Gas Laws Ch 14 Gases Kinetic Molecular Theory









- Slides: 15

Gas Laws Ch. 14

Gases • Kinetic Molecular Theory (KMT) says: – Gases have massdemo – Gases are easily compressed – Gases fill their container completely – Gases move through each other easily (diffusion) – Gases exert pressure (balloons, wind, atmosphere) – Collisions of gas particles are perfectly elastic

Gases, cont. • In order to describe a gas completely, four variables must be considered: • n = amount of gas (moles) • V = volume of gas (Liters) • T = temperature of the gas (Kelvin) • P = pressure of/on the gas (usually atm) • 1 atm = 101, 325 Pa = 760 mm. Hg = 760 torr = 14. 7 psi = 1. 013 bar • STP = standard temp. & pressure (0°C and 1 atm)

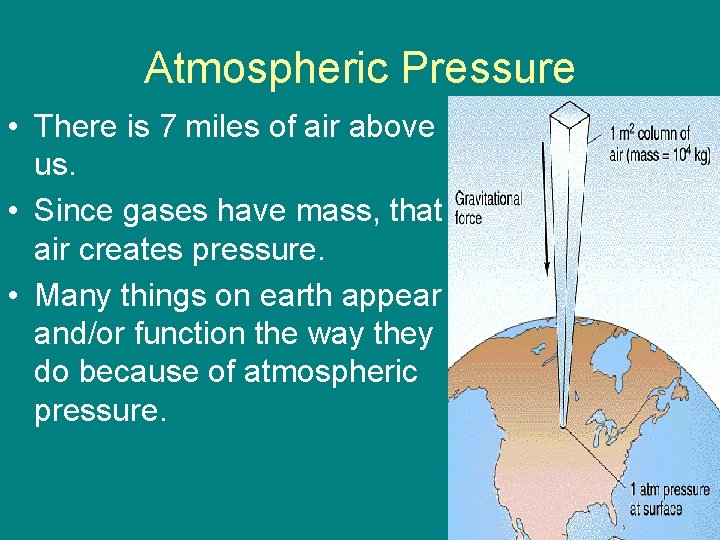
Atmospheric Pressure • There is 7 miles of air above us. • Since gases have mass, that air creates pressure. • Many things on earth appear and/or function the way they do because of atmospheric pressure.

Boyle’s Law • Inverse relationship between pressure and volume • With temp. constant, as pressure of a gas increases, the volume decreases (and vice versa) • P 1 x V 1 = k • P 2 x V 2 = k • So, P 1 V 1 = P 2 V 2

Charles’ Law • Direct relationship between volume and temperature. • As the temperature of a gas increases, the volume increases; if pressure is constant. • V 1 ÷ T 1 = k • V 2 ÷ T 2 = k • So, (V 1 ÷ T 1) = (V 2 ÷ T 2)



Dalton’s Law of Partial Pressures • Pertains to mixtures of gases. • The sum of the partial pressures of all the component gases in a mixture is equal to the total pressure of the mixture. • PT = p 1 + p 2 + p 3 …. .

Avogadro’s Law • Equal volumes of different gases at the same temp. and pressure contain equal numbers of particles (equal # of moles) • Volume is directly proportional to # of moles. • V 1 ÷ n 1 = V 2 ÷ n 2 • 1 mole of any gas at STP occupies 22. 4 L of volume.

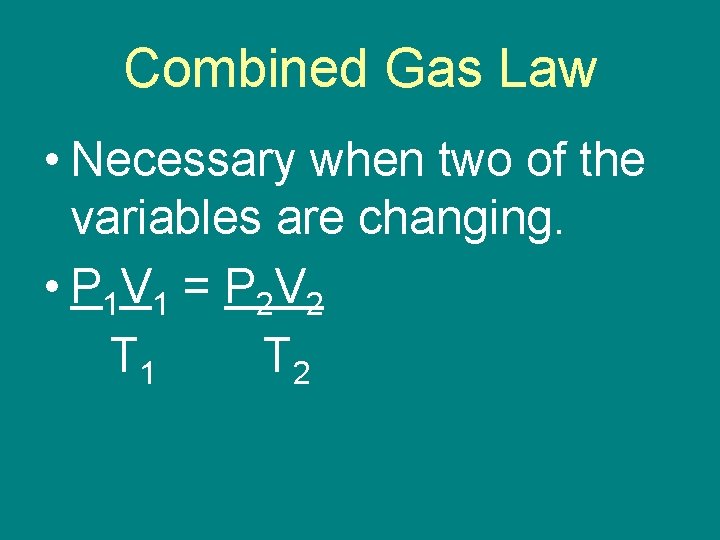
Combined Gas Law • Necessary when two of the variables are changing. • P 1 V 1 = P 2 V 2 T 1 T 2

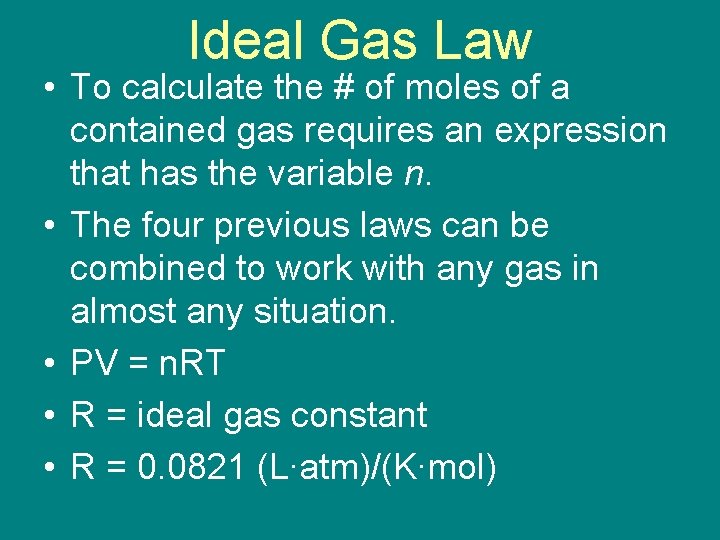
Ideal Gas Law • To calculate the # of moles of a contained gas requires an expression that has the variable n. • The four previous laws can be combined to work with any gas in almost any situation. • PV = n. RT • R = ideal gas constant • R = 0. 0821 (L∙atm)/(K∙mol)

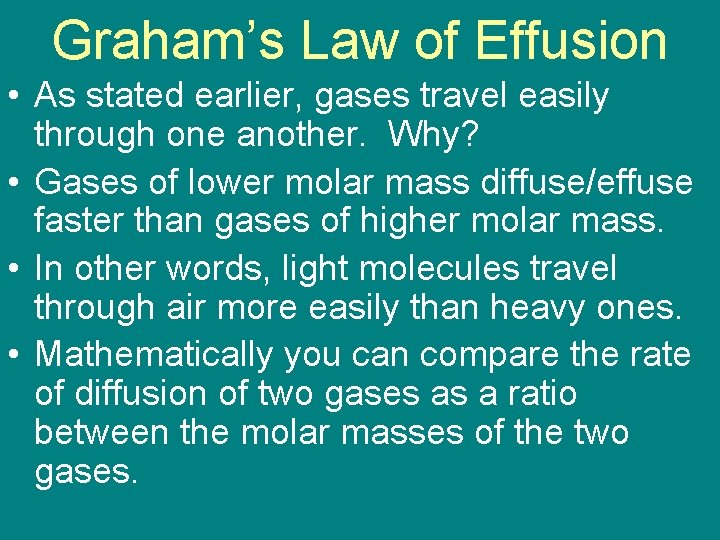
Graham’s Law of Effusion • As stated earlier, gases travel easily through one another. Why? • Gases of lower molar mass diffuse/effuse faster than gases of higher molar mass. • In other words, light molecules travel through air more easily than heavy ones. • Mathematically you can compare the rate of diffusion of two gases as a ratio between the molar masses of the two gases.

• So, which gas would effuse/diffuse faster, hydrogen (H 2)or sulfur hexafluoride (SF 6)? • Which gas would effuse/diffuse faster, hydrogen (H 2) or oxygen (O 2)? • You can be more precise than that: How much faster would hydrogen travel through air than oxygen?

• What to Know for Gas Laws Test • Know your notes, and all of the new vocabulary • Have each of the gas laws memorized, by name and by formula • After reading a problem, recognize what gas law to use to solve it.