GAS LAWS COMBINED GAS LAW l STP T
GAS LAWS
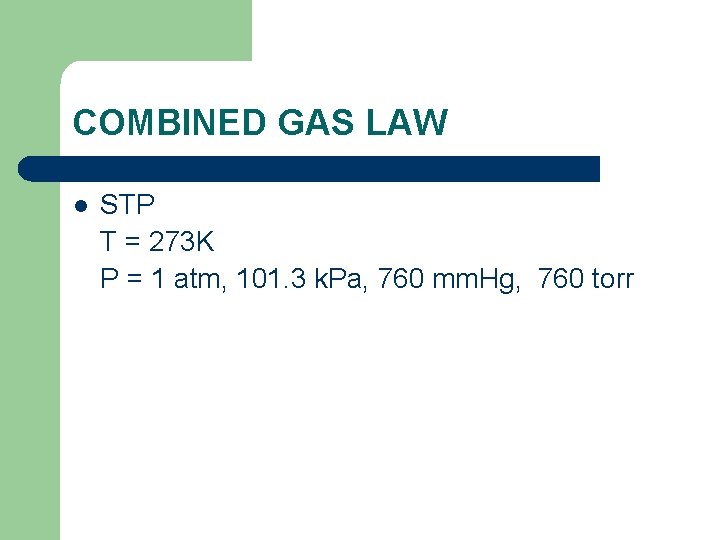
COMBINED GAS LAW l STP T = 273 K P = 1 atm, 101. 3 k. Pa, 760 mm. Hg, 760 torr

l 10. 0 cm 3 volume of a gas measured 75. 6 k. Pa and 60. 0 o. C is to be corrected to correspond to the volume it would occupy at STP.

l Methane is compressed in a closed 15. 8 dm 3 container at 101. 3 KPa. If the volume drops to 8. 7 dm 3 and the temperature begins at 25 o. C and then drops to 18 o. C , what will the pressure of the gas be?

IDEAL GAS LAW l P V = n R T l Calculate R if pressure is in atm.

l Calculate R if pressure is in k. Pa
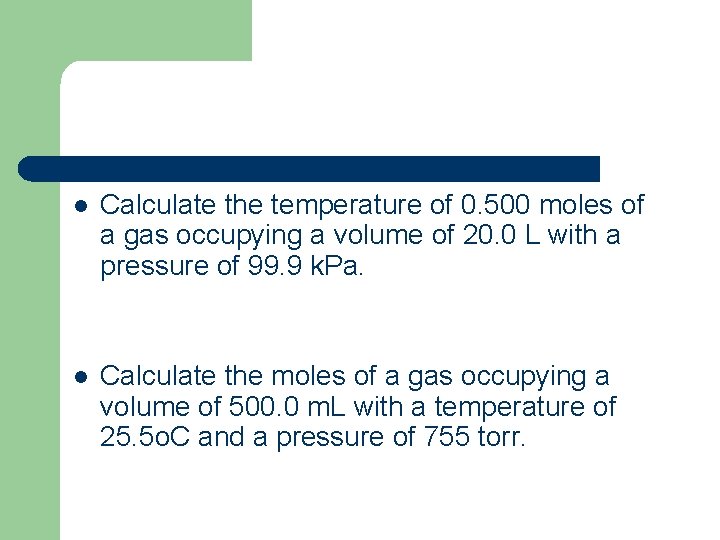
l Calculate the temperature of 0. 500 moles of a gas occupying a volume of 20. 0 L with a pressure of 99. 9 k. Pa. l Calculate the moles of a gas occupying a volume of 500. 0 m. L with a temperature of 25. 5 o. C and a pressure of 755 torr.

l If carbon dioxide gas occupies a volume of 56. 2 ml at a pressure of 750 torr and 25 o. C, how many grams of carbon dioxide gas do you have?
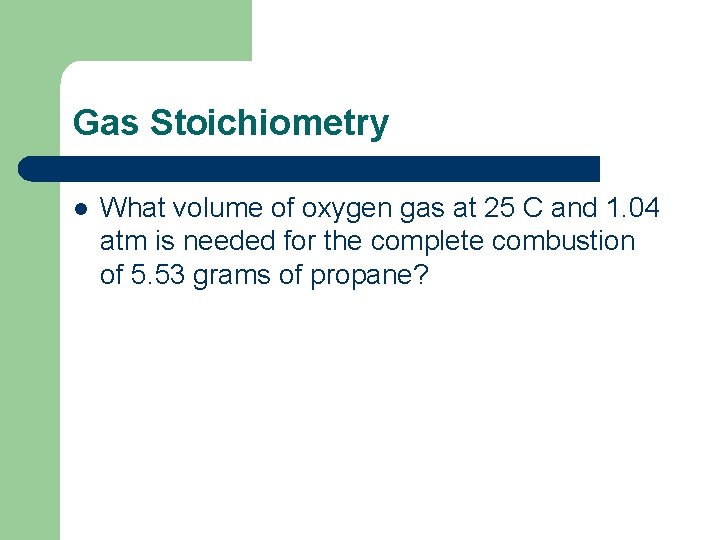
Gas Stoichiometry l What volume of oxygen gas at 25 C and 1. 04 atm is needed for the complete combustion of 5. 53 grams of propane?
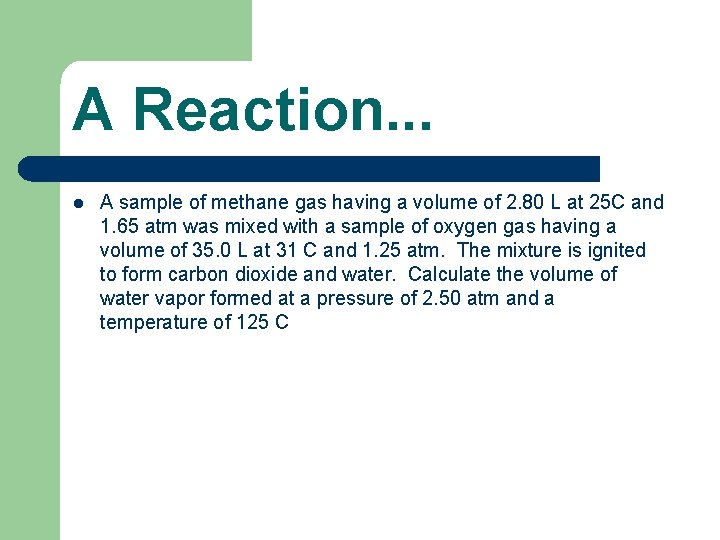
A Reaction. . . l A sample of methane gas having a volume of 2. 80 L at 25 C and 1. 65 atm was mixed with a sample of oxygen gas having a volume of 35. 0 L at 31 C and 1. 25 atm. The mixture is ignited to form carbon dioxide and water. Calculate the volume of water vapor formed at a pressure of 2. 50 atm and a temperature of 125 C

Dalton’s Law of Partial Pressure l l The total pressure in a container is the sum of the partial pressures of all the gases in the container Ptotal = P 1 + P 2 + …

l A mixture of gasses is collected at a pressure of 101. 3 k. Pa. If the gas is 70. % nitrogen, 20. % oxygen and 10% carbon dioxide, what is the partial pressure of each gas in the mixture?
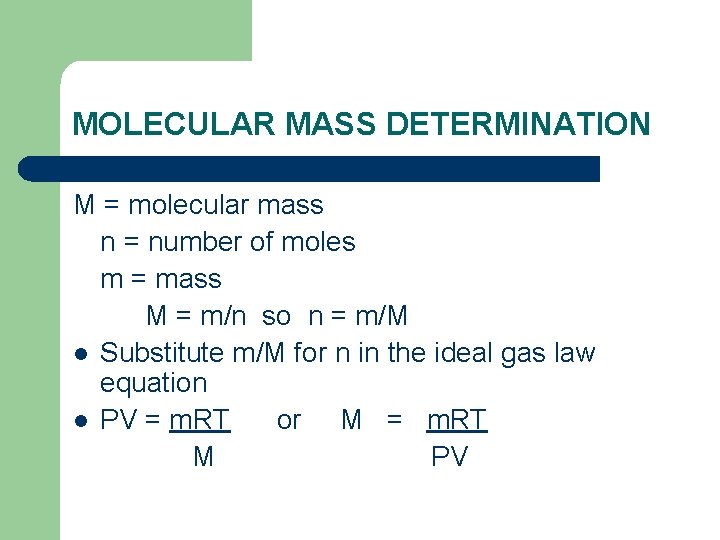
MOLECULAR MASS DETERMINATION M = molecular mass n = number of moles m = mass M = m/n so n = m/M l Substitute m/M for n in the ideal gas law equation l PV = m. RT or M = m. RT M PV
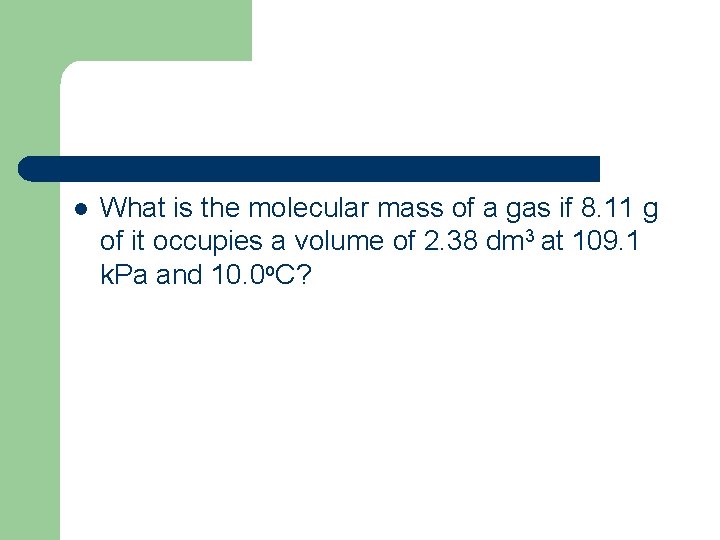
l What is the molecular mass of a gas if 8. 11 g of it occupies a volume of 2. 38 dm 3 at 109. 1 k. Pa and 10. 0 o. C?

l A gas is collected over water at 21. 0 o. C at a pressure of 750 mm. Hg. The gas had a mass of 0. 128 g and the volume is 100. cm 3. What is the molar mass of the gas?
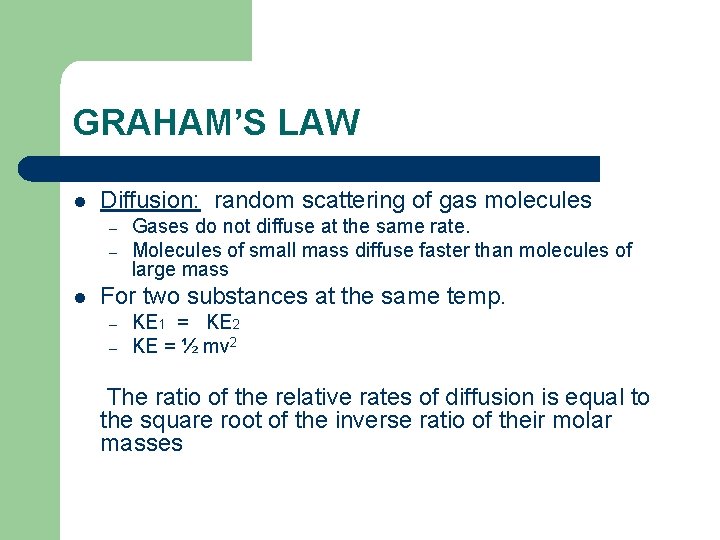
GRAHAM’S LAW l Diffusion: random scattering of gas molecules – – l Gases do not diffuse at the same rate. Molecules of small mass diffuse faster than molecules of large mass For two substances at the same temp. – – KE 1 = KE 2 KE = ½ mv 2 The ratio of the relative rates of diffusion is equal to the square root of the inverse ratio of their molar masses

l Find the relative rates of diffusion for the gases krypton and bromine. l Find the relative rates of diffusion for the gases hydrogen and nitrogen.

Hydrogen gas diffuses 3. 724 times faster than gas A. What is the molar mass for gas A and what is gas A?
- Slides: 18