Gas Laws Atmospheric Sciences 101 Autumn 2020 The
















- Slides: 32

Gas Laws Atmospheric Sciences 101 Autumn 2020 The atmosphere is made up of gases so we need to know the basic laws of how gases behave

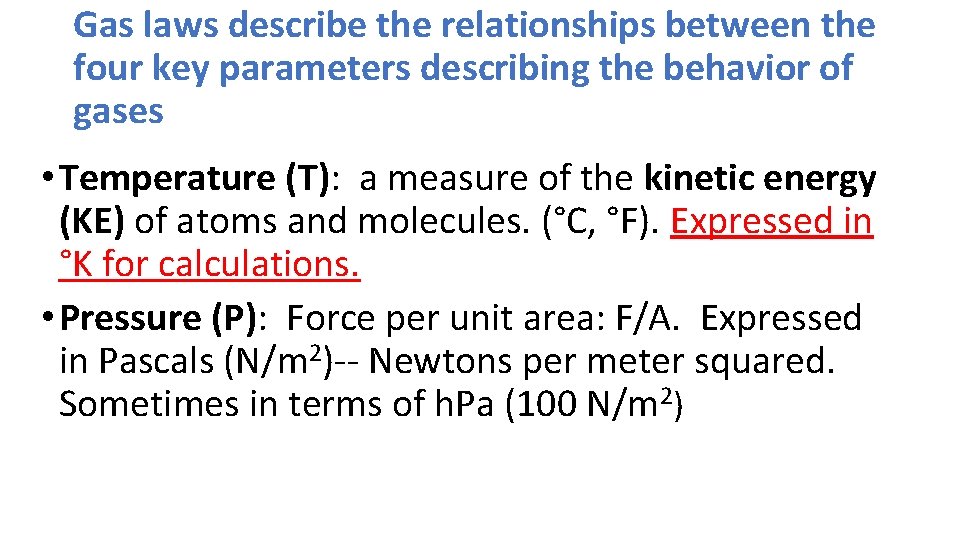
Gas laws describe the relationships between the four key parameters describing the behavior of gases • Temperature (T): a measure of the kinetic energy (KE) of atoms and molecules. (°C, °F). Expressed in °K for calculations. • Pressure (P): Force per unit area: F/A. Expressed in Pascals (N/m 2)-- Newtons per meter squared. Sometimes in terms of h. Pa (100 N/m 2)

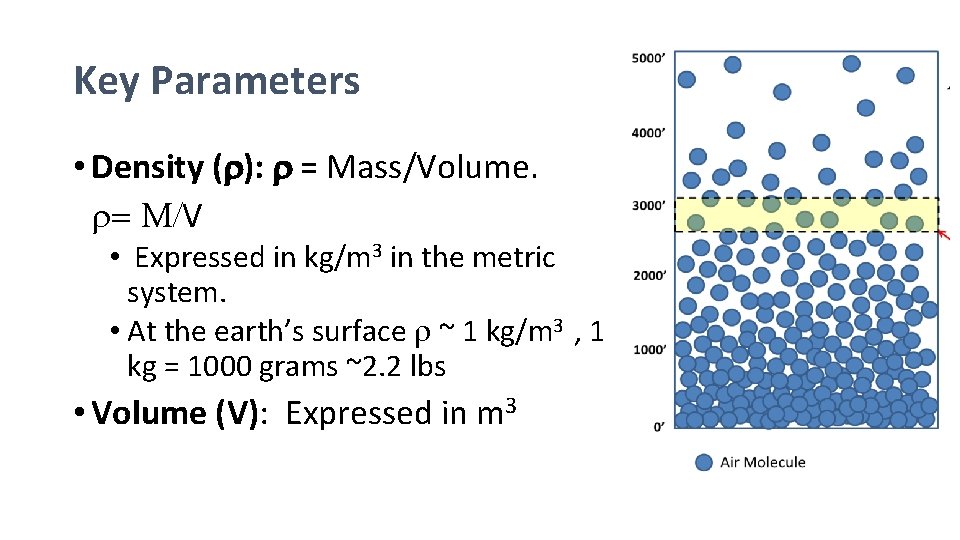
Key Parameters • Density (r): r = Mass/Volume. r= M/V • Expressed in kg/m 3 in the metric system. • At the earth’s surface r ~ 1 kg/m 3 , 1 kg = 1000 grams ~2. 2 lbs • Volume (V): Expressed in m 3

The Key Gas Parameters are Related by GAS LAWS

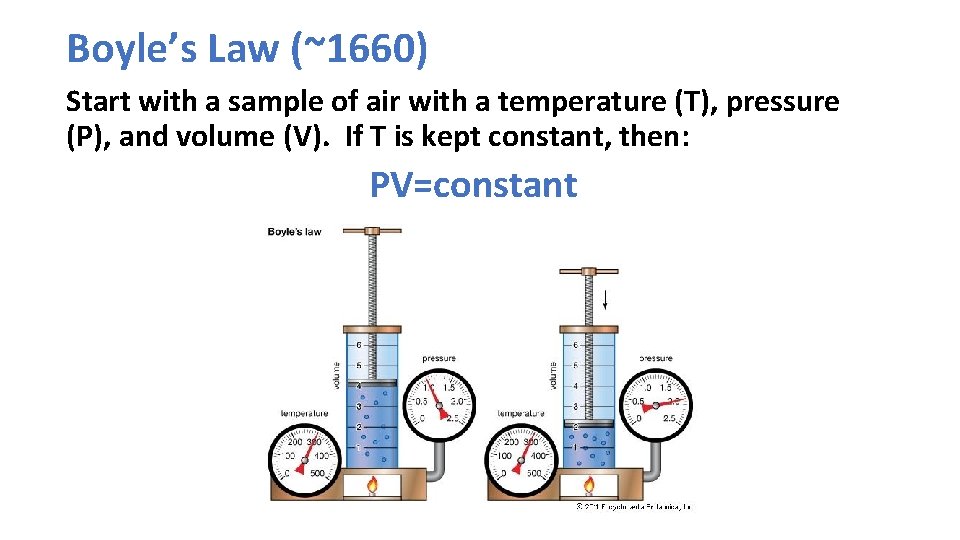
Boyle’s Law (~1660) Start with a sample of air with a temperature (T), pressure (P), and volume (V). If T is kept constant, then: PV=constant

Boyles Law Basis of air pumps we all use

We are all familiar with Boyle’s Law in our daily lives.

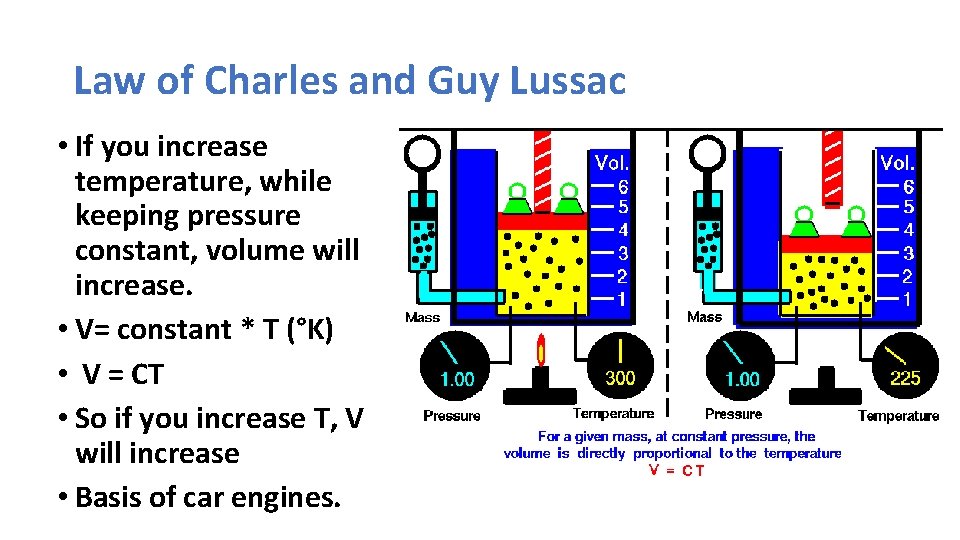
Law of Charles and Guy Lussac • If you increase temperature, while keeping pressure constant, volume will increase. • V= constant * T (°K) • V = CT • So if you increase T, V will increase • Basis of car engines.


Why use two gas laws when you can use one?

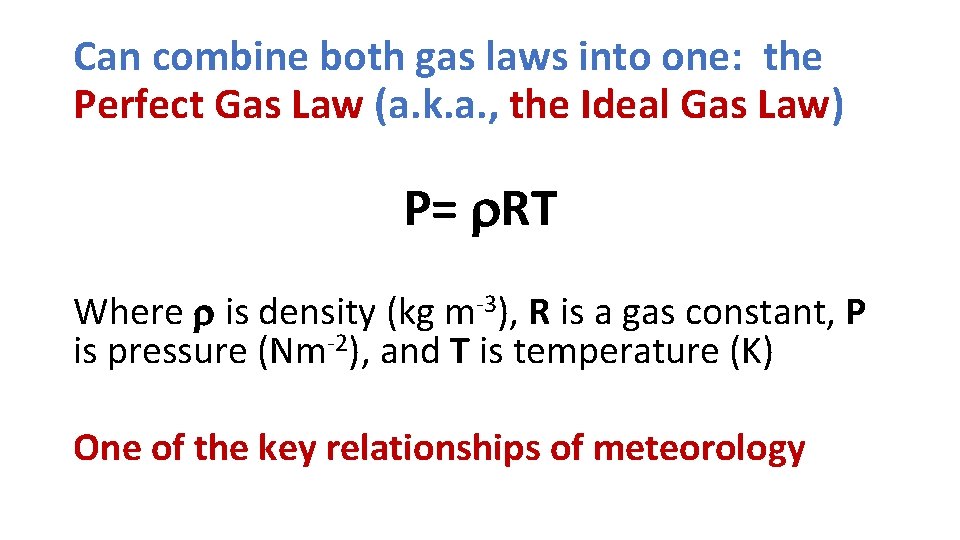
Can combine both gas laws into one: the Perfect Gas Law (a. k. a. , the Ideal Gas Law) P= r. RT Where r is density (kg m-3), R is a gas constant, P is pressure (Nm-2), and T is temperature (K) One of the key relationships of meteorology

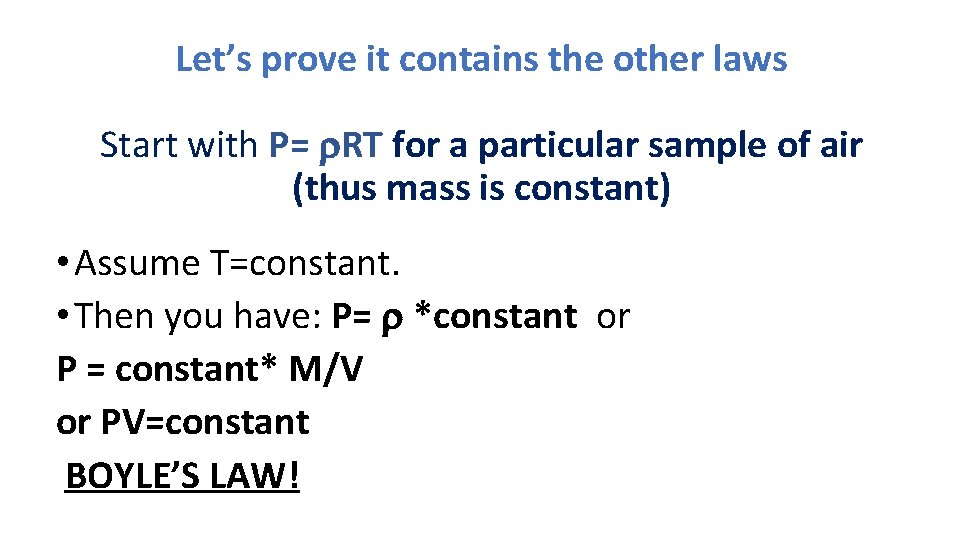
Let’s prove it contains the other laws Start with P= r. RT for a particular sample of air (thus mass is constant) • Assume T=constant. • Then you have: P= r *constant or P = constant* M/V or PV=constant BOYLE’S LAW!

More fun: Law of Charles and Guy Lussac Again start with P= r. RT Assume P=constant. Then you have: Constant=(M/V)*R*T Since Mass and R are constants: Constant= T/V V = constant*T: Law of Charles and Guy Lussac

Gas Law Importance • We will these gas laws to explain many weather features • The perfect gas law is one of the key relationships used in computer weather forecast models.

Adiabatic Processes • Clouds form as air cools when it rises. • But why does rising air cool? • Air warms as it forced to sink. Why? • Why does the spray of aerosol cans feel cold? • And why are bike pumps warm after use?

Explained by adiabatic processes! • Air parcel: An identifiable collection of air that stays together. Think of the air in balloon. • An adiabatic process is a processes that occurs WITHOUT the exchange of energy with the surroundings. • In an meteorological context, an adiabatic process is one in which there is no exchange of energy between an air parcel and the surrounding air.

Think of an insulated box

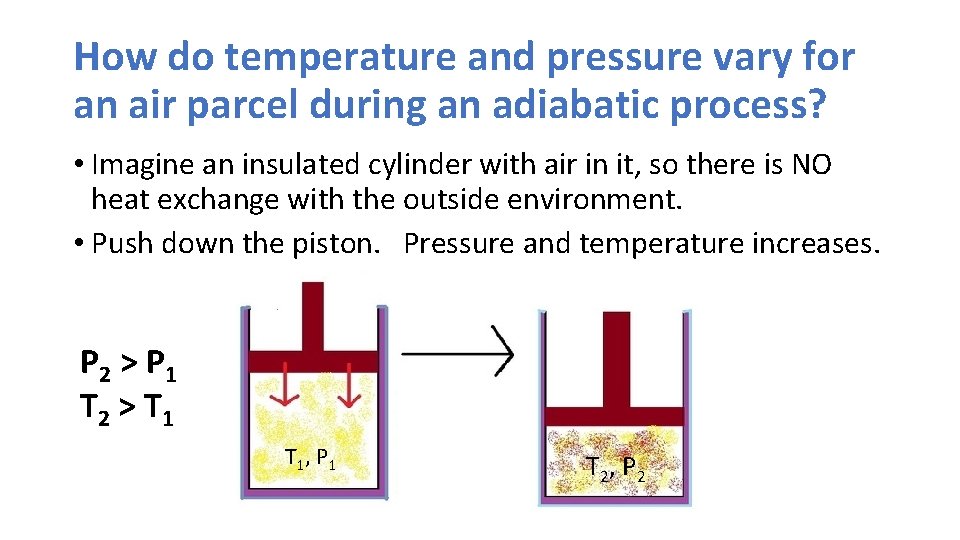
How do temperature and pressure vary for an air parcel during an adiabatic process? • Imagine an insulated cylinder with air in it, so there is NO heat exchange with the outside environment. • Push down the piston. Pressure and temperature increases. P 2 > P 1 T 2 > T 1 , P 1 T 2 , P 2

Adiabatic processes • So if an air parcel is compressed adiabatically, temperature will rise • Similarly, if you expand an air parcel, its temperature will fall. • Why? • When compressing air, you are doing work on the molecules, giving them a “shove” and making them go faster. • If you allow the air parcel to expand, it takes energy to push the boundaries of the parcel out (or to push the piston out), resulting in cooling.

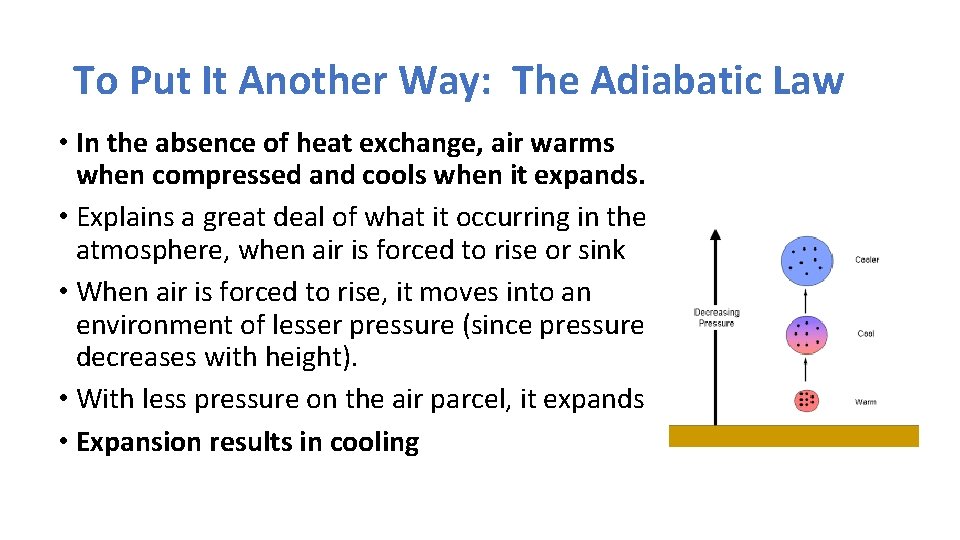
To Put It Another Way: The Adiabatic Law • In the absence of heat exchange, air warms when compressed and cools when it expands. • Explains a great deal of what it occurring in the atmosphere, when air is forced to rise or sink • When air is forced to rise, it moves into an environment of lesser pressure (since pressure decreases with height). • With less pressure on the air parcel, it expands • Expansion results in cooling

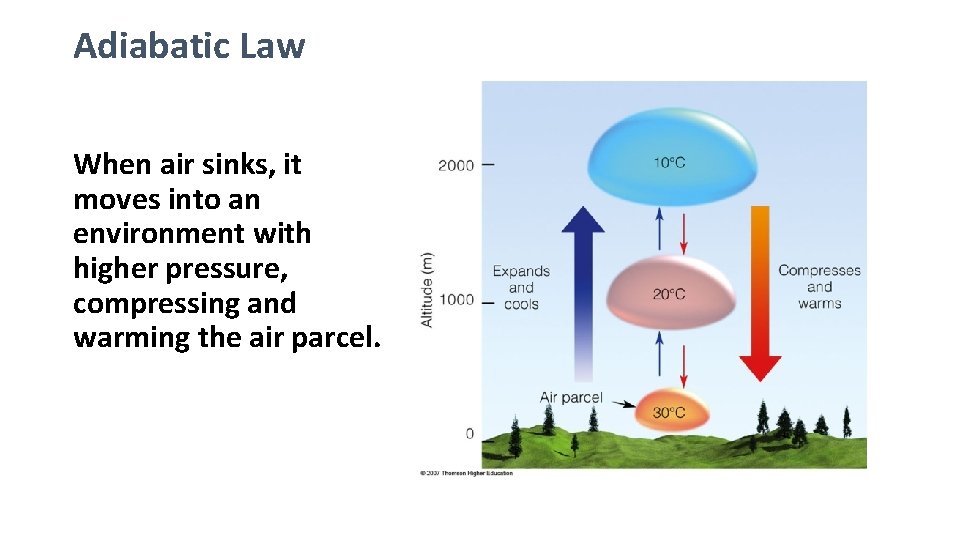
Adiabatic Law When air sinks, it moves into an environment with higher pressure, compressing and warming the air parcel.

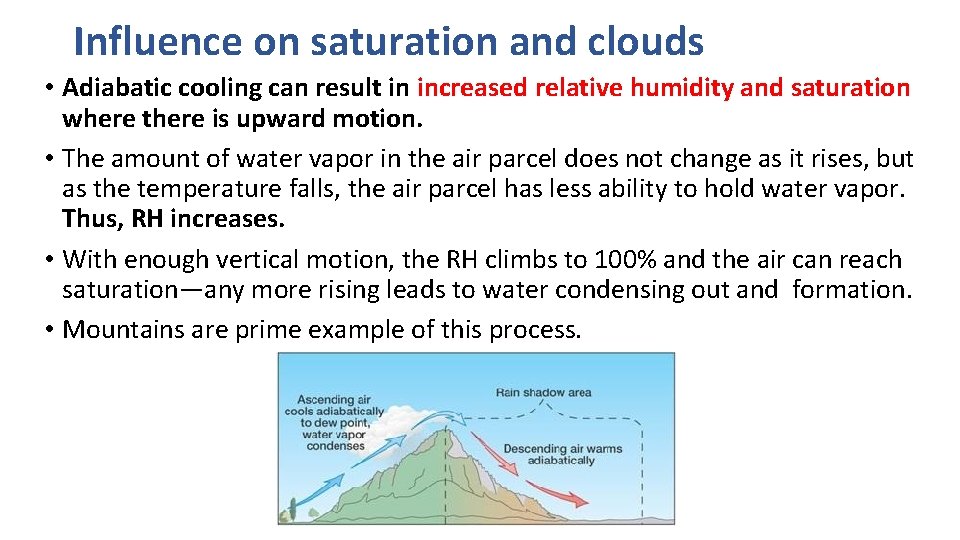
Influence on saturation and clouds • Adiabatic cooling can result in increased relative humidity and saturation where there is upward motion. • The amount of water vapor in the air parcel does not change as it rises, but as the temperature falls, the air parcel has less ability to hold water vapor. Thus, RH increases. • With enough vertical motion, the RH climbs to 100% and the air can reach saturation—any more rising leads to water condensing out and formation. • Mountains are prime example of this process.

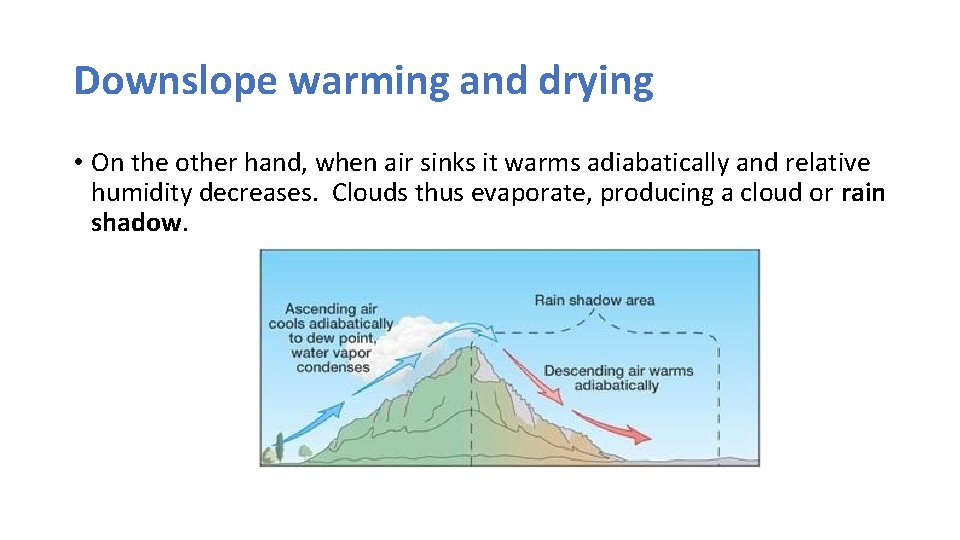
Downslope warming and drying • On the other hand, when air sinks it warms adiabatically and relative humidity decreases. Clouds thus evaporate, producing a cloud or rain shadow.

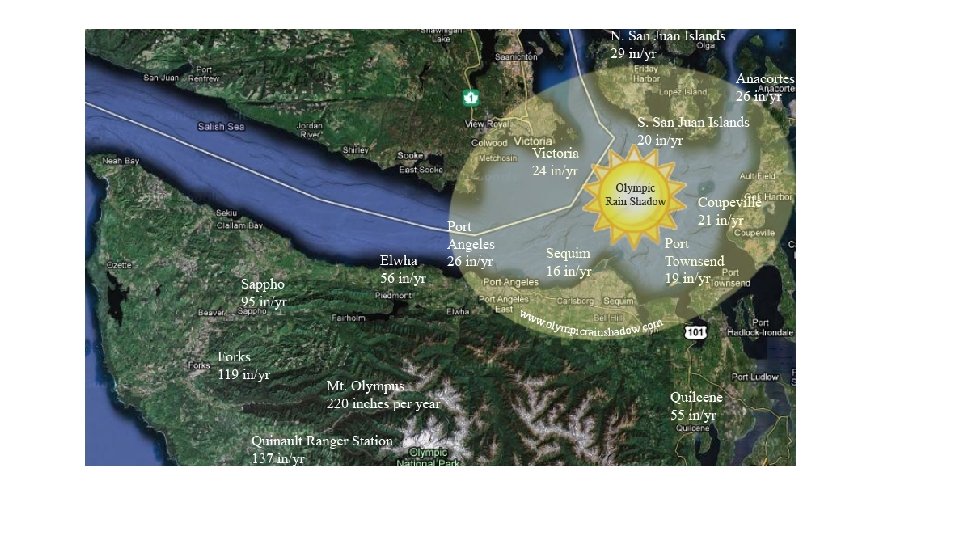
Great examples here in Washington



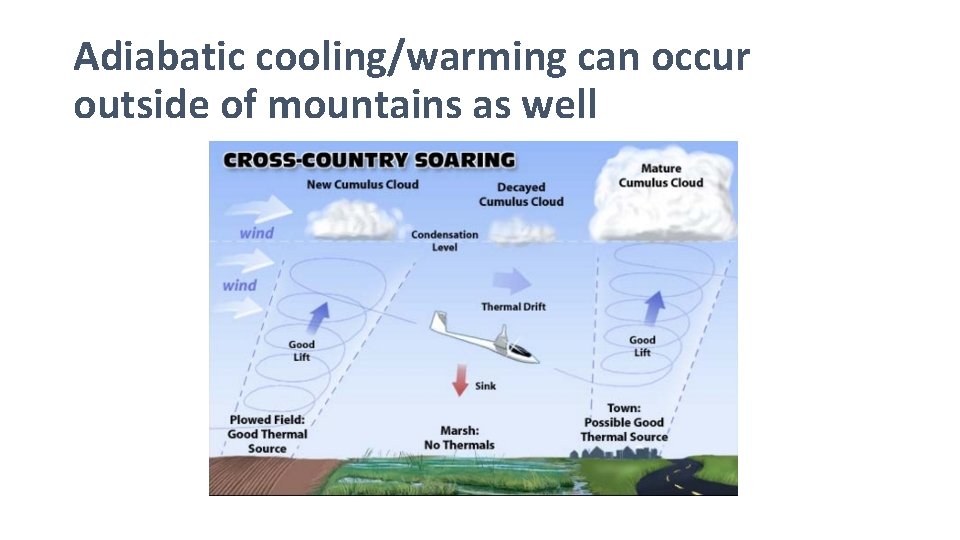
Adiabatic cooling/warming can occur outside of mountains as well

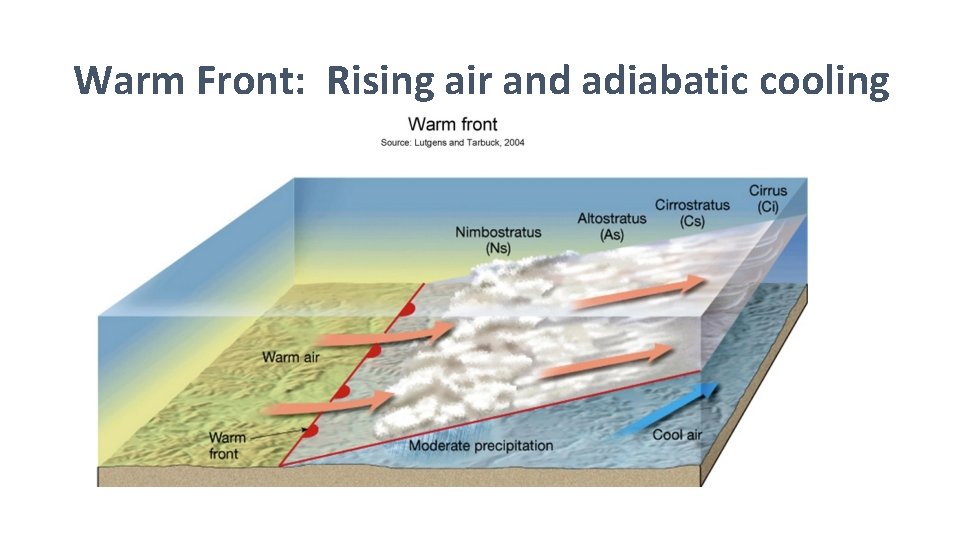
Warm Front: Rising air and adiabatic cooling

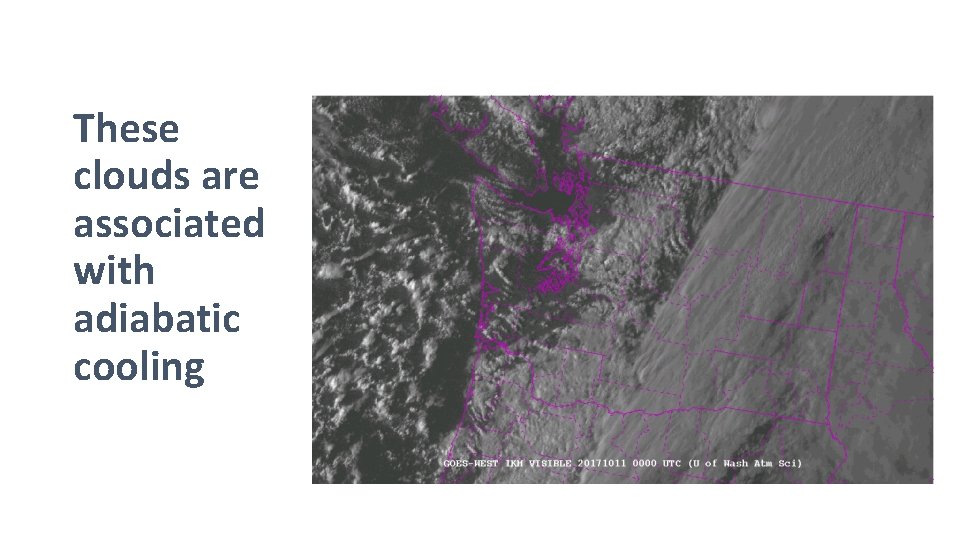
These clouds are associated with adiabatic cooling

And an example that many of you have experienced—a very cold spray from aerosol can due to adiabatic expansion and cooling

Or a heat pump: uses adiabatic principles

 Human sciences tok definition
Human sciences tok definition Facts about montesquieu
Facts about montesquieu Gas laws crash course
Gas laws crash course Do pressure and volume have a direct relationship
Do pressure and volume have a direct relationship Stp gas laws
Stp gas laws Gas law equations
Gas law equations All the gas laws
All the gas laws Different gas laws
Different gas laws Pivnert
Pivnert Conceptual gas law questions
Conceptual gas law questions Example of boyle's law problem
Example of boyle's law problem Chapter 13 gas laws worksheet answer key
Chapter 13 gas laws worksheet answer key 3 gas laws
3 gas laws Different gas laws
Different gas laws Combined gas law worksheet
Combined gas law worksheet Gas laws
Gas laws State charle's law.
State charle's law. Empirical gas laws
Empirical gas laws Ap chemistry gas laws
Ap chemistry gas laws Gas laws graphic organizer
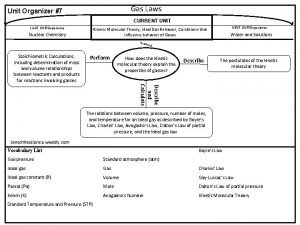
Gas laws graphic organizer Gas laws hot air balloon
Gas laws hot air balloon Pressure at stp
Pressure at stp Kmt gas laws
Kmt gas laws Gas law formulas
Gas law formulas Empirical gas law
Empirical gas law Combined gas law
Combined gas law Avogadro's law formula
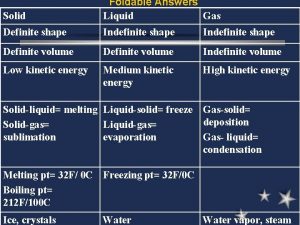
Avogadro's law formula Solid liquid gas foldable
Solid liquid gas foldable Gas 101
Gas 101 Gas 101
Gas 101 Gas 101
Gas 101 Gas 101
Gas 101 Atp denver
Atp denver