Gas Absorption Line Broadening April 6 From last

Gas Absorption Line Broadening April 6

From last time… Summary in Words of Gas Transitions • 3 types of quantized transitions important to us: • Electronic (highest energy: UV-Vis) • Vibrational (medium energy: Vis-NIR-Thermal IR) • Rotational (Far IR & Microwave) • Other types of absorption are not quantized: • Photo-Ionization : Ripping electronic off to make ion (Occurs when photon energy > ionization energy of molecule) • Photo-Dissociation: Tearing an atom off a molecule (E. g. O 3 O 2 + O* - critical for stratospheric chemistry) (Occurs when photon energy > dissociation energy of molecule) • Pure rotational transitions can happen ONLY if molecule has a permanent electric dipole moment: (e. g. H 2 O, CO, O 3). Symmetric linear molecules (N 2, CO 2, N 2 O) do not have a permanent dipole moment. •
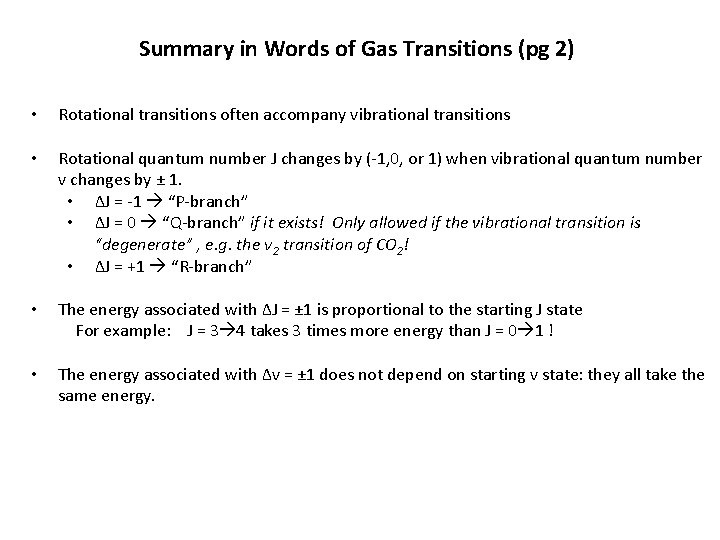
Summary in Words of Gas Transitions (pg 2) • Rotational transitions often accompany vibrational transitions • Rotational quantum number J changes by (-1, 0, or 1) when vibrational quantum number v changes by ± 1. • ΔJ = -1 “P-branch” • ΔJ = 0 “Q-branch” if it exists! Only allowed if the vibrational transition is “degenerate” , e. g. the ν 2 transition of CO 2! • ΔJ = +1 “R-branch” • The energy associated with ΔJ = ± 1 is proportional to the starting J state For example: J = 3 4 takes 3 times more energy than J = 0 1 ! • The energy associated with Δv = ± 1 does not depend on starting v state: they all take the same energy.
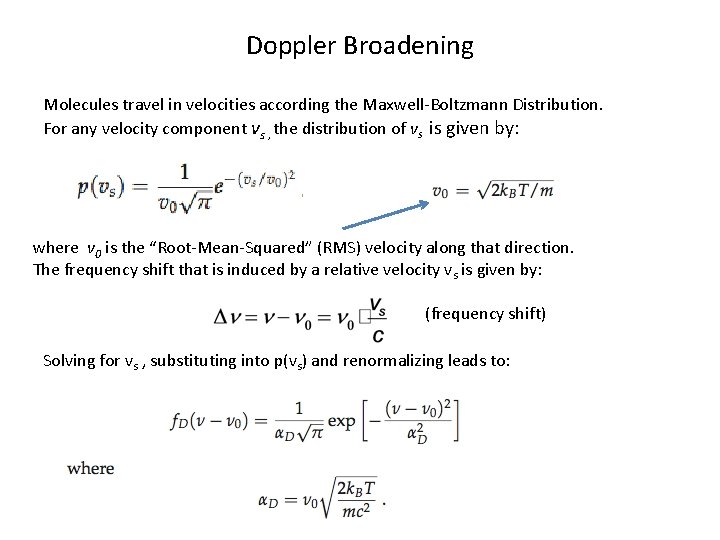
Doppler Broadening Molecules travel in velocities according the Maxwell-Boltzmann Distribution. For any velocity component vs , the distribution of vs is given by: where v 0 is the “Root-Mean-Squared” (RMS) velocity along that direction. The frequency shift that is induced by a relative velocity vs is given by: (frequency shift) Solving for vs , substituting into p(vs) and renormalizing leads to:
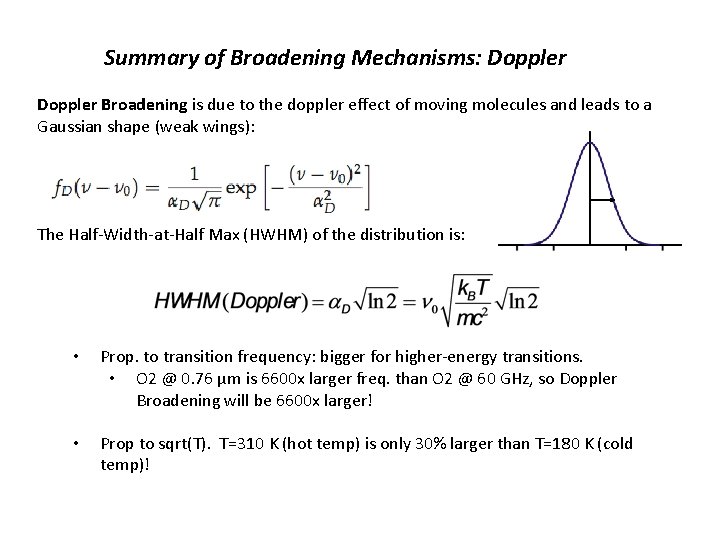
Summary of Broadening Mechanisms: Doppler Broadening is due to the doppler effect of moving molecules and leads to a Gaussian shape (weak wings): The Half-Width-at-Half Max (HWHM) of the distribution is: • Prop. to transition frequency: bigger for higher-energy transitions. • O 2 @ 0. 76 μm is 6600 x larger freq. than O 2 @ 60 GHz, so Doppler Broadening will be 6600 x larger! • Prop to sqrt(T). T=310 K (hot temp) is only 30% larger than T=180 K (cold temp)!
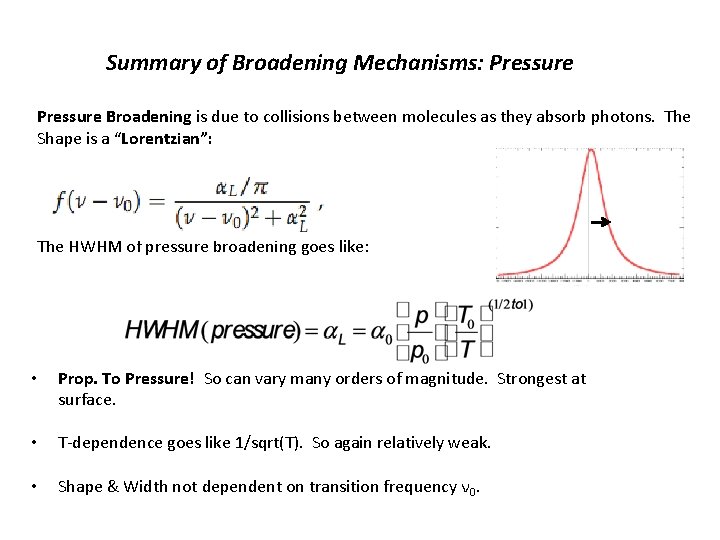
Summary of Broadening Mechanisms: Pressure Broadening is due to collisions between molecules as they absorb photons. The Shape is a “Lorentzian”: The HWHM of pressure broadening goes like: • Prop. To Pressure! So can vary many orders of magnitude. Strongest at surface. • T-dependence goes like 1/sqrt(T). So again relatively weak. • Shape & Width not dependent on transition frequency ν 0.
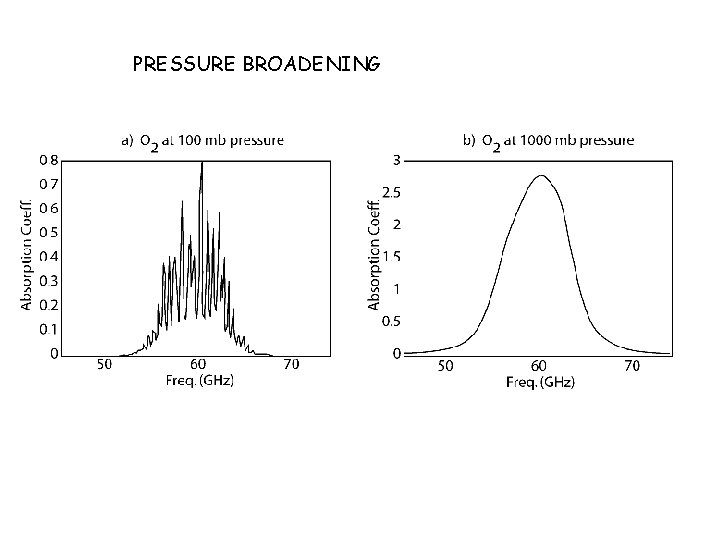
PRESSURE BROADENING
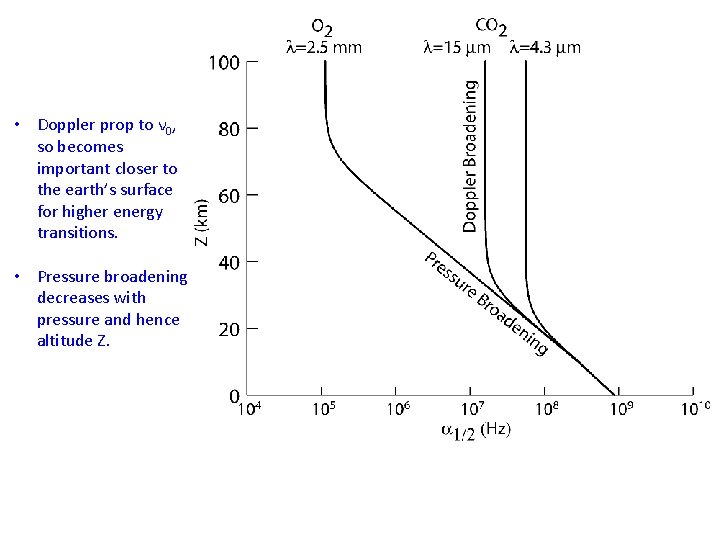
• Doppler prop to ν 0, so becomes important closer to the earth’s surface for higher energy transitions. • Pressure broadening decreases with pressure and hence altitude Z.
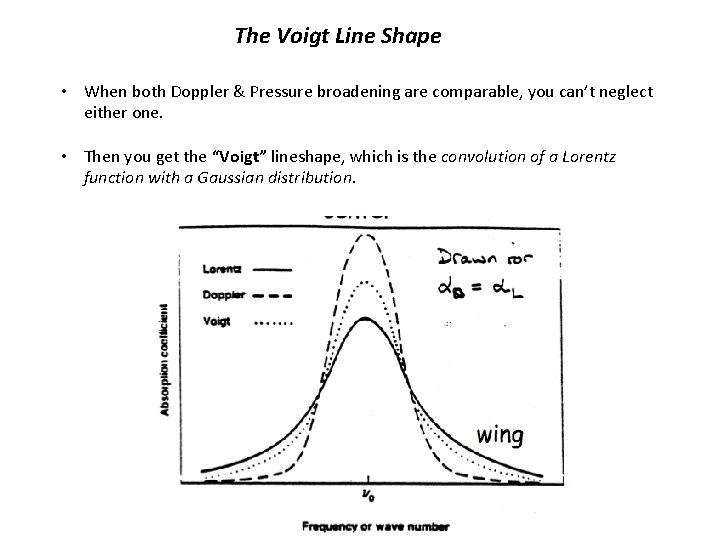
The Voigt Line Shape • When both Doppler & Pressure broadening are comparable, you can’t neglect either one. • Then you get the “Voigt” lineshape, which is the convolution of a Lorentz function with a Gaussian distribution.
Summary in Words of Gas Transitions • 3 types of quantized transitions important to us: • Electronic (highest energy: UV-Vis) • Vibrational (medium energy: Vis-NIR-Thermal IR) • Rotational (Far IR & Microwave) • Other types of absorption are not quantized: • Photo-Ionization : Ripping electronic off to make ion (Occurs when photon energy > ionization energy of molecule) • Photo-Dissociation: Tearing an atom off a molecule (E. g. O 3 O 2 + O* - critical for stratospheric chemistry) (Occurs when photon energy > dissociation energy of molecule) • Pure rotational transitions can happen if molcule has permanent electric dipole moment: (e. g. H 2 O, CO, O 3). Symmetric linear molecules (N 2, CO 2, N 2 O) do not have permanent dipole moment.
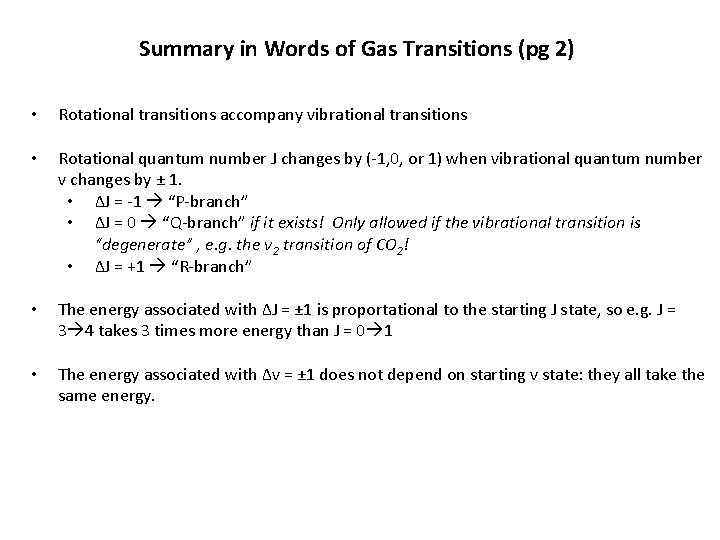
Summary in Words of Gas Transitions (pg 2) • Rotational transitions accompany vibrational transitions • Rotational quantum number J changes by (-1, 0, or 1) when vibrational quantum number v changes by ± 1. • ΔJ = -1 “P-branch” • ΔJ = 0 “Q-branch” if it exists! Only allowed if the vibrational transition is “degenerate” , e. g. the ν 2 transition of CO 2! • ΔJ = +1 “R-branch” • The energy associated with ΔJ = ± 1 is proportational to the starting J state, so e. g. J = 3 4 takes 3 times more energy than J = 0 1 • The energy associated with Δv = ± 1 does not depend on starting v state: they all take the same energy.
- Slides: 11