Gas Experiments Slide Show Data To do these







- Slides: 17

Gas Experiments Slide Show & Data To do these experiments we needed to modify a soda bottle. The bottle cap gets drilled, and a “valve stem” from a car tire is inserted. This allows us to put the cap on tightly, then attach a bicycle air pump on, and pump the bottle up like a tire. The pressure is increased inside the bottle, up to about 80 psi or about 550 k. Pa, or about 5 atm. This pressure can be measured by a hand held pressure gauge.

In experiment 1 we pump up the bottle as fast as we can to about 80 psi (or 5 atm). We use the bicycle pump gauge to keep track – and not over pump the bottle, lest it explode. Holding the bottle like a football in our forearm, we can feel the bottle get hotter the more it’s pumped up. What is happening?

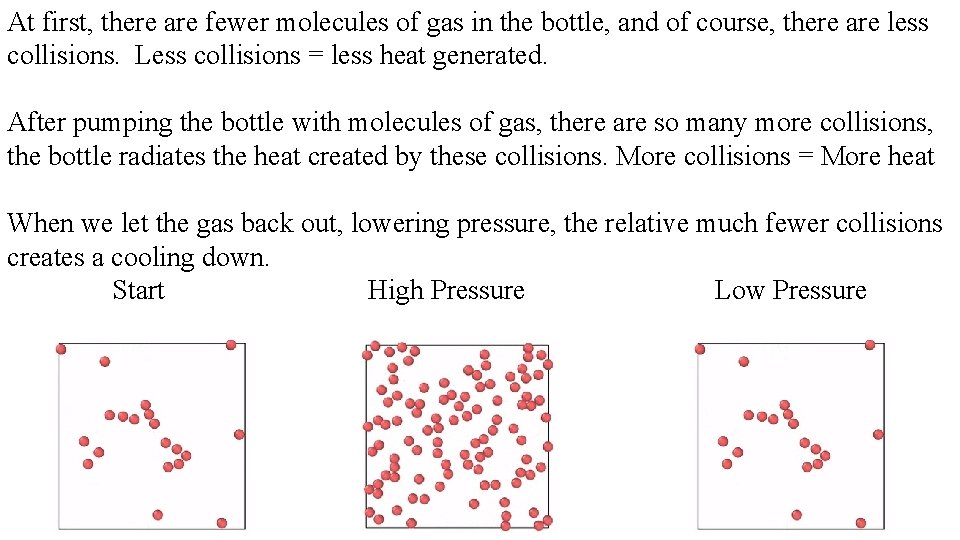
At first, there are fewer molecules of gas in the bottle, and of course, there are less collisions. Less collisions = less heat generated. After pumping the bottle with molecules of gas, there are so many more collisions, the bottle radiates the heat created by these collisions. More collisions = More heat When we let the gas back out, lowering pressure, the relative much fewer collisions creates a cooling down. Start High Pressure Low Pressure

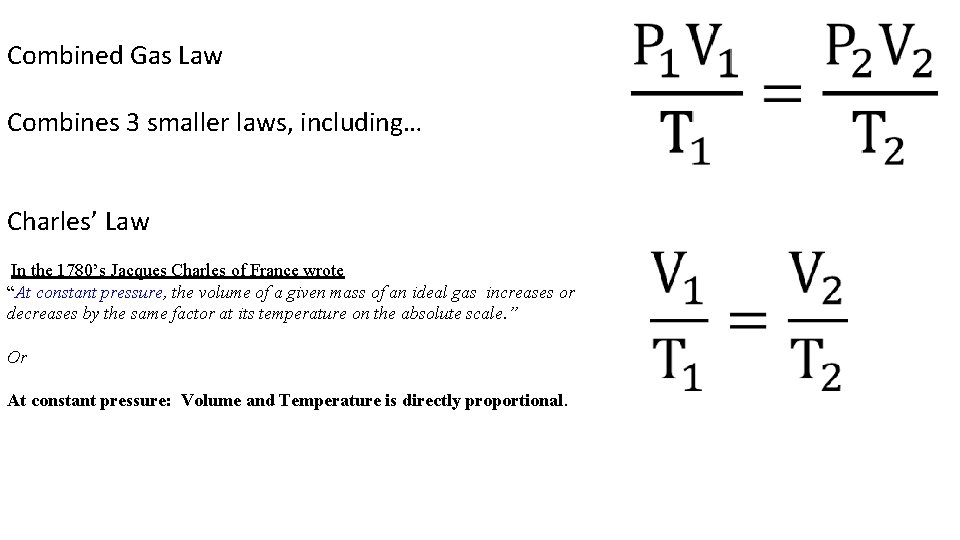
Combined Gas Law Combines 3 smaller laws, including… Charles’ Law In the 1780’s Jacques Charles of France wrote “At constant pressure, the volume of a given mass of an ideal gas increases or decreases by the same factor at its temperature on the absolute scale. ” Or At constant pressure: Volume and Temperature is directly proportional.

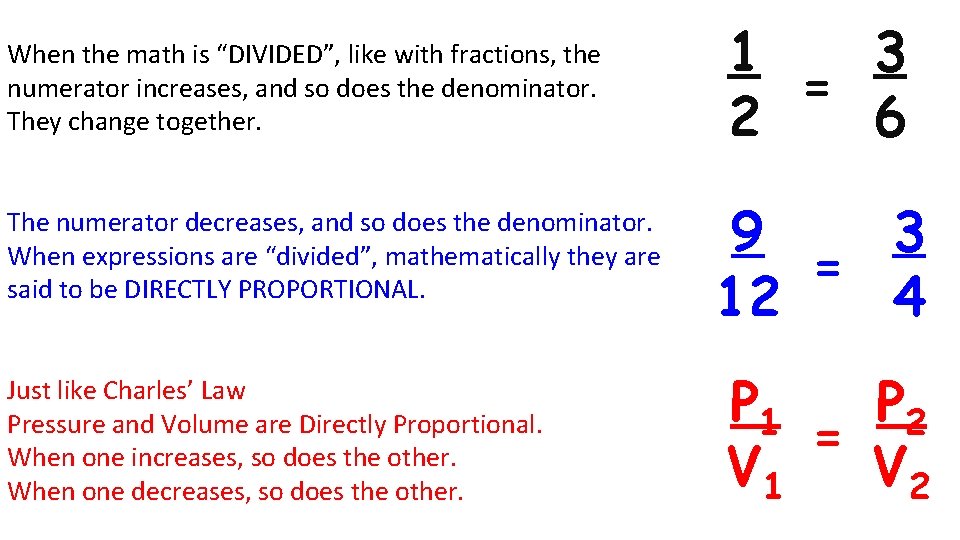
When the math is “DIVIDED”, like with fractions, the numerator increases, and so does the denominator. They change together. The numerator decreases, and so does the denominator. When expressions are “divided”, mathematically they are said to be DIRECTLY PROPORTIONAL. Just like Charles’ Law Pressure and Volume are Directly Proportional. When one increases, so does the other. When one decreases, so does the other. 1 3 = 2 6 9 3 = 12 4 P 1 P 2 = V 1 V 2

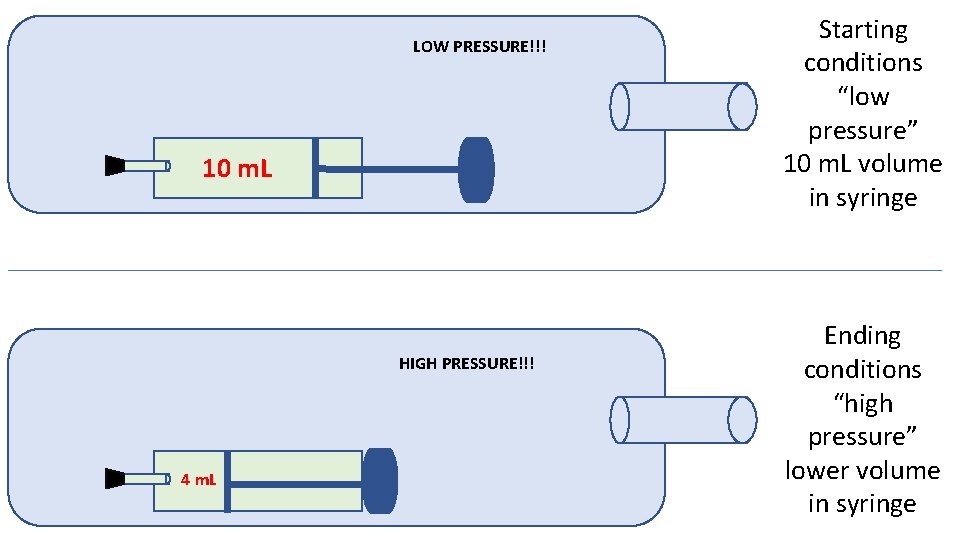
Experiment 2 Pressure and Volume of Gases In this experiment we will open up a plastic syringe (no needles!) to 10 m. L and put a stopper on the end. That seals the 10 m. L of gas inside the syringe. Next, that syringe goes into the bottle and we pump the bottle up with the bicycle air pump, all the way to about 80 psi.

LOW PRESSURE!!! 10 m. L HIGH PRESSURE!!! 4 m. L Starting conditions “low pressure” 10 m. L volume in syringe Ending conditions “high pressure” lower volume in syringe

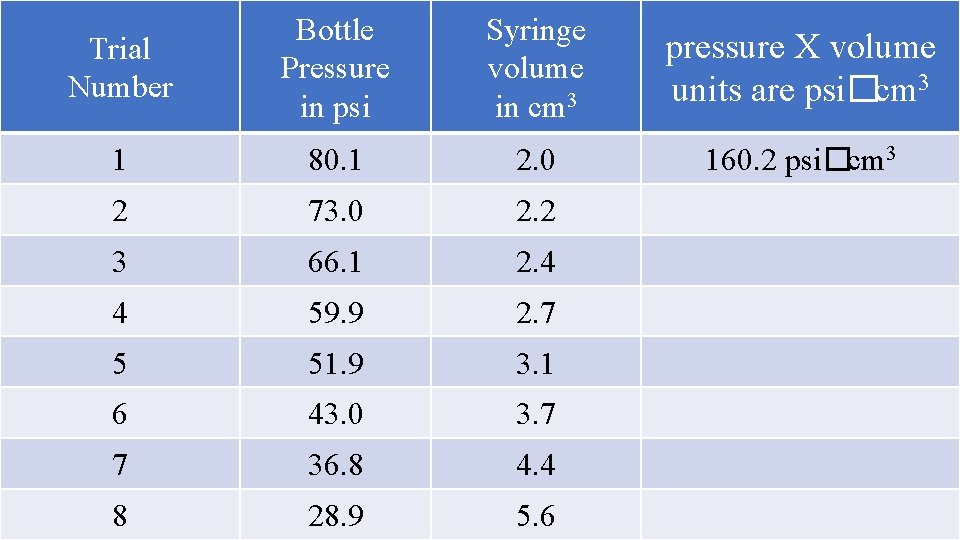
Once the bottle is pumped up to high pressure, the gas collisions SQUEEZE the syringe smaller. When we let out a little bit of gas, lowering the pressure a little, the syringe volume increases. When we let out a little more gas, lowering the pressure more, the syringe volume increases more. High pressure in bottle = low volume in syringe Lower pressure in bottle = higher volume in syringe Look at the data table that follows… Pressure and volume do the “opposite”. As one increases, the other decreases. As one decreases, the other increases. Pressure and volume are INVERSELY PROPORTIONAL.

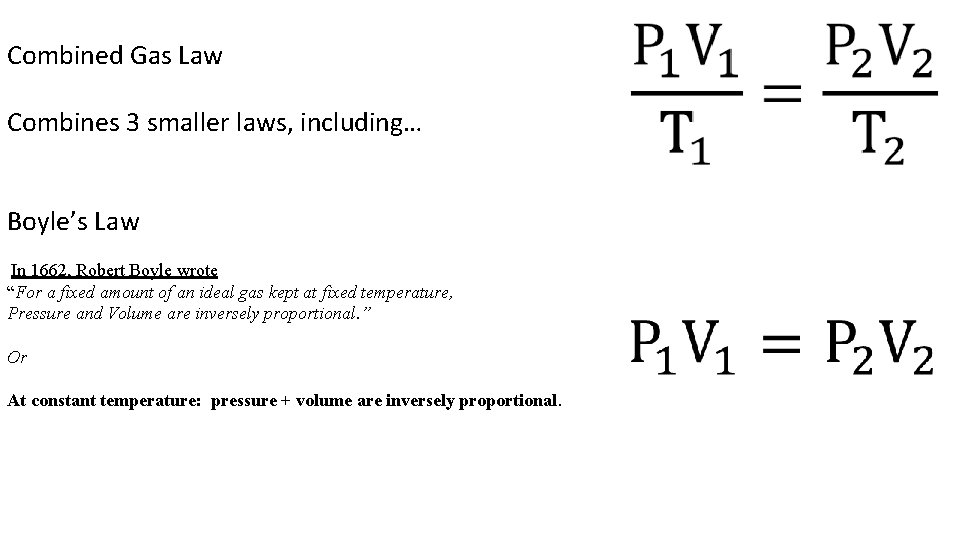
Combined Gas Law Combines 3 smaller laws, including… Boyle’s Law In 1662, Robert Boyle wrote “For a fixed amount of an ideal gas kept at fixed temperature, Pressure and Volume are inversely proportional. ” Or At constant temperature: pressure + volume are inversely proportional.

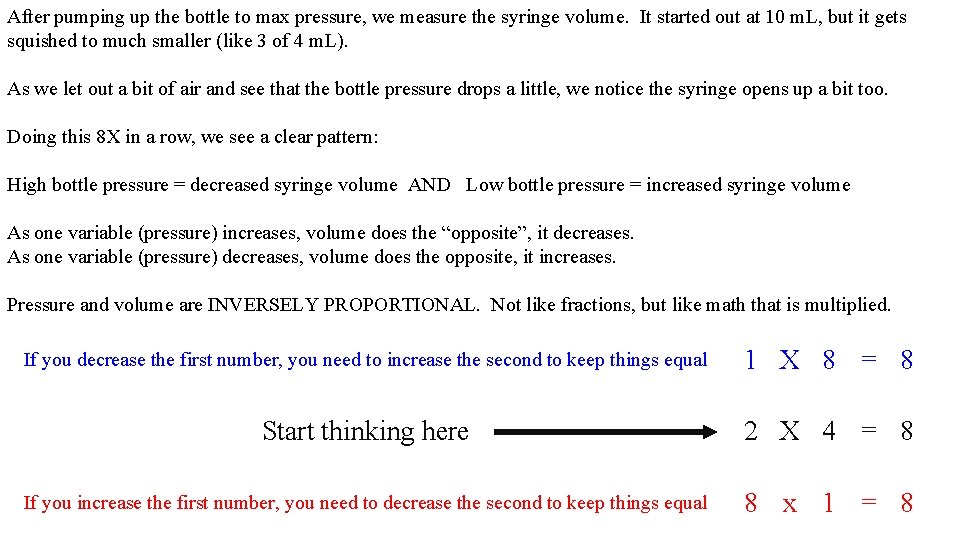
After pumping up the bottle to max pressure, we measure the syringe volume. It started out at 10 m. L, but it gets squished to much smaller (like 3 of 4 m. L). As we let out a bit of air and see that the bottle pressure drops a little, we notice the syringe opens up a bit too. Doing this 8 X in a row, we see a clear pattern: High bottle pressure = decreased syringe volume AND Low bottle pressure = increased syringe volume As one variable (pressure) increases, volume does the “opposite”, it decreases. As one variable (pressure) decreases, volume does the opposite, it increases. Pressure and volume are INVERSELY PROPORTIONAL. Not like fractions, but like math that is multiplied. If you decrease the first number, you need to increase the second to keep things equal 1 X 8 = 8 Start thinking here 2 X 4 = 8 If you increase the first number, you need to decrease the second to keep things equal 8 x 1 = 8

Trial Number Bottle Pressure in psi Syringe volume in cm 3 pressure X volume units are psi�cm 3 1 80. 1 2. 0 160. 2 psi�cm 3 2 73. 0 2. 2 3 66. 1 2. 4 4 59. 9 2. 7 5 51. 9 3. 1 6 43. 0 3. 7 7 36. 8 4. 4 8 28. 9 5. 6

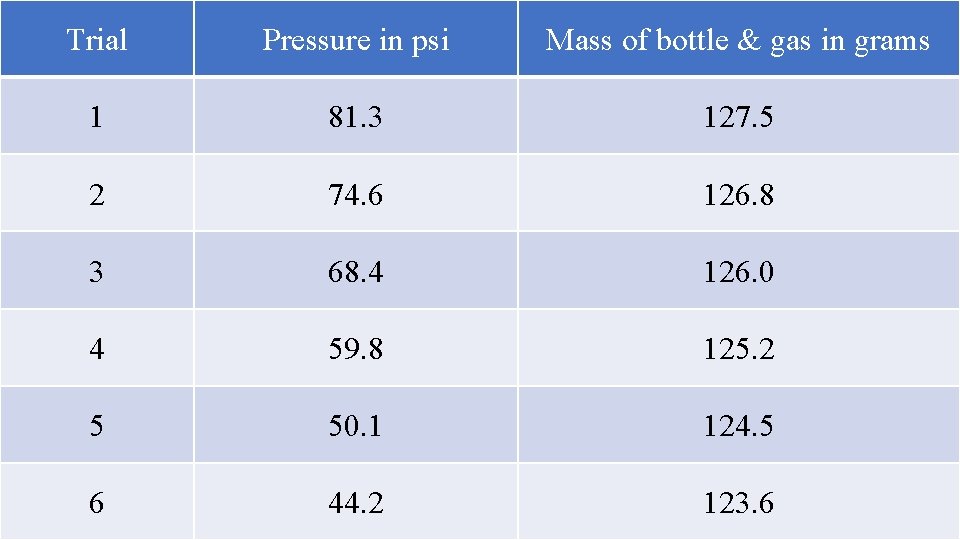
Experiment 3 – Mass and Pressure There is NO mass in the combined gas law, but you can think, so that’s what is needed now. When you pump the bottle up to high pressure, the bottle weighs the most grams. As you let some gas out, lowering pressure, the mass decreases. As you let more gas out, lowering pressure more, the mass continues to decrease. What are you doing when you pump up the bottle, what is happening with the pump & the bottle? Time to think!

Trial Pressure in psi Mass of bottle & gas in grams 1 81. 3 127. 5 2 74. 6 126. 8 3 68. 4 126. 0 4 59. 8 125. 2 5 50. 1 124. 5 6 44. 2 123. 6

When you pump up the bottle, you are increasing the pressure. HOW? You are adding more gas, which means you are adding more particles. These extra particles cause more collisions which presents as higher pressure. These particles are small (atomic and molecular in size). But you are adding a lot of them. Small as they are, the each have mass. You can’t put one molecule of anything onto a scale, but you can measure billions of them at once, and that’s how many particles you pump into the bottle with the bicycle pump. When you let air out, pressure drops. Why? Less collisions. What causes collisions? The particles. Fewer particles have less mass than more particles.

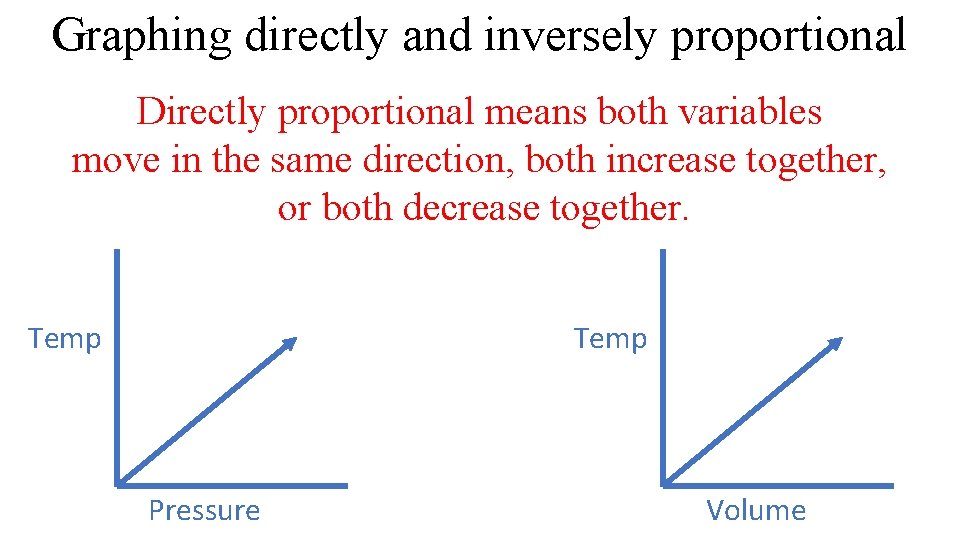
Graphing directly and inversely proportional Directly proportional means both variables move in the same direction, both increase together, or both decrease together. Temp Pressure Volume

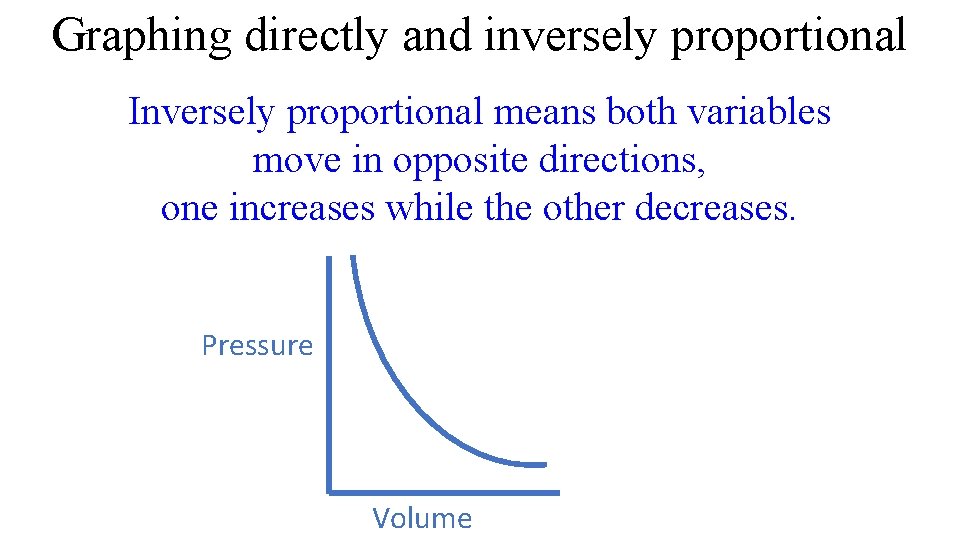
Graphing directly and inversely proportional Inversely proportional means both variables move in opposite directions, one increases while the other decreases. Pressure Volume

When ever you do gas chem math you can ALWAYS use the combined gas law formula. P is pressure, V is volume, and T is temperature. If you happen to have constant T: use just P and V or If you happen to have constant P: use just V and T or If you happen to have constant V: use just V and T Keep units consistent on both sides of the = sign. You must always use Kelvin temp, no matter what, or you GO TO JAIL Do not pass go, do not collect $200.