Gas Chromatography Presented by Mr Pritam Jain 1

Gas Chromatography Presented by: Mr. Pritam Jain 1

ØTheory and Principle Gas Chromatography ØInstrumentation Øadvantages 2

Theory and Principle: It is divided into two classes depending upon the nature of the stationary phase since the mobile phase is always gas 1) Gas solid chromatography (GSC) 2) Gas liquid chromatography (GLC) Gas Solid chromatography: St. Phase is solid adsorptive material Gas Liquid chromatography: St. Phase is thin layer of liquid, usually as a coating on the surface of an inert particle. 3

Theory: In 1950, Dutch chemical engineers began study of processes which caused band broadening in chromatography. Derived an expression called Van Deempter equation related to HETP to a no. of expt. Parameters like 1. diameter of st. phase particles, 2. diff. coefficient of solute in st. and mobile phase 3. flow rate of mobile phase. Theoretical plate : Each single equilibrium between two phases is known as Theoretical plate. And the length of column required for one equilibrium is called as Height equivalent to theoretical plate(HETP). 4
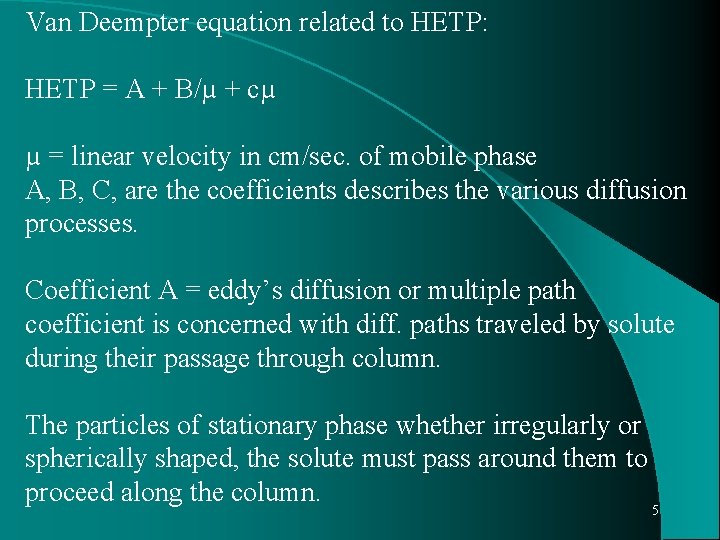
Van Deempter equation related to HETP: HETP = A + B/µ + cµ µ = linear velocity in cm/sec. of mobile phase A, B, C, are the coefficients describes the various diffusion processes. Coefficient A = eddy’s diffusion or multiple path coefficient is concerned with diff. paths traveled by solute during their passage through column. The particles of stationary phase whether irregularly or spherically shaped, the solute must pass around them to proceed along the column. 5

Some molecules of same kind will reach the end of column before others. Faster molecules are found in the leading edge of the peak and slower ones forms the trailing edge. Net effect is band broadening. In modern chromatographic technique, column is packed using small, uniformly sized particles, the value of A is minimal. Contribution of this term in increasing HETP is negligible. 6

Coefficient B = coefficient of longitudinal diffusion. The Concentration of solute is lower at edges of the band than in center and during the travel of band through column, solute is diffusing continually through the mobile phase away from the center of band. This phenomenon occurs at both the leading and trailing edge of the peak causing band broadening. 7

The equation predicts that, the contribution to HETP of this term is inversely proportional to mobile phase velocity, the effect is more pronounced at low flow rates. The contribution of Longitudinal diffusion to band broadening can be reduced by proper adjustment of flow rate and by increasing the viscosity of mobile phase. 8
Coefficient C = coefficient of mass transfer concerned with transfer of solute between two phases. The mobile phase is moving rapidly, equilibrium between two phases may not be attained. Therefore, some solute molecules in mobile phase are not transferred to stationary phase quickly enough and as result carried ahead of centre of band. So, the solutes which stationary phase retained too long and lag behind. 9

In contrast to longitudinal diffusion, the contribution to HETP of this term is directly proportional to flow rate, so compromise flow rate is necessary. Mass transfer effects also reduced by using very thin coating of stationary phase, so the area in contact with mobile phase is maximized, and diffusion in stationary phase is reduced. 10

Principles of Separation 1. Column is selected, packed with Liquid Phase, and installed. 2. Sample injected with microliter syringe into the injection port where it is vaporized and mixed into the Carrier Gas stream (helium, nitrogen, argon). 3. Sample vapor becomes partitioned between Moving Gas Phase and Stationary Liquid Phase. 4. The time the different compounds in the sample spend in the Vapor Phase is a function of their Vapor Pressure. 5. The more volatile (Low Boiling Point / Higher Vapor Pressure) compounds arrive at the end of the column first and pass into the detector. 11
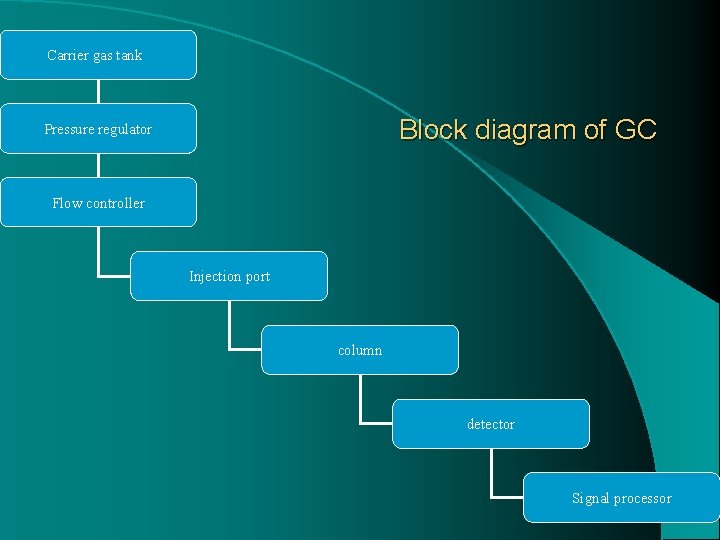
Carrier gas tank Block diagram of GC Pressure regulator Flow controller Injection port column detector Signal processor 12

Gas Chromatography Filters/Traps Data system H RESET Regulators Syringe/Sampler Inlets Detectors Gas Carrier Hydrogen Air Column gas system inlet l column l detector l data system l 13
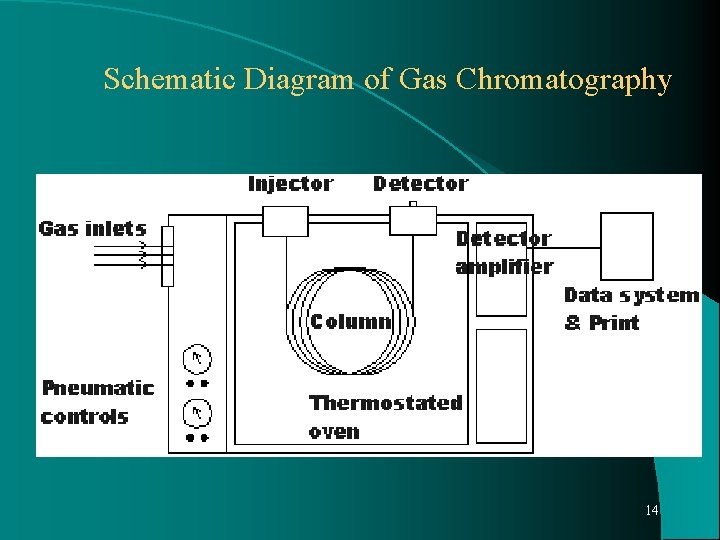
Schematic Diagram of Gas Chromatography 14
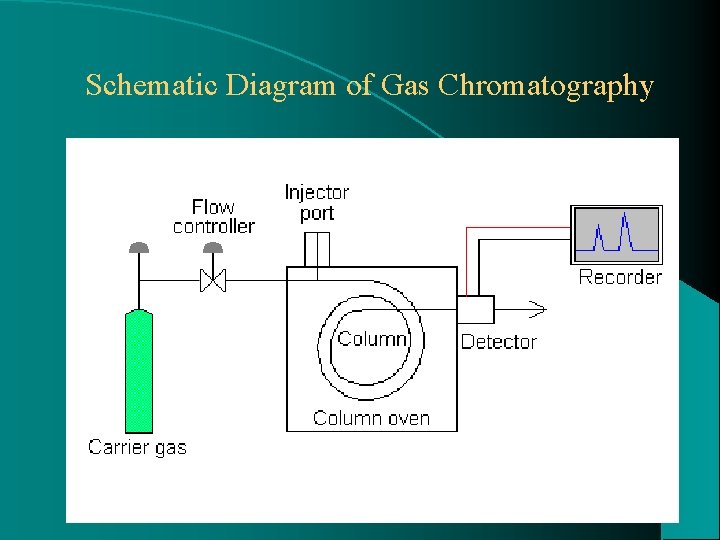
Schematic Diagram of Gas Chromatography 15

Carrier gas: Serves as a mobile phase supplied in steel tanks under high pressure. At a pressure of 40 to 80 psi passes into flow controllers which allows the operator to adjust flow rate to desired operating level (50 to 100 m. L/min. ) Usually nitrogen and helium are used Occasionally hydrogen and argon are used 16

Purity of gas is very important because it deposits impurities in the column. It should be inert in respect with the sample component, column packing material. Low viscosity gases as H 2 and helium allow higher flow rates, while high viscosity gas as nitrogen useful in reducing longitudinal diffusion. Disadvantage is that in GC the mobile phase is limited 17

Sample Injection port: It is a small chamber, usually separately heated to a temp. slightly above that of column. In this the analytical sample is made to vaporize rapidly before entering the column. Sample is introduced into the flowing gas stream through a self sealing rubber or silicon septum using a microlitre syringe. Sample may be injected into chamber directly on the beginning of the column. Samples may be pure liquids, solids dissolved in liquid solvents or gases. 18

Stationary phase The interior of column contains either an uncoated solid material for GSC or an inert solid support coated with a thin layer of liquid phase for GLC. Particles of packing material are very small (80 – 120 mesh) to reduces the void volume, while sometime providing large surface area for interaction with the solutes. 19

In GSC Adsorbents used are : Charcoal , silica gel , alumina or glass beads. In GLC Solid support material used : Diatomaceous earth treated with acid and base to remove impurities and then calcinated to activate the surface. Glass mocrobeads are also used. The liquid phase is coated on uniformly on the surface of solid support at levels 1 -5% by weight. The liquid must be chemically stable, have low vapor pressure. 15 -20 highly purified liquid differs in polarity are used. Most of these are based on silicon polymers with substituents such as phenyl, cyano or trifluoropropyl introduced in order to affect polarity. Ethylene glycol polymers are used for separation of polar compounds such as alcohols and amines. 20

Columns Stainless steel or glass tube of 1 -3 m in length and 2 -4. 6 mm in diameter. Attached at either end of injection port and the detector, using compression fittings to achieve gas tight seal. Shorter columns may be straight or U shaped but longer ones are coiled into spiral. Also prepared from other metals like copper and aluminum. (Adv. - flexible so may be made in any shape , less expensive but less durable and reactive) 21

For certain compds. , like steroids which are highly susceptible to degradation, glass columns are used. (Disadvantage - Difficult to get gas tight seal at injector and detector connections) Recent development – Use of nickel tubing for fabrication of column. It is inert and having good strength and durability. Narrow diameter column are used. Increase in efficiency can be carried out by: Using tubing with an inner diameter of 1 mm. 22

Greater efficiency obtained by: Use of capillary column. These are tubes of glass or highly purified fused silica with internal diameter of 0. 2 to 0. 75 mm. Most commonly used columns are 1. Wall coated open tubular column (WCOT): stationary phase is deposited as an extremely thin layer directly on inner surface of the tube. 2. Support coated open tubular column (SCOT): the inner surface of tube is coated with a layer of inert support onto which the liquid is coated. Disadvantage of capillary column: their low capacities due to small volume of stationary phase. Therefore injection volumes must be very small (less than 0. 1 microlitre)23

Operating condition Isothermal mode (Temp. of instrument maintained const. ) This method is unsatisfactory for complex mixtures, when both volatile and non volatile solutes are present. For these mixtures, if column operated at high temp. , low boiling solutes eluted rapidly but not resolved, while less volatile substances separated satisfactorily. So resolution can not achieved, separation can not obtained. 24

To prevent this problem, temp. programming technique is used. Temperature of column is raised at a preset rate beginning at the time of sample injection The programming rate must be constant during the whole run. Result of temp. programming is: Chromatogram with evenly spaced peaks having good heights resulting in all saving of time. Initial temp. should be chosen in order to minimize the retention time for least retained solute. Final temp. must be sufficient to elute the least volatile compound in reasonable time. 25

DETECTORS: Two general classifications: 1. Mass flow rate detectors- sensitive to rate of flow of solute through the detector. 2. Concentration sensitive detector- respond to the concentration of solute in mobile phase in the detector. Flame Ionization Detector: Mass flow detector, most frequently used in GC, because highly sensitive and able to detect microgram quantity of solutes and almost universal detector. Insensitive to water and inorganic substances. 26

Mechanism: Hydrogen and oxygen are introduced into the column effluent stream. Mixture is ignited and as a result of energy of flame. Electrons are stripped from the solute and ions are formed. These charged particles migrate to a pair of oppositely charged collector electrodes in the chamber and gives a small electrical current to flow. Current is amplified is proportional to flow rate of solute through the detector. 27
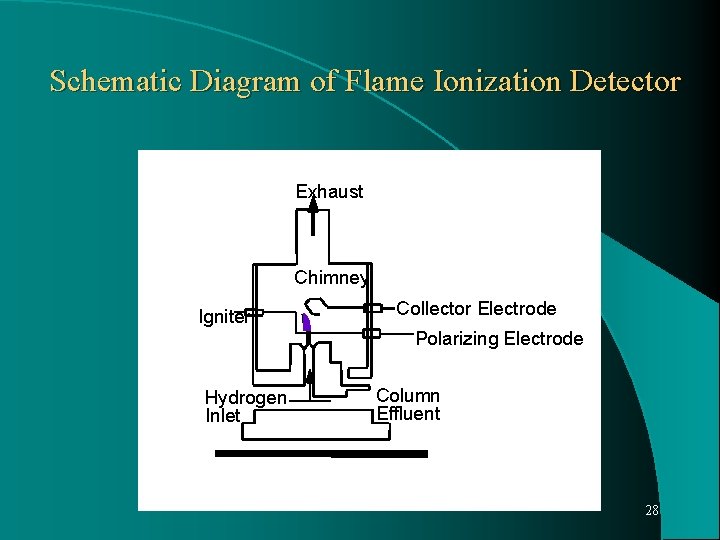
Schematic Diagram of Flame Ionization Detector Exhaust Chimney Igniter Collector Electrode Polarizing Electrode Hydrogen Inlet Column Effluent 28
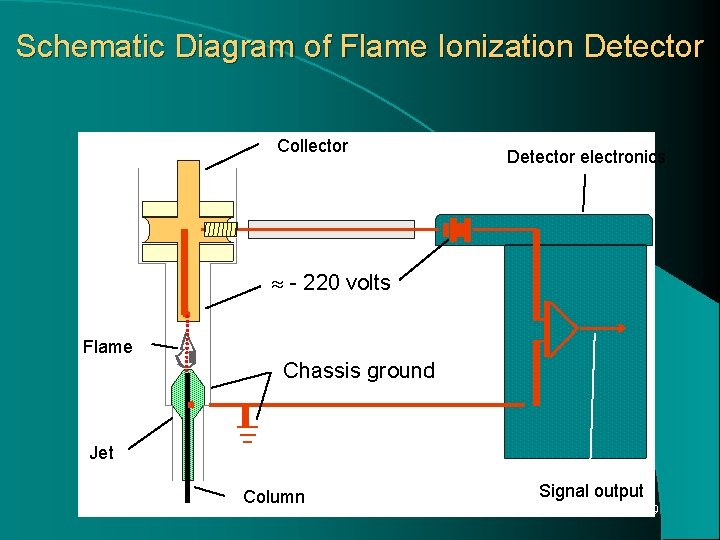
Schematic Diagram of Flame Ionization Detector Collector Detector electronics - 220 volts Flame Chassis ground Jet Column Signal output 29

Thermal Conductivity Detector: Also called Hot Wire Detector (HWD) or katharometer. Concentration sensitive detector, widely used because respond to all solutes. A coil of fine wire made from tungsten – rhenium alloy, resides in a small chamber into which column effluent flows. Most TC detectors consist of matched pair of wires, one is placed in gas stream before it enter in column, and other is at the end of column. 30

An electrical potential is placed across the filament and they heat up due to their resistance. The resistance is directly proportional to temp. When only carrier gas is flowing through the chamber, filament maintains a steady temp. which is determined by thermal conductivity of gas. When binary mixture of solute and carrier gas emerges from column, the mixture has a different thermal conductivity and heat is conducted from the sample filament at greater or lesser rate. This changes the resistance of the wire and change in resistance or current is a measure of concentration of solute in the detector. 31
Thermal Conductivity Detector • Responds to all compounds • Adequate sensitivity for many compounds • Good linear range of signal • Simple construction • Nondestructive detection 32
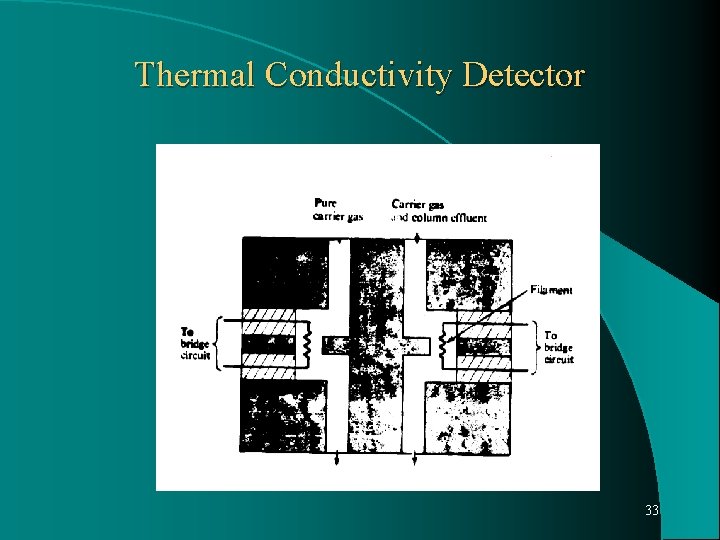
Thermal Conductivity Detector 33

Electron Capture Detector Most sensitive detector respond to nanogram or picogram, quantities of material having functional group possess high electron affinity, such as halogens or nitro group. A radioactive source 63 Ni, emits beta particles which interact with the carrier gas molecules to form positive ions and electrons. These migrate to oppositely charged electrodes in the detector chamber to produce a standing current. When a solute elutes from the column, it is able to capture some portion of the electrons, lowering the standing current. 34

Decrease in the current, detected electronically and proportional to amount of solute. ECD extremely sensitive to halogenated compounds eg. Pesticides. 4. Thermionic Specific detector (TSD) Also called as Nitrogen Phosphorous detector (NPD) Modified form of FID, shows response to compounds containing nitrogen and phosphorous. Consist of standard FID with an electrically heated bead of alkali metal compound, such as rubidium silicate. The nitrogen and phosphorous containing compounds are detected at the collector electrodes as in FID. Its sensitivity for nitrogen and phosphorous is 103 to 104 greater than 35 for other organic compounds.
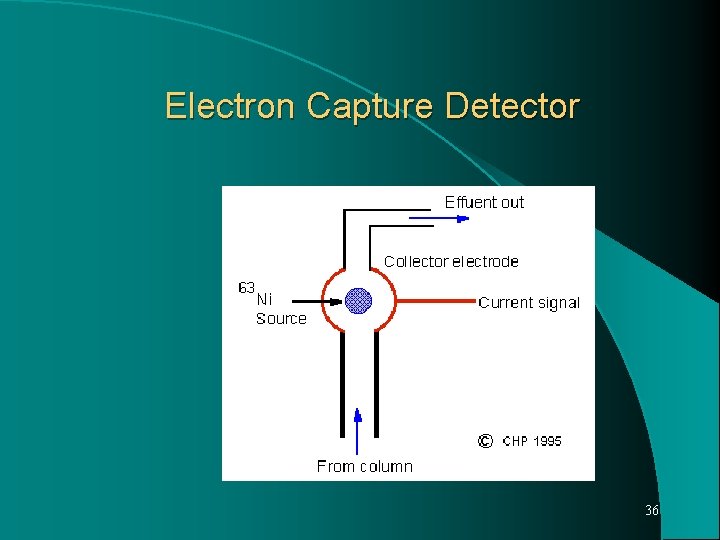
Electron Capture Detector 36
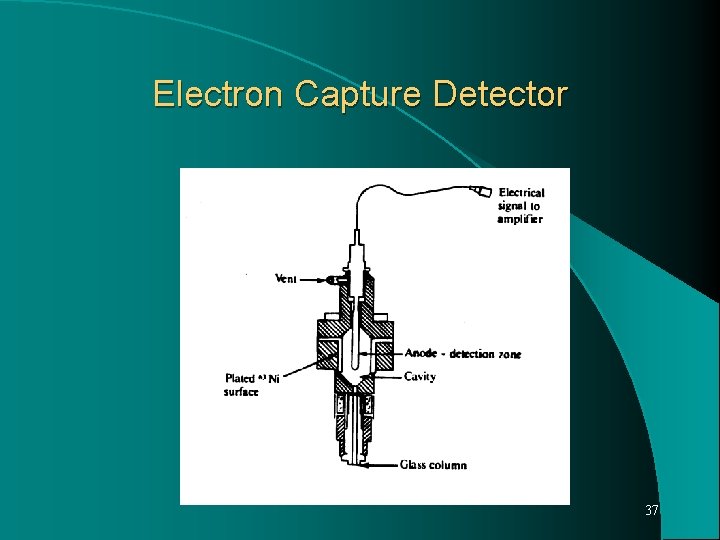
Electron Capture Detector 37

Another modification of FID , has increased selectivity for sulfur and phosphorous containing compounds is flame photometric detectors (FPD). The eluted compounds are first burned in FID flame, from which the products of pyrolysis pass to another flame, where sulfur and phosphorous atoms are excited to higher energy state and detected by emission spectroscopy. Sensitivity to S and P is 105 times greater than that for carbon compounds 38

GLC ADVANTAGES 1. Very good separation 2. Time (analysis is short) 3. Small sample is needed - ml 4. Good detection system 5. Quantitatively analyzed 39
- Slides: 39