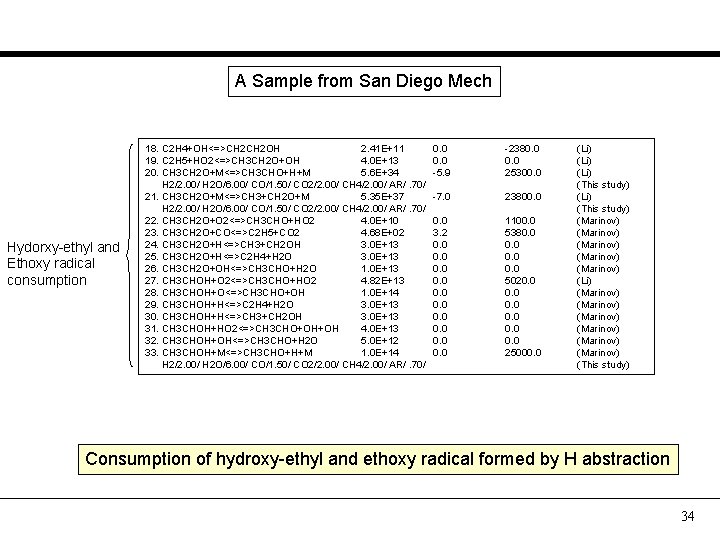
A Sample from San Diego Mech Hydorxy-ethyl and Ethoxy radical consumption 18. C 2 H 4+OH<=>CH 2 OH 2. 41 E+11 19. C 2 H 5+HO 2<=>CH 3 CH 2 O+OH 4. 0 E+13 20. CH 3 CH 2 O+M<=>CH 3 CHO+H+M 5. 6 E+34 H 2/2. 00/ H 2 O/6. 00/ CO/1. 50/ CO 2/2. 00/ CH 4/2. 00/ AR/. 70/ 21. CH 3 CH 2 O+M<=>CH 3+CH 2 O+M 5. 35 E+37 H 2/2. 00/ H 2 O/6. 00/ CO/1. 50/ CO 2/2. 00/ CH 4/2. 00/ AR/. 70/ 22. CH 3 CH 2 O+O 2<=>CH 3 CHO+HO 2 4. 0 E+10 23. CH 3 CH 2 O+CO<=>C 2 H 5+CO 2 4. 68 E+02 24. CH 3 CH 2 O+H<=>CH 3+CH 2 OH 3. 0 E+13 25. CH 3 CH 2 O+H<=>C 2 H 4+H 2 O 3. 0 E+13 26. CH 3 CH 2 O+OH<=>CH 3 CHO+H 2 O 1. 0 E+13 27. CH 3 CHOH+O 2<=>CH 3 CHO+HO 2 4. 82 E+13 28. CH 3 CHOH+O<=>CH 3 CHO+OH 1. 0 E+14 29. CH 3 CHOH+H<=>C 2 H 4+H 2 O 3. 0 E+13 30. CH 3 CHOH+H<=>CH 3+CH 2 OH 3. 0 E+13 31. CH 3 CHOH+HO 2<=>CH 3 CHO+OH+OH 4. 0 E+13 32. CH 3 CHOH+OH<=>CH 3 CHO+H 2 O 5. 0 E+12 33. CH 3 CHOH+M<=>CH 3 CHO+H+M 1. 0 E+14 H 2/2. 00/ H 2 O/6. 00/ CO/1. 50/ CO 2/2. 00/ CH 4/2. 00/ AR/. 70/ 0. 0 -5. 9 -2380. 0 25300. 0 -7. 0 23800. 0 3. 2 0. 0 0. 0 1100. 0 5380. 0 5020. 0 0. 0 25000. 0 (Li) (This study) (Marinov) (Marinov) (Li) (Marinov) (Marinov) (This study) Consumption of hydroxy-ethyl and ethoxy radical formed by H abstraction 34
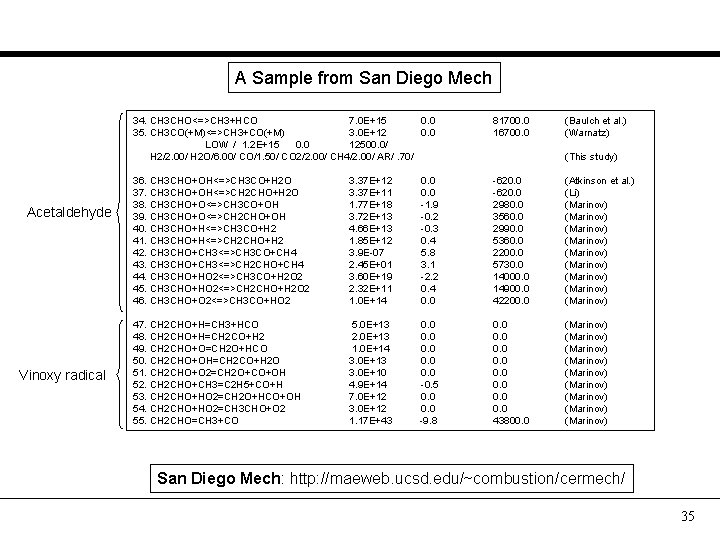
A Sample from San Diego Mech Acetaldehyde Vinoxy radical 34. CH 3 CHO<=>CH 3+HCO 7. 0 E+15 0. 0 35. CH 3 CO(+M)<=>CH 3+CO(+M) 3. 0 E+12 0. 0 LOW / 1. 2 E+15 0. 0 12500. 0/ H 2/2. 00/ H 2 O/6. 00/ CO/1. 50/ CO 2/2. 00/ CH 4/2. 00/ AR/. 70/ 81700. 0 16700. 0 (Baulch et al. ) (Warnatz) 36. CH 3 CHO+OH<=>CH 3 CO+H 2 O 37. CH 3 CHO+OH<=>CH 2 CHO+H 2 O 38. CH 3 CHO+O<=>CH 3 CO+OH 39. CH 3 CHO+O<=>CH 2 CHO+OH 40. CH 3 CHO+H<=>CH 3 CO+H 2 41. CH 3 CHO+H<=>CH 2 CHO+H 2 42. CH 3 CHO+CH 3<=>CH 3 CO+CH 4 43. CH 3 CHO+CH 3<=>CH 2 CHO+CH 4 44. CH 3 CHO+HO 2<=>CH 3 CO+H 2 O 2 45. CH 3 CHO+HO 2<=>CH 2 CHO+H 2 O 2 46. CH 3 CHO+O 2<=>CH 3 CO+HO 2 3. 37 E+11 1. 77 E+18 3. 72 E+13 4. 66 E+13 1. 85 E+12 3. 9 E-07 2. 45 E+01 3. 60 E+19 2. 32 E+11 1. 0 E+14 0. 0 -1. 9 -0. 2 -0. 3 0. 4 5. 8 3. 1 -2. 2 0. 4 0. 0 -620. 0 2980. 0 3560. 0 2990. 0 5360. 0 2200. 0 5730. 0 14000. 0 14900. 0 42200. 0 (Atkinson et al. ) (Li) (Marinov) (Marinov) (Marinov) 47. CH 2 CHO+H=CH 3+HCO 48. CH 2 CHO+H=CH 2 CO+H 2 49. CH 2 CHO+O=CH 2 O+HCO 50. CH 2 CHO+OH=CH 2 CO+H 2 O 51. CH 2 CHO+O 2=CH 2 O+CO+OH 52. CH 2 CHO+CH 3=C 2 H 5+CO+H 53. CH 2 CHO+HO 2=CH 2 O+HCO+OH 54. CH 2 CHO+HO 2=CH 3 CHO+O 2 55. CH 2 CHO=CH 3+CO 5. 0 E+13 2. 0 E+13 1. 0 E+14 3. 0 E+13 3. 0 E+10 4. 9 E+14 7. 0 E+12 3. 0 E+12 1. 17 E+43 0. 0 0. 0 -0. 5 0. 0 -9. 8 0. 0 0. 0 43800. 0 (Marinov) (Marinov) (Marinov) (This study) San Diego Mech: http: //maeweb. ucsd. edu/~combustion/cermech/ 35
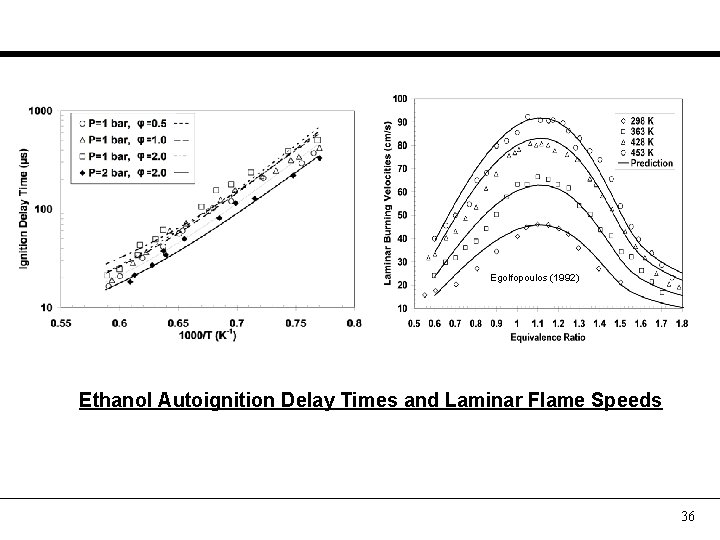
Egolfopoulos (1992) Ethanol Autoignition Delay Times and Laminar Flame Speeds 36
Combustion Process 37
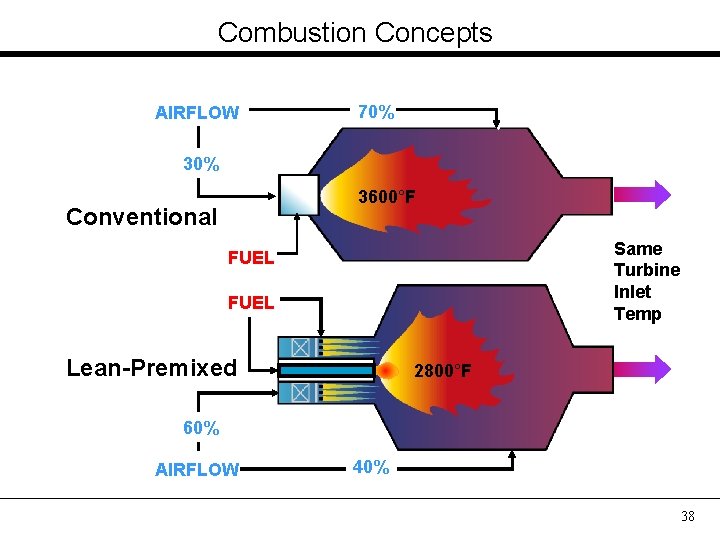
Combustion Concepts AIRFLOW 70% 3600°F Conventional Same Turbine Inlet Temp FUEL Lean-Premixed 2800°F 60% AIRFLOW 40% 38
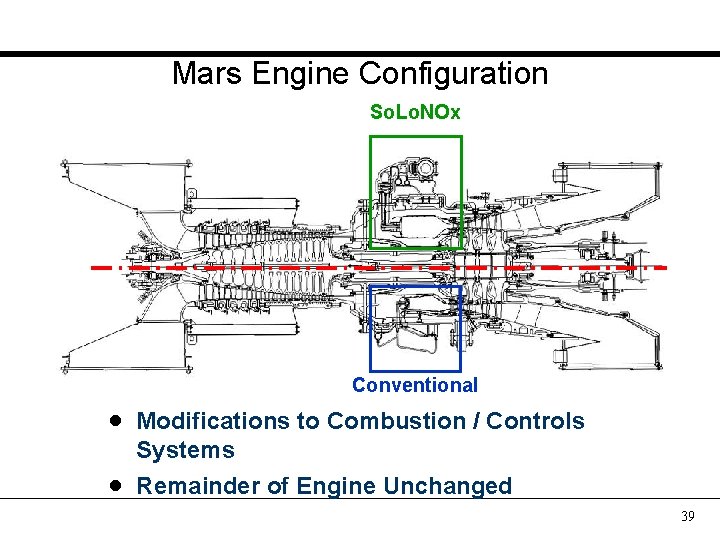
Mars Engine Configuration So. Lo. NOx Conventional · · Modifications to Combustion / Controls Systems Remainder of Engine Unchanged 39
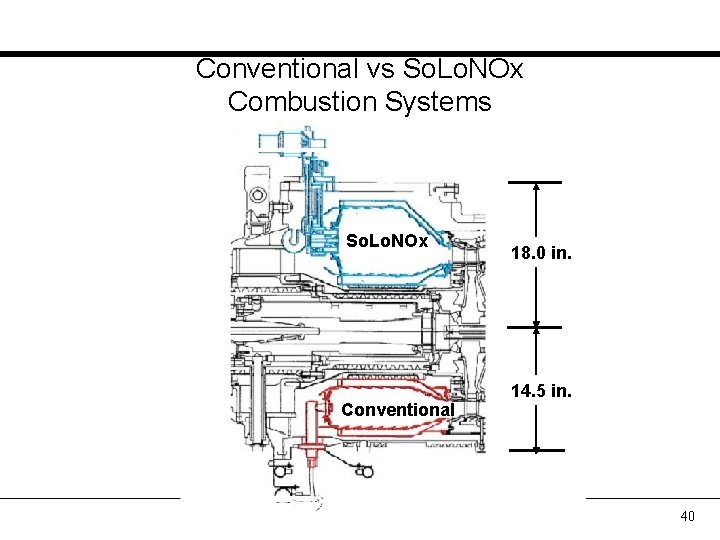
Conventional vs So. Lo. NOx Combustion Systems So. Lo. NOx Conventional 18. 0 in. 14. 5 in. 40
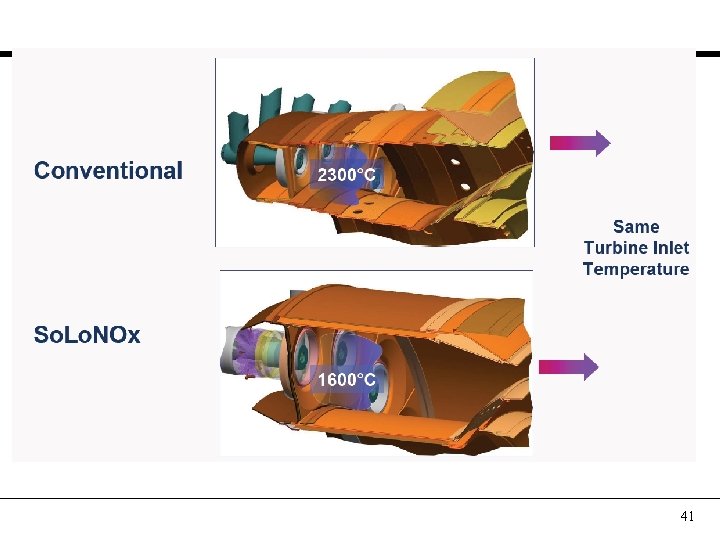
41
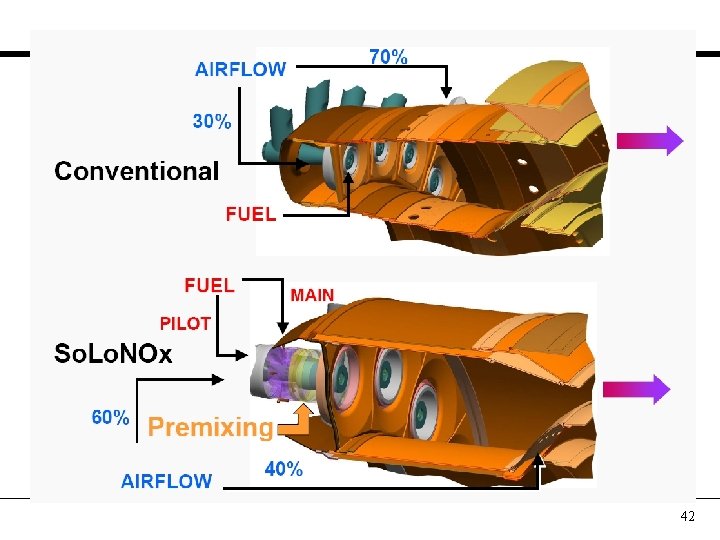
42
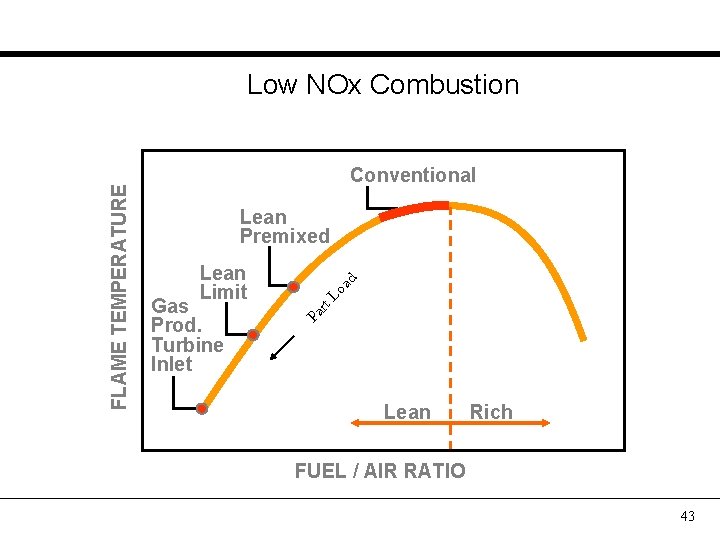
Conventional Lo Gas Prod. Turbine Inlet rt Lean Limit ad Lean Premixed Pa FLAME TEMPERATURE Low NOx Combustion Lean Rich FUEL / AIR RATIO 43
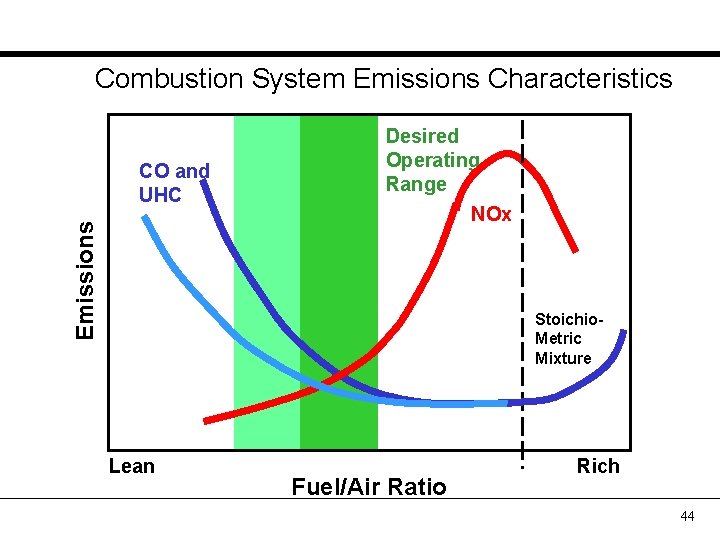
Combustion System Emissions Characteristics Emissions CO and UHC Desired Operating Range NOx Stoichio. Metric Mixture Lean Fuel/Air Ratio Rich 44
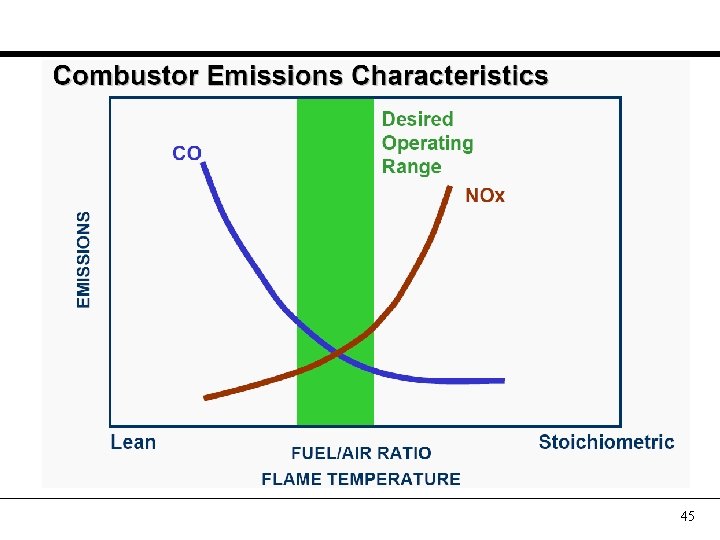
45
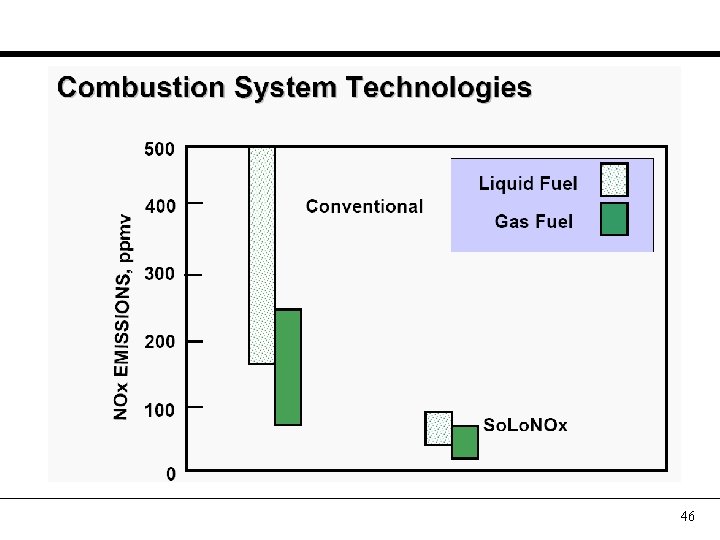
46
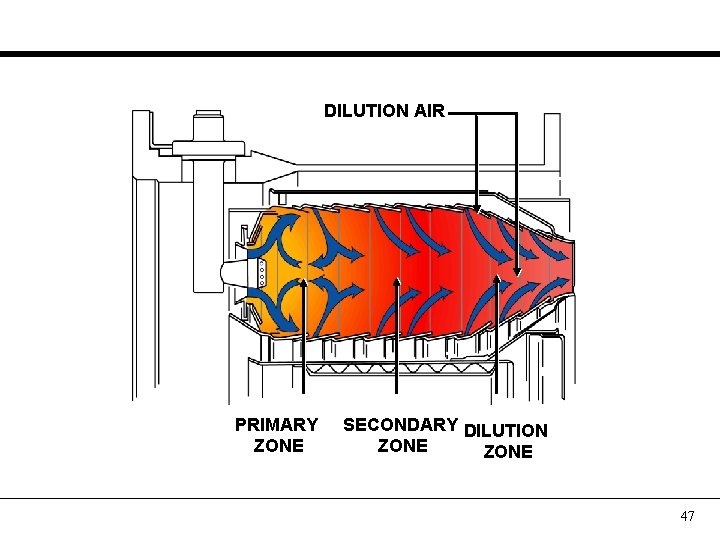
DILUTION AIR PRIMARY ZONE SECONDARY DILUTION ZONE 47
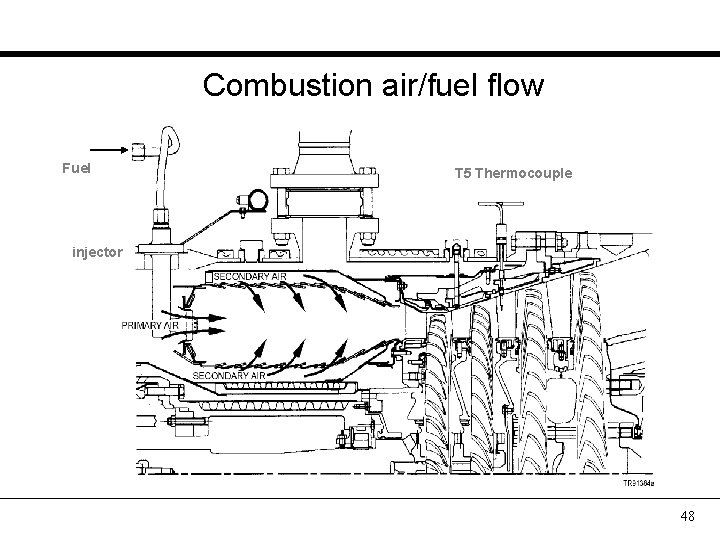
Combustion air/fuel flow Fuel T 5 Thermocouple injector 48
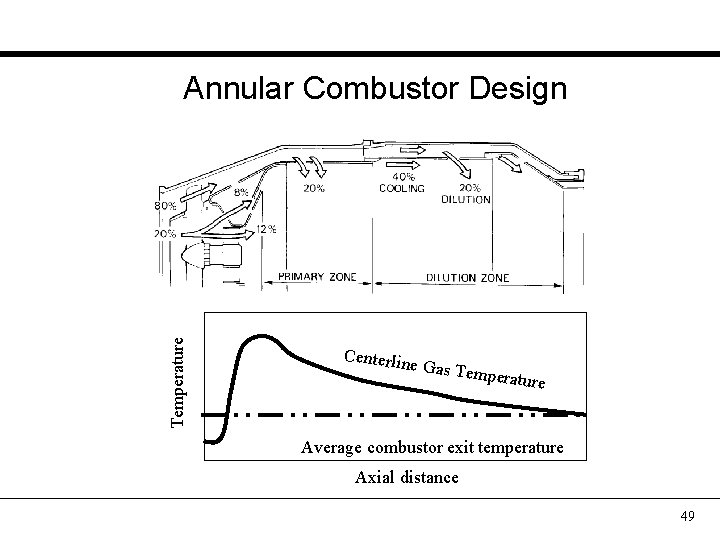
Temperature Annular Combustor Design Centerlin e Gas Tem perature Average combustor exit temperature Axial distance 49
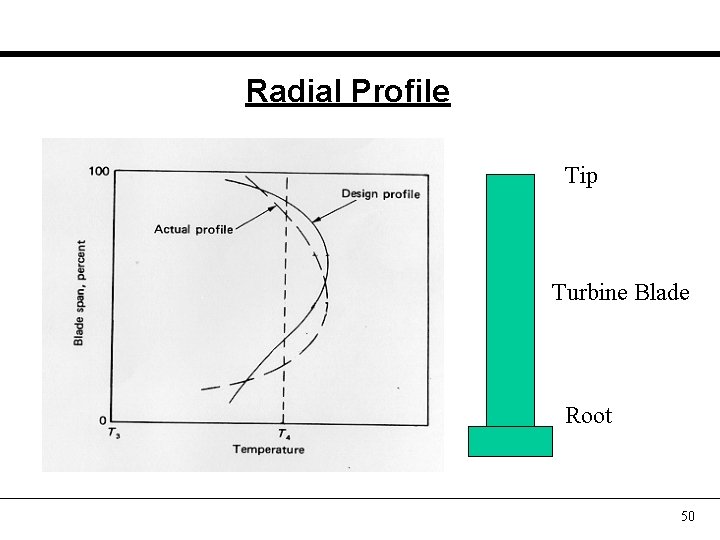
Radial Profile Tip Turbine Blade Root 50
Rig Test Measurements • • Exit temperature pattern and profile Liner metal temperatures Emissions Pressure Loss Stability Limits Combustion Efficiency Ignition Loop 51
Fuels 52
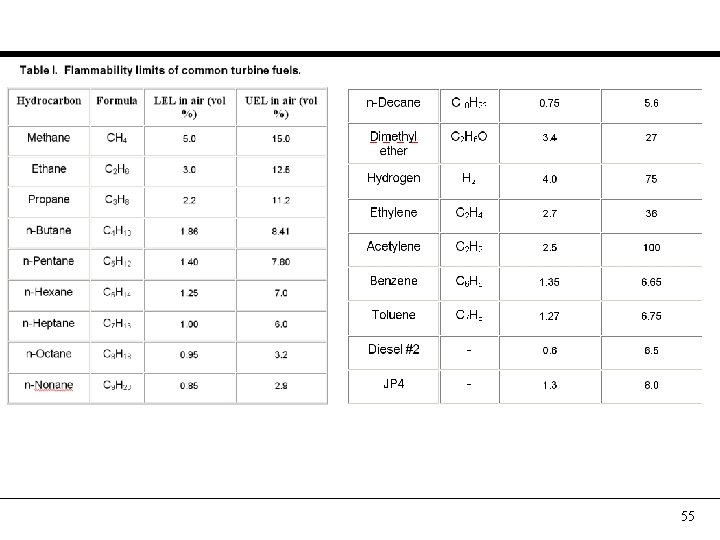
Gaseous fuel molecules 1. 2. 3. 4. 5. 6. 7. 8. 9. Methane (CH 4) Ethane (C 2 H 6) Propane (C 3 H 8) Butane (C 4 H 10) Pentane (C 5 H 12) Hexane (C 6 H 14), Heptane (C 7 H 16) and higher Carbon monoxide (CO) Hydrogen (H 2) Nitrogen (N 2), Carbon Di-oxide (CO 2), Water (H 2 O) 53
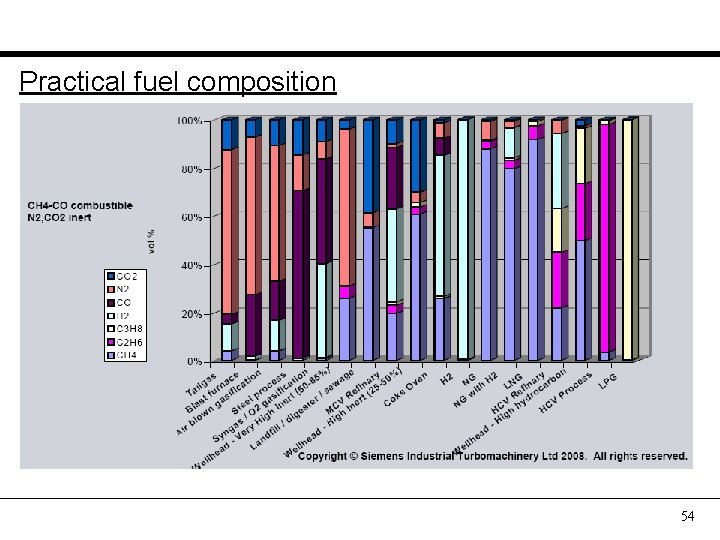
Practical fuel composition 54
55
Important Fuel Properties • • Heating Value/Wobbe Index Dew Point Blowout Flashback Laminar and Turbulent Flame Speed Autoignition Delay Time Ratio of Flammability Limits Flame Temperature 56
Gaseous Fuel Analysis • Accurate Fuel Composition – (CO, H 2 S, CO 2, H 2, C 2 H 4, O 2) Safety/Leakage • Molecular Weight/Sp. Gr. • Flammability/Explosion Limits • Heating Value, Wobbe Index Energy Input/Fuel Flow • Flame Temperature Emissions Single Phase • Dew Point • Flame Speed Reaction in Premixer • Autoignition Turbine • Contaminants Durability 57
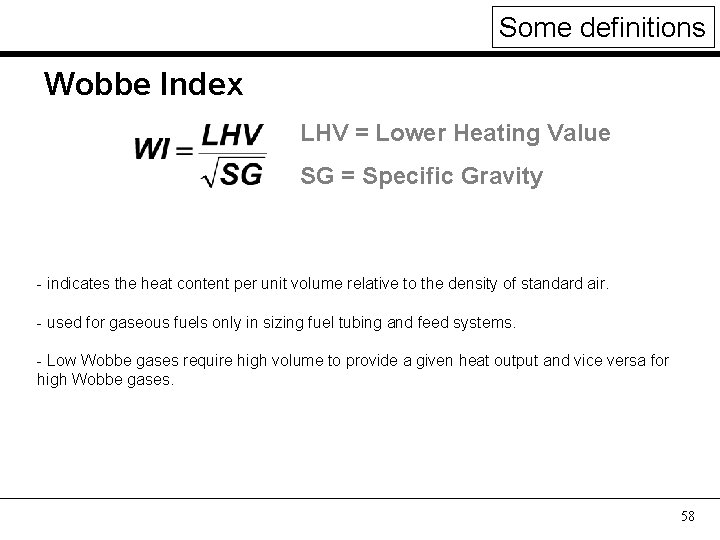
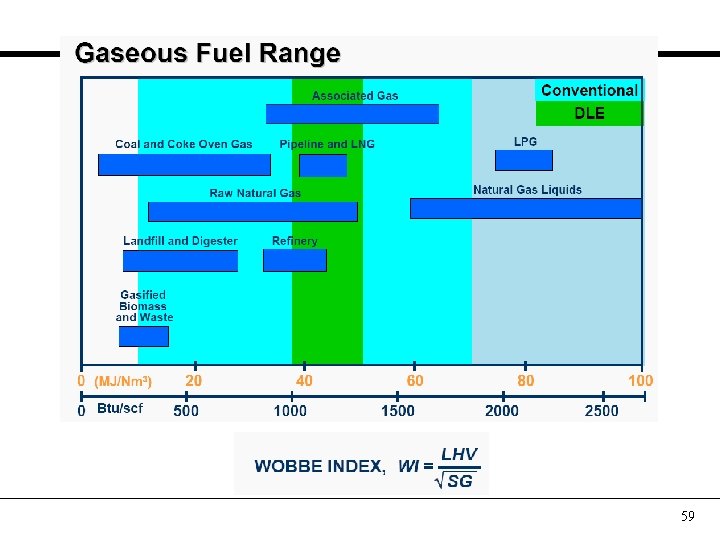
Some definitions Wobbe Index LHV = Lower Heating Value SG = Specific Gravity - indicates the heat content per unit volume relative to the density of standard air. - used for gaseous fuels only in sizing fuel tubing and feed systems. - Low Wobbe gases require high volume to provide a given heat output and vice versa for high Wobbe gases. 58
59
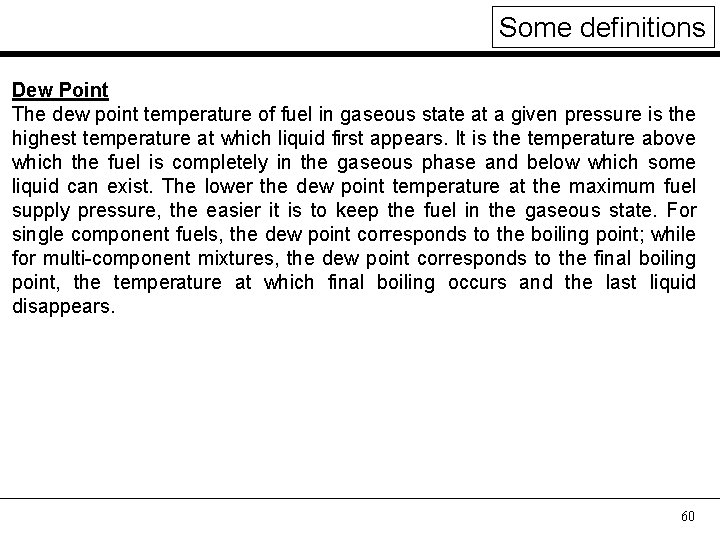
Some definitions Dew Point The dew point temperature of fuel in gaseous state at a given pressure is the highest temperature at which liquid first appears. It is the temperature above which the fuel is completely in the gaseous phase and below which some liquid can exist. The lower the dew point temperature at the maximum fuel supply pressure, the easier it is to keep the fuel in the gaseous state. For single component fuels, the dew point corresponds to the boiling point; while for multi-component mixtures, the dew point corresponds to the final boiling point, the temperature at which final boiling occurs and the last liquid disappears. 60
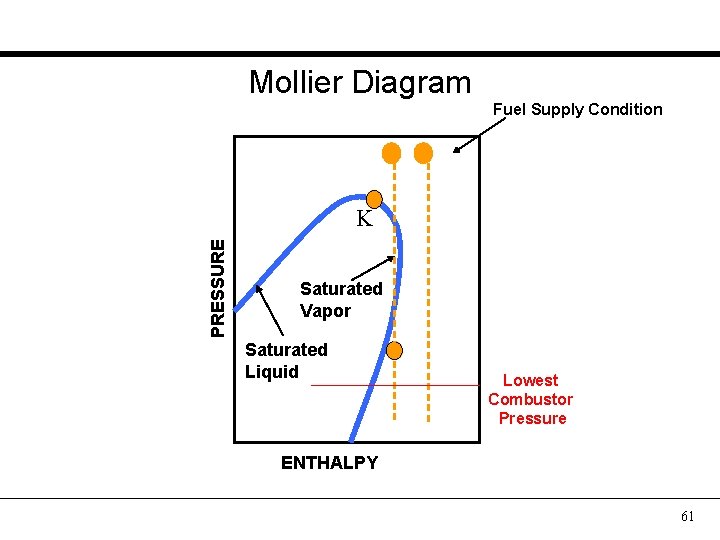
Mollier Diagram Fuel Supply Condition PRESSURE K Saturated Vapor Saturated Liquid Lowest Combustor Pressure ENTHALPY 61
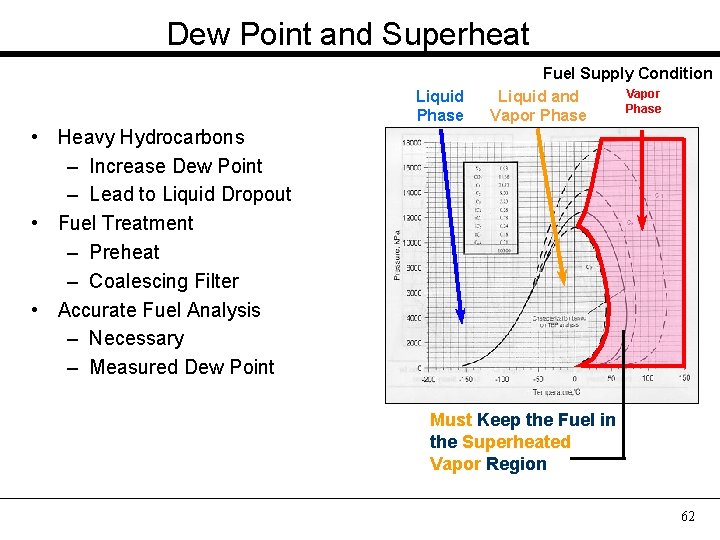
Dew Point and Superheat Liquid Phase Fuel Supply Condition Vapor Liquid and Phase Vapor Phase • Heavy Hydrocarbons – Increase Dew Point – Lead to Liquid Dropout • Fuel Treatment – Preheat – Coalescing Filter • Accurate Fuel Analysis – Necessary – Measured Dew Point Must Keep the Fuel in the Superheated Vapor Region 62
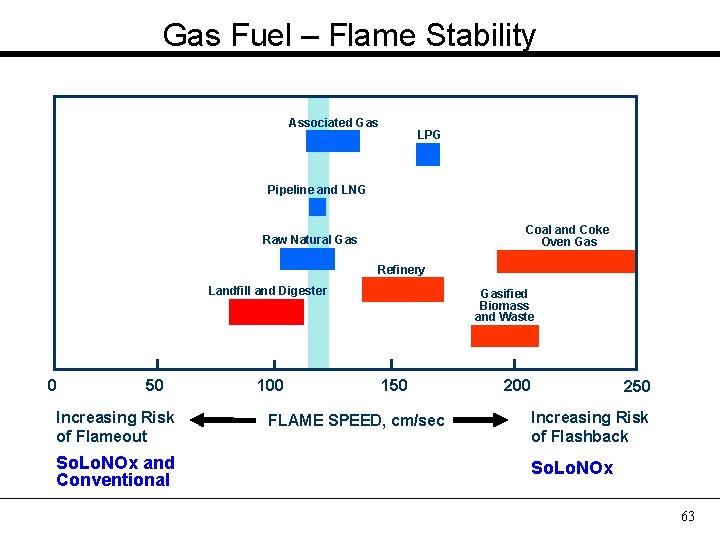
Gas Fuel – Flame Stability Associated Gas LPG Pipeline and LNG Coal and Coke Oven Gas Raw Natural Gas Refinery Landfill and Digester 0 50 Increasing Risk of Flameout So. Lo. NOx and Conventional 100 Gasified Biomass and Waste 150 FLAME SPEED, cm/sec 200 250 Increasing Risk of Flashback So. Lo. NOx 63
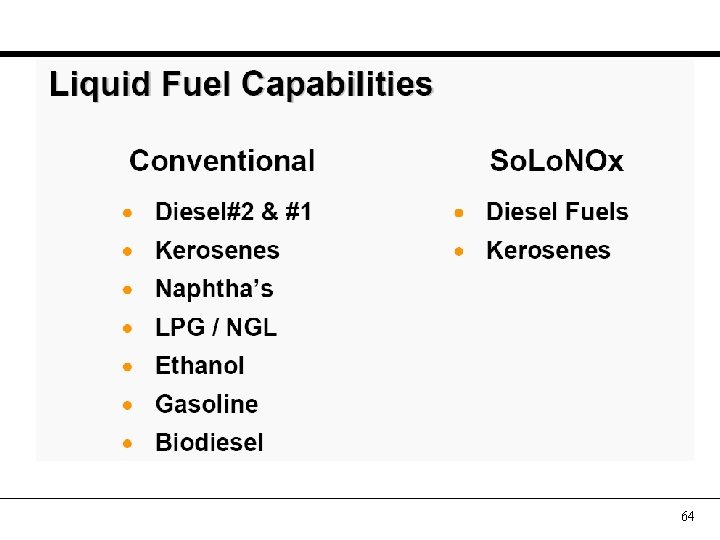
64
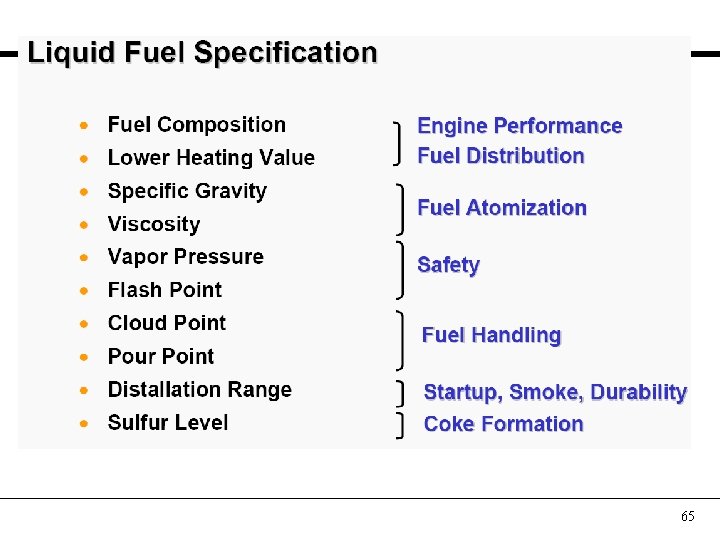
65
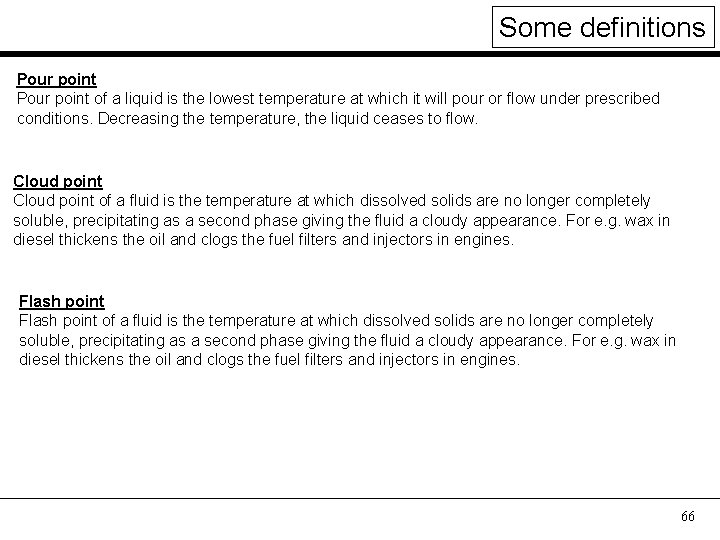
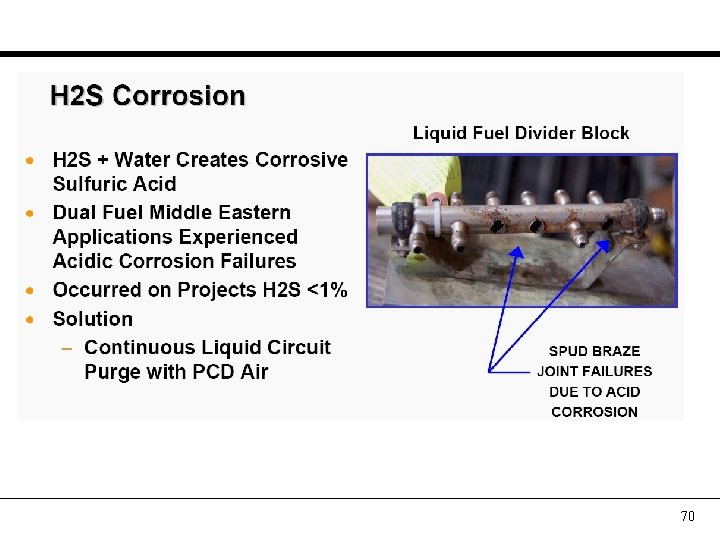
Some definitions Pour point of a liquid is the lowest temperature at which it will pour or flow under prescribed conditions. Decreasing the temperature, the liquid ceases to flow. Cloud point of a fluid is the temperature at which dissolved solids are no longer completely soluble, precipitating as a second phase giving the fluid a cloudy appearance. For e. g. wax in diesel thickens the oil and clogs the fuel filters and injectors in engines. Flash point of a fluid is the temperature at which dissolved solids are no longer completely soluble, precipitating as a second phase giving the fluid a cloudy appearance. For e. g. wax in diesel thickens the oil and clogs the fuel filters and injectors in engines. 66
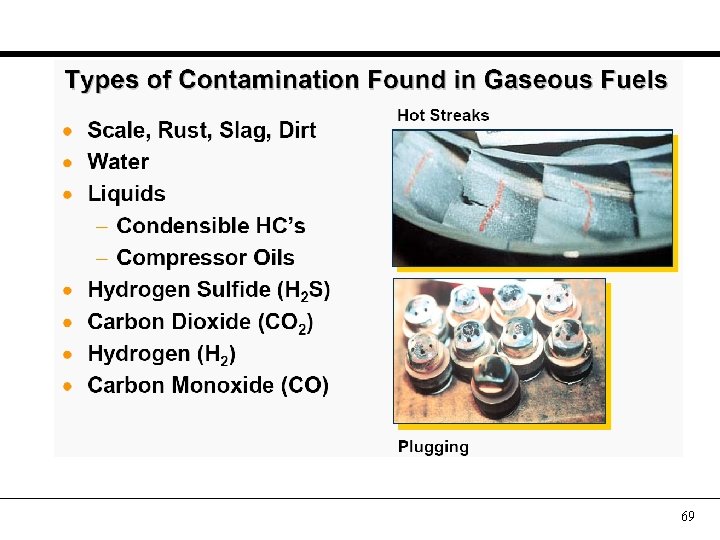
Fuel: Contaminants • • Solids Water Heavy gases present as liquids Other combustibles (H 2, CO) Oils (e. g from upstream compressors) Hydrogen Sulfide (H 2 S) Carbon Dioxide (CO 2) 67

68
69
70

71

Hot Corrosion on a Turbine Rotor 72
Emissions 73
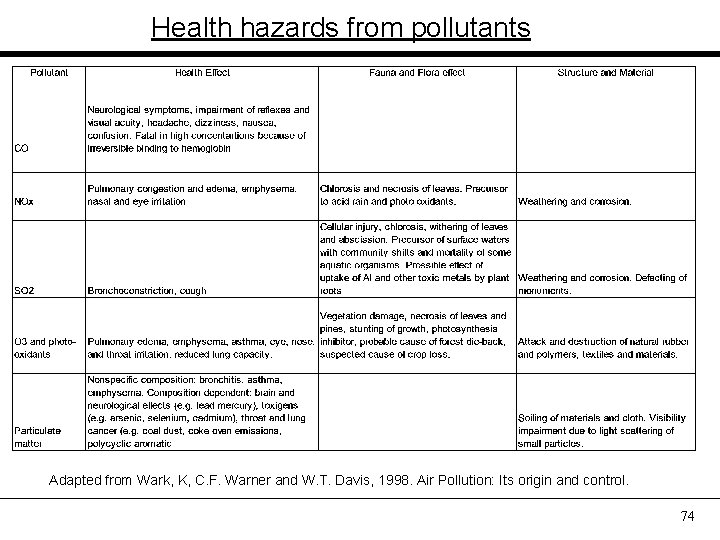
Health hazards from pollutants Adapted from Wark, K, C. F. Warner and W. T. Davis, 1998. Air Pollution: Its origin and control. 74
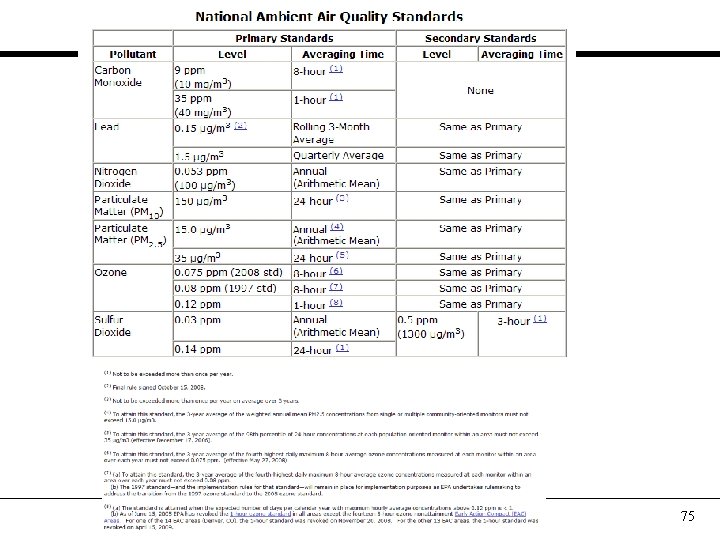
75
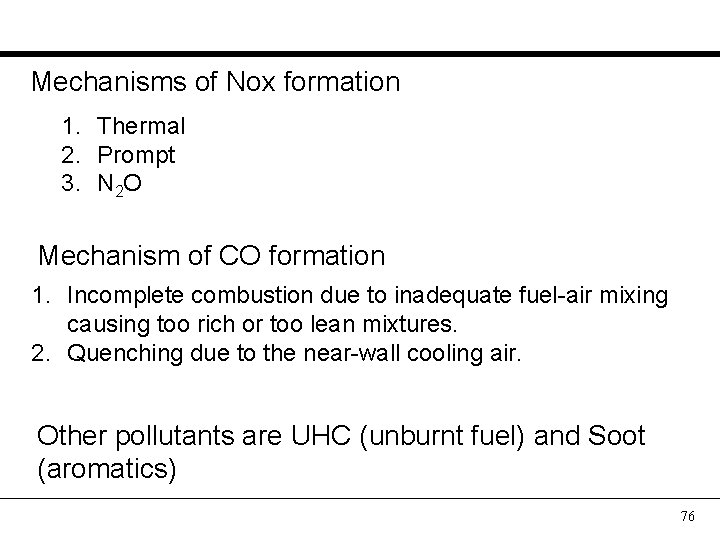
Mechanisms of Nox formation 1. Thermal 2. Prompt 3. N 2 O Mechanism of CO formation 1. Incomplete combustion due to inadequate fuel-air mixing causing too rich or too lean mixtures. 2. Quenching due to the near-wall cooling air. Other pollutants are UHC (unburnt fuel) and Soot (aromatics) 76
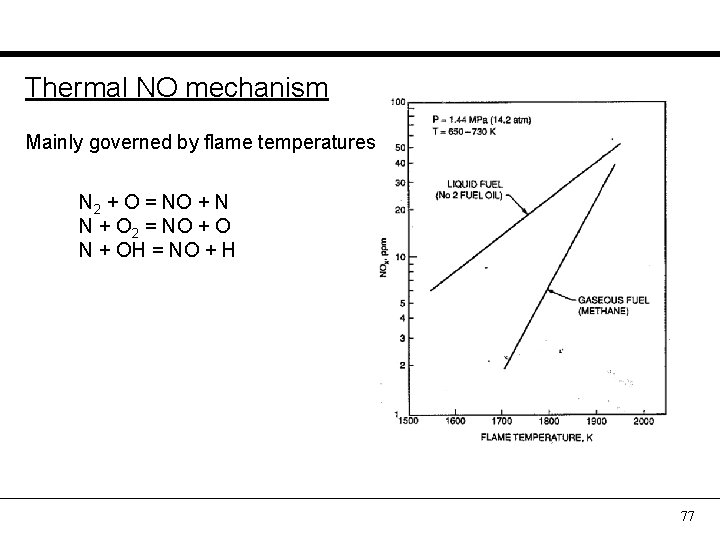
Thermal NO mechanism Mainly governed by flame temperatures N 2 + O = NO + N N + O 2 = NO + O N + OH = NO + H 77
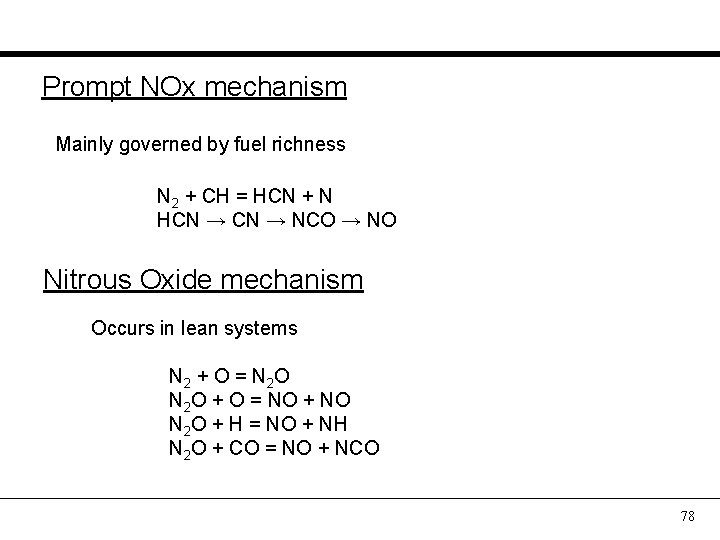
Prompt NOx mechanism Mainly governed by fuel richness N 2 + CH = HCN + N HCN → NCO → NO Nitrous Oxide mechanism Occurs in lean systems N 2 + O = N 2 O N 2 O + O = NO + NO N 2 O + H = NO + NH N 2 O + CO = NO + NCO 78
Technologies to reduce the production of NOx in Industrial Gas Turbines • Water, steam or inert gas injection in conventional systems • Dry Low NOx (lean premixed combustion) • Catalytic reduction - In combustion chamber (leaner mixtures) - Exhaust treatment (NH 3 reacting NOx forming N 2 and H 2 O 79
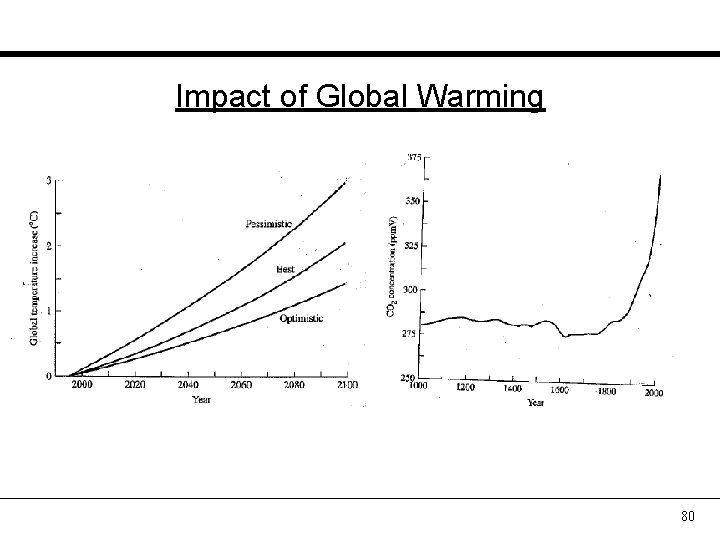
Impact of Global Warming 80
Oscillations 81
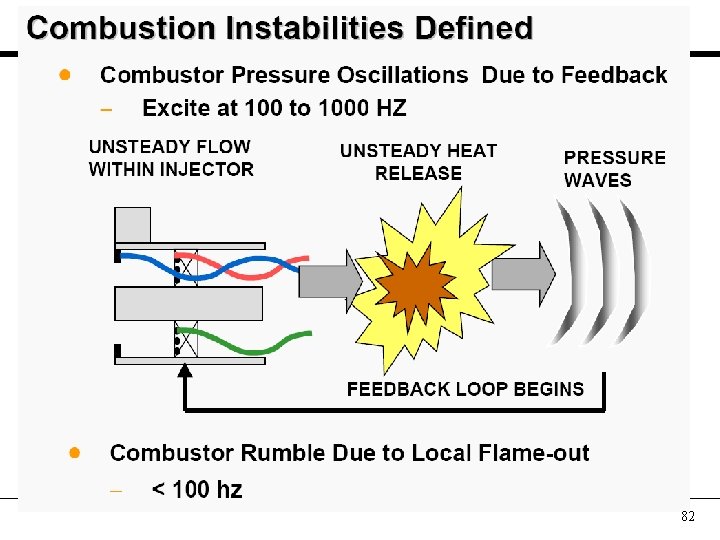
82
Some cooling concepts 83
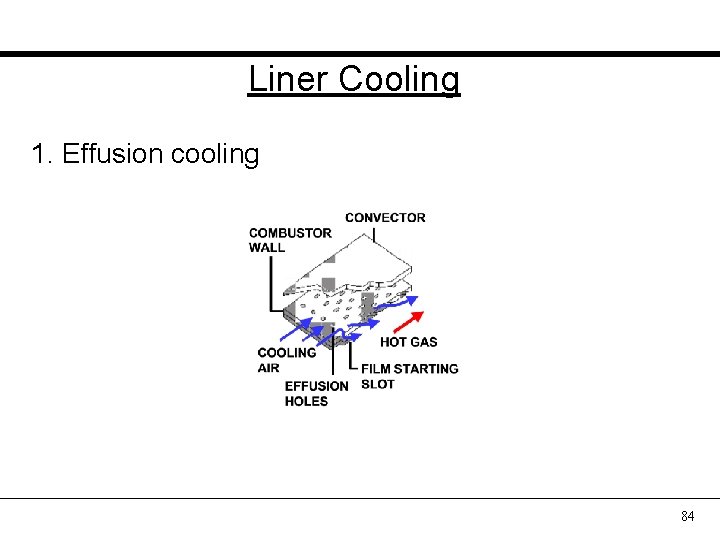
Liner Cooling 1. Effusion cooling 84
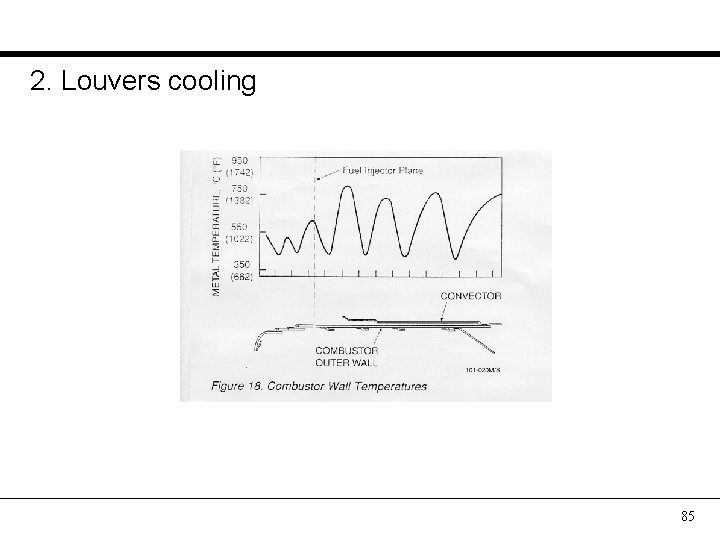
2. Louvers cooling 85
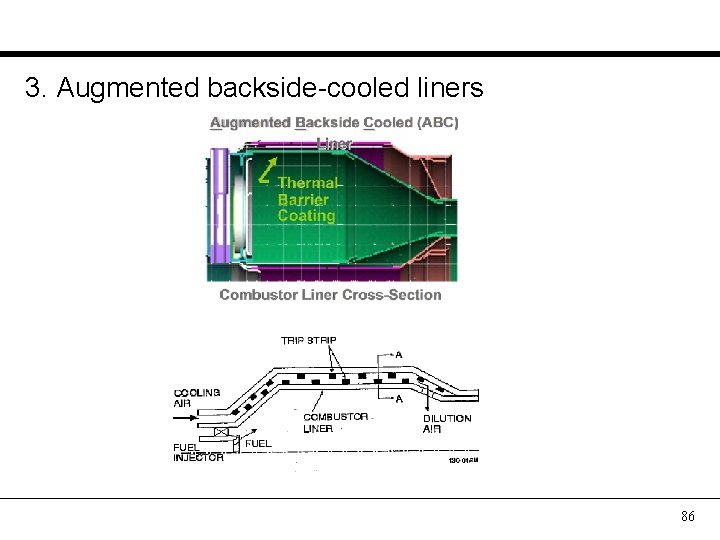
3. Augmented backside-cooled liners 86
87
- Slides: 87