Gametogenesis and Sporogenesis Scott Russell Office 210 NML













- Slides: 40

Gametogenesis and Sporogenesis Scott Russell Office: 210 NML / Lab: 143 GLCH Phone: 325 -4391 (office) 325 -6234 (lab) srussell@ou. edu

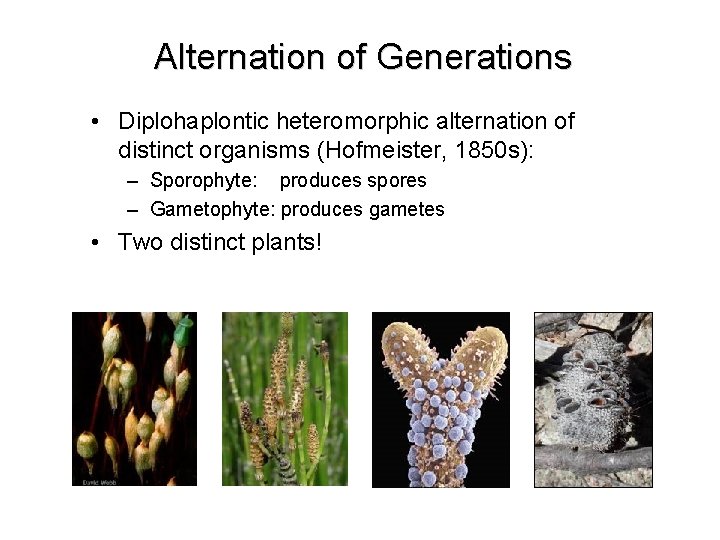
Alternation of Generations • Diplohaplontic heteromorphic alternation of distinct organisms (Hofmeister, 1850 s): – Sporophyte: produces spores – Gametophyte: produces gametes • Two distinct plants!

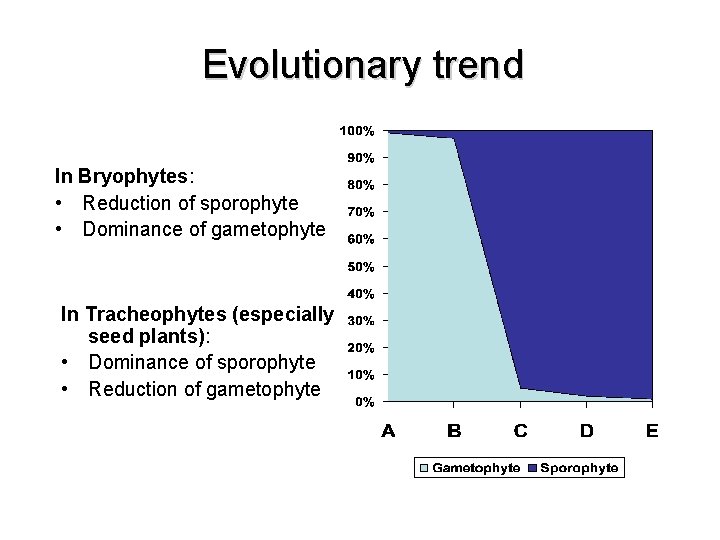
Evolutionary trend In Bryophytes: • Reduction of sporophyte • Dominance of gametophyte In Tracheophytes (especially seed plants): • Dominance of sporophyte • Reduction of gametophyte

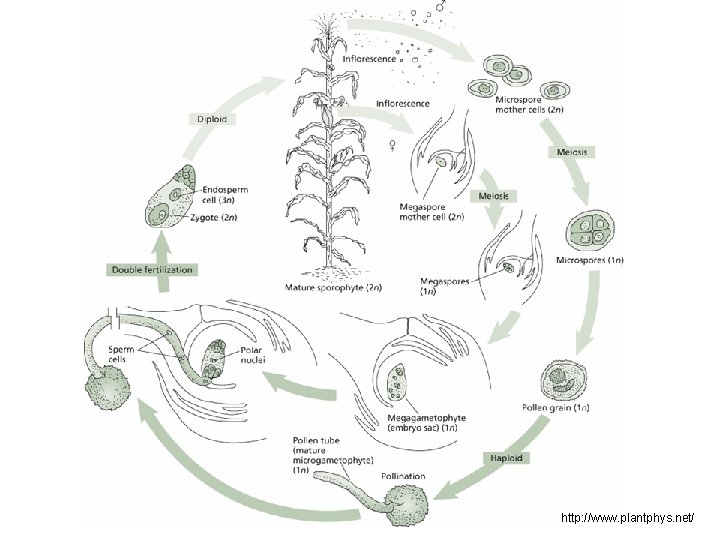
Angiosperm Life Cycle

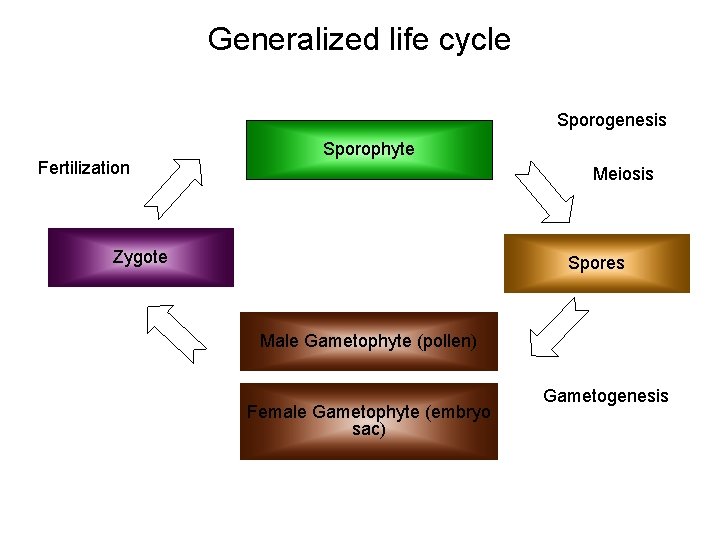
Generalized life cycle Sporogenesis Fertilization Sporophyte Meiosis Zygote Spores Male Gametophyte (pollen) Female Gametophyte (embryo sac) Gametogenesis

Gamete formation Two processes: • Sporogenesis: - making of the spore • Gametogenesis: - making of the gamete

Sporogenesis • • Organogenesis of anther, ovary and ovules Sporogenous cells – cytoplasmically rich, conspicuous nucleus Sporocytes – cells committed to meiosis Meiosis – First part • • • Pairing of homologous chromosomes Alignment of DNA and synapsis Crossing-over Conclusion of prophase I Independent assortment of homologous chromosomes Halving of genome – Second part • Separation of chromatids • Formation of four spores – In males, all four are used (except Cyperaceae) – In females, one, two or all four spore products may be used

http: //www. plantphys. net/

Gametogenesis • Spore nucleus divides mitotically beginning the gametophyte stage – In male gametophyte, also termed the microgametophyte or more commonly pollen grain, the spore nucleus divides twice – In female gametophyte, also known as the megagametophyte, or more commonly embryo sac, the spore nucleus also divides (different number of divisions for different embryo sac types)

Male Gametophyte

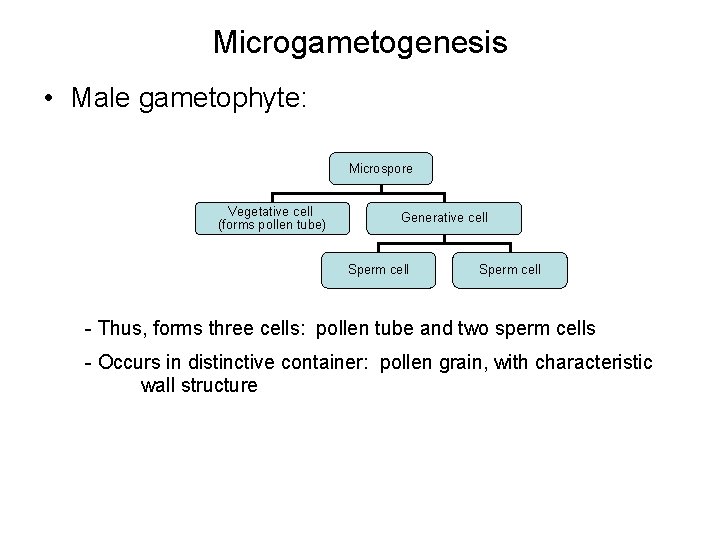
Microgametogenesis • Male gametophyte: Microspore Vegetative cell (forms pollen tube) Generative cell Sperm cell - Thus, forms three cells: pollen tube and two sperm cells - Occurs in distinctive container: pollen grain, with characteristic wall structure

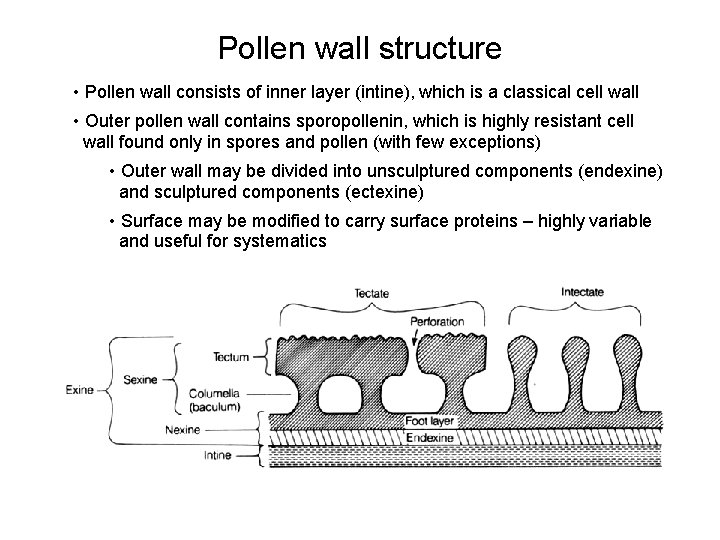
Pollen wall structure • Pollen wall consists of inner layer (intine), which is a classical cell wall • Outer pollen wall contains sporopollenin, which is highly resistant cell wall found only in spores and pollen (with few exceptions) • Outer wall may be divided into unsculptured components (endexine) and sculptured components (ectexine) • Surface may be modified to carry surface proteins – highly variable and useful for systematics

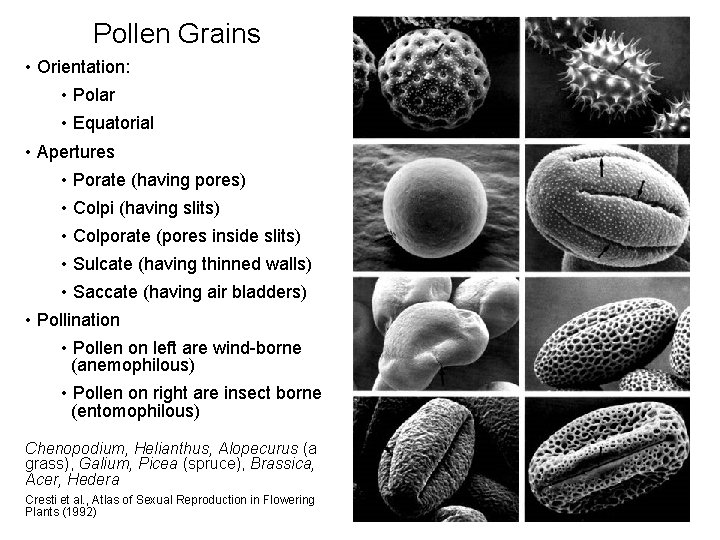
Pollen Grains • Orientation: • Polar • Equatorial • Apertures • Porate (having pores) • Colpi (having slits) • Colporate (pores inside slits) • Sulcate (having thinned walls) • Saccate (having air bladders) • Pollination • Pollen on left are wind-borne (anemophilous) • Pollen on right are insect borne (entomophilous) Chenopodium, Helianthus, Alopecurus (a grass), Galium, Picea (spruce), Brassica, Acer, Hedera Cresti et al. , Atlas of Sexual Reproduction in Flowering Plants (1992)

Male gametophytes: bicellular and tricellular

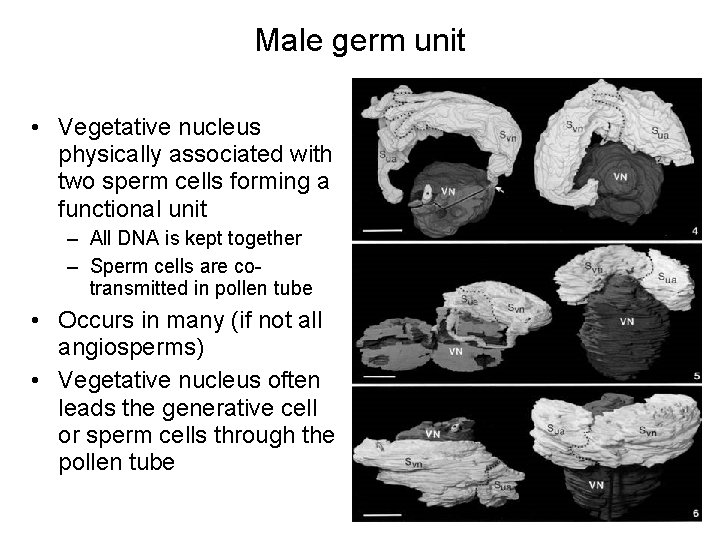
Male germ unit • Vegetative nucleus physically associated with two sperm cells forming a functional unit – All DNA is kept together – Sperm cells are cotransmitted in pollen tube • Occurs in many (if not all angiosperms) • Vegetative nucleus often leads the generative cell or sperm cells through the pollen tube
Female Gametophyte

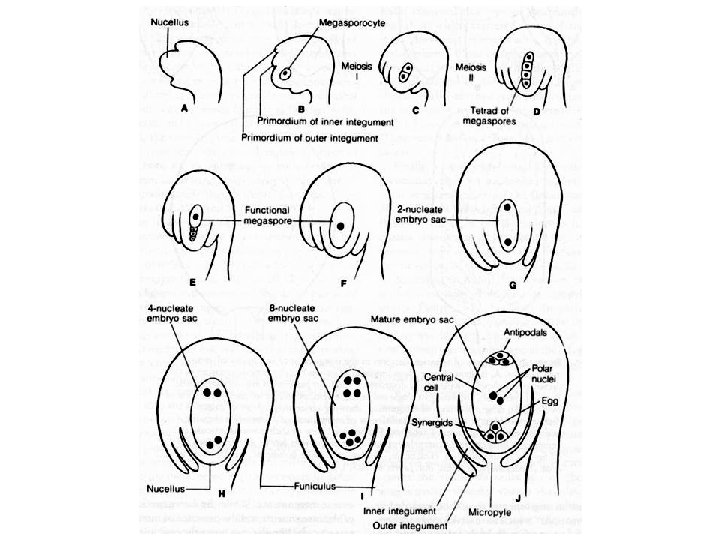
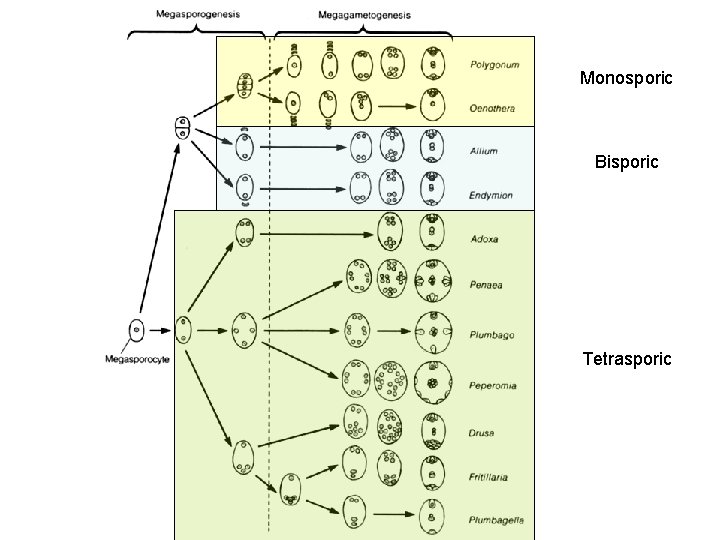
• Female gametophyte (three major patterns): – Monosporic: one spore product participates (others abort) – Bisporic: two spore products participate (others abort) – Tetrasporic: all four spores participate (common coenocyte) • Most common is so-called Polygonum type: Megaspore Synergid Egg Polar Nucleus Megaspore Antipodal


Monosporic Bisporic Tetrasporic

Male-Female Interactions

Fertilization • • • Gametogenesis Pollination Gamete maturation and communication Gamete adhesion, recognition, fusion Fusion product activation

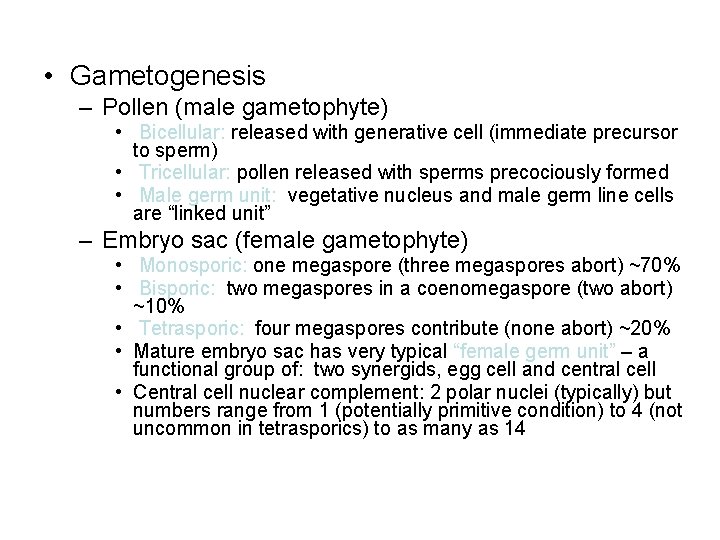
• Gametogenesis – Pollen (male gametophyte) • Bicellular: released with generative cell (immediate precursor to sperm) • Tricellular: pollen released with sperms precociously formed • Male germ unit: vegetative nucleus and male germ line cells are “linked unit” – Embryo sac (female gametophyte) • Monosporic: one megaspore (three megaspores abort) ~70% • Bisporic: two megaspores in a coenomegaspore (two abort) ~10% • Tetrasporic: four megaspores contribute (none abort) ~20% • Mature embryo sac has very typical “female germ unit” – a functional group of: two synergids, egg cell and central cell • Central cell nuclear complement: 2 polar nuclei (typically) but numbers range from 1 (potentially primitive condition) to 4 (not uncommon in tetrasporics) to as many as 14

Pollination

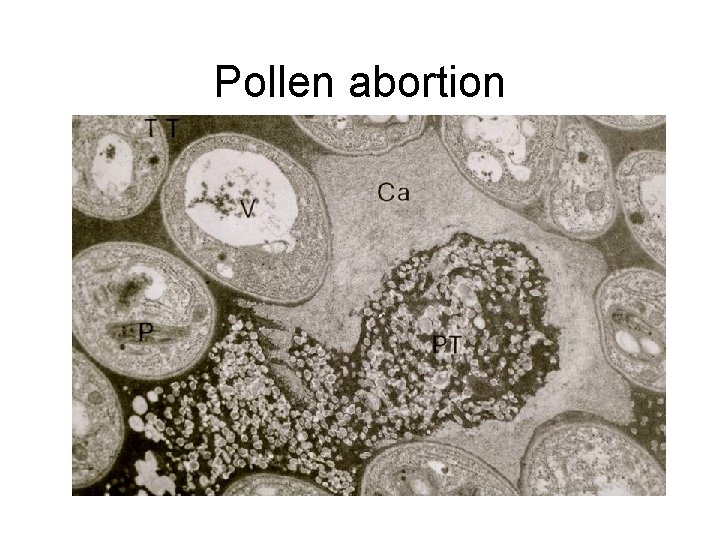
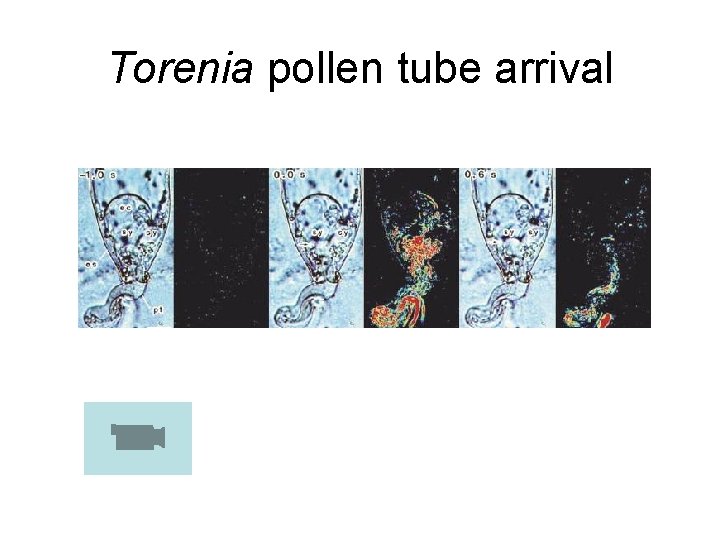
• Pollination – Adhesion, hydration, germination (recognition/selfincompatibility) – Pollen tube elongation (often not called growth) • Bicellular pollen species (tobacco, Torenia, lily, orchids, ~2/3 of flowering plants): – 1 st phase: slow elongation, culture conditions usually easily met in vitro – 2 nd phase: sperm formation: nutritional demands high, sperm formation often is faulty – 3 rd phase: rapid elongation terminating with embryo sac penetration, fertilization • Tricellular pollen species (grasses, Arabidopsis, composites, ~1/3 of angiosperms) – Rapid elongation terminating with embryo sac penetration, fertilization – Pollen selection mechanisms: number of pollen tubes ultimately reduced to the number of available ovules • Some pollen tubes cease elongation or burst • Nutrients may be depleted; pollen tube vigor may be “lost” • Distinction between active and passive mechanisms of pollen tube abortion may be lost

Pollen abortion

• Gamete maturation and communication – Gametes undergo a maturation period – Female gametes: • 1 st stage: cellularization (nucleus is centered and cell axially polarized) • 2 nd stage: vacuolization (often many small vacuoles are present) • 3 rd stage: nuclear migration (nuclear-cytoplasmic polarization) – Male gametes: • Progressive surface modifications • Programmatic changes – Gametes may be either at G 1 or G 2 for fusion • Bicellular and tricellular pollen species may have G 1 or G 2 fusion (only about 10 species surveyed so far!) • Cell progression between male and female gametes is synchronized by male in tobacco

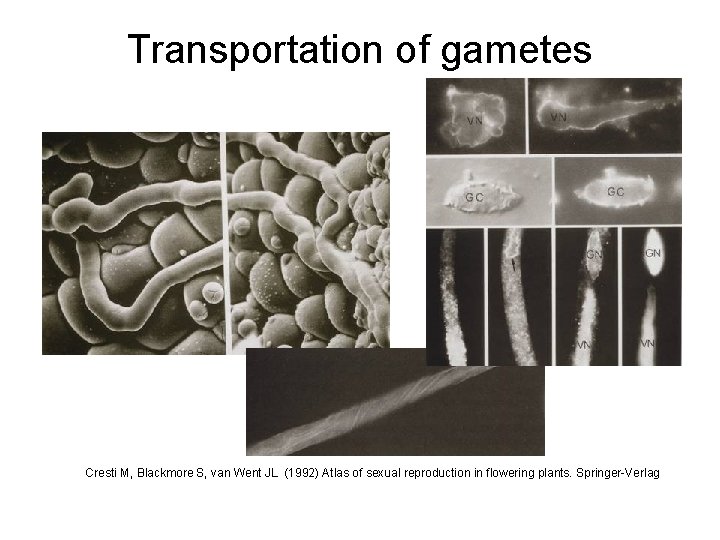
Transportation of gametes Cresti M, Blackmore S, van Went JL (1992) Atlas of sexual reproduction in flowering plants. Springer-Verlag

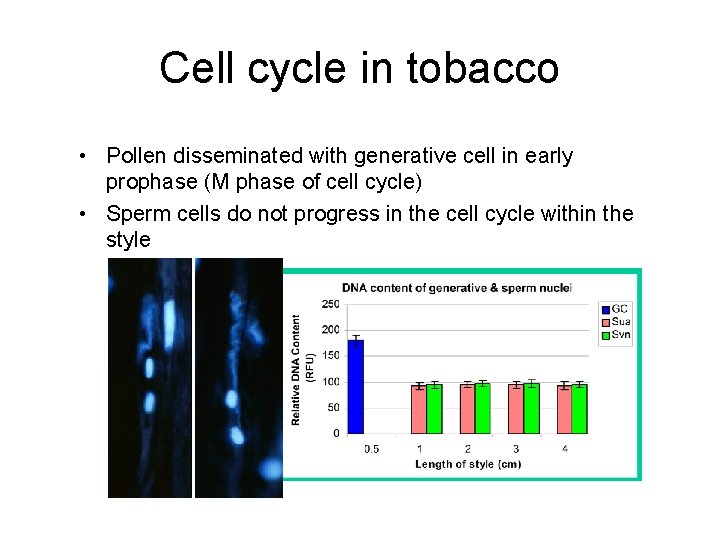
Cell cycle in tobacco • Pollen disseminated with generative cell in early prophase (M phase of cell cycle) • Sperm cells do not progress in the cell cycle within the style

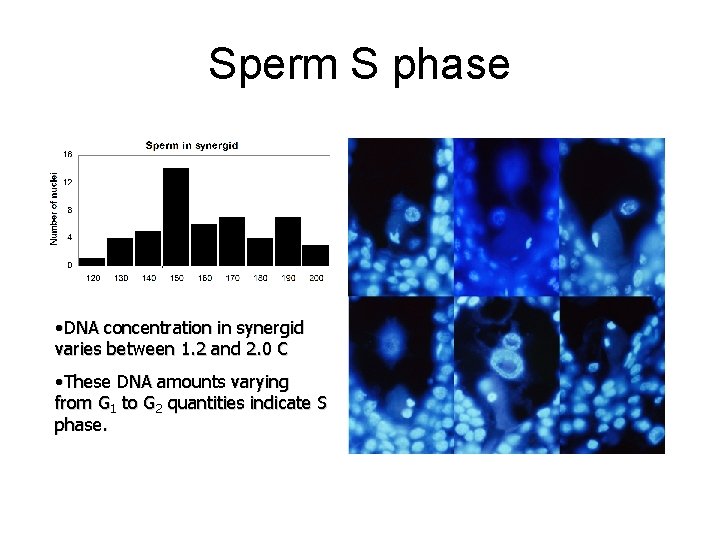
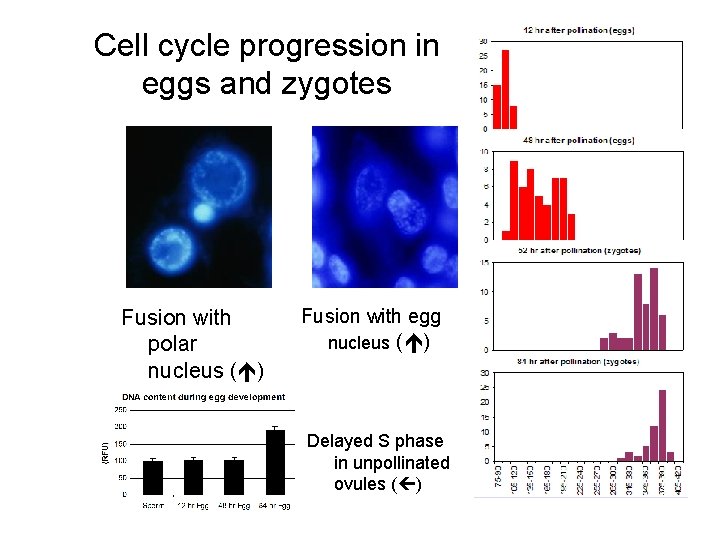
Sperm S phase • DNA concentration in synergid varies between 1. 2 and 2. 0 C • These DNA amounts varying from G 1 to G 2 quantities indicate S phase.

Cell cycle progression in eggs and zygotes Fusion with polar nucleus ( ) Fusion with egg nucleus ( ) Delayed S phase in unpollinated ovules ( )

pollination Nicotiana generative cell interphase pollen grain generative cell mitosis sperm formed pollen tube sperm G 1 sperm m S G 2 synergid

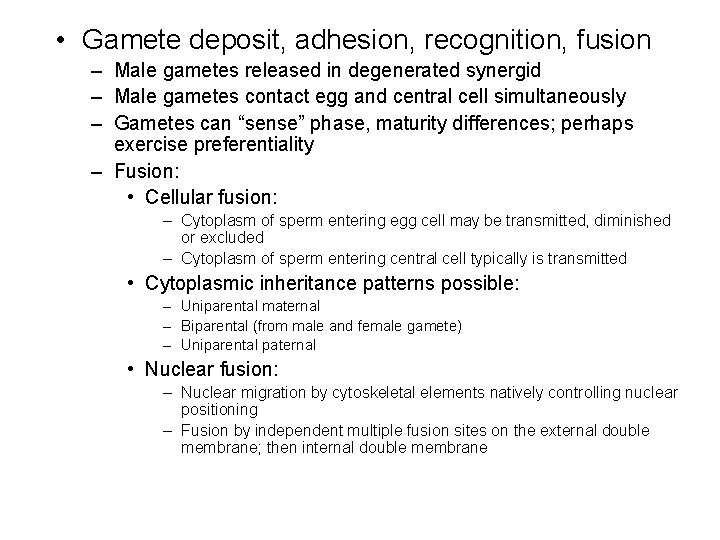
• Gamete deposit, adhesion, recognition, fusion – Male gametes released in degenerated synergid – Male gametes contact egg and central cell simultaneously – Gametes can “sense” phase, maturity differences; perhaps exercise preferentiality – Fusion: • Cellular fusion: – Cytoplasm of sperm entering egg cell may be transmitted, diminished or excluded – Cytoplasm of sperm entering central cell typically is transmitted • Cytoplasmic inheritance patterns possible: – Uniparental maternal – Biparental (from male and female gamete) – Uniparental paternal • Nuclear fusion: – Nuclear migration by cytoskeletal elements natively controlling nuclear positioning – Fusion by independent multiple fusion sites on the external double membrane; then internal double membrane

Torenia pollen tube arrival

Sperm au naturel

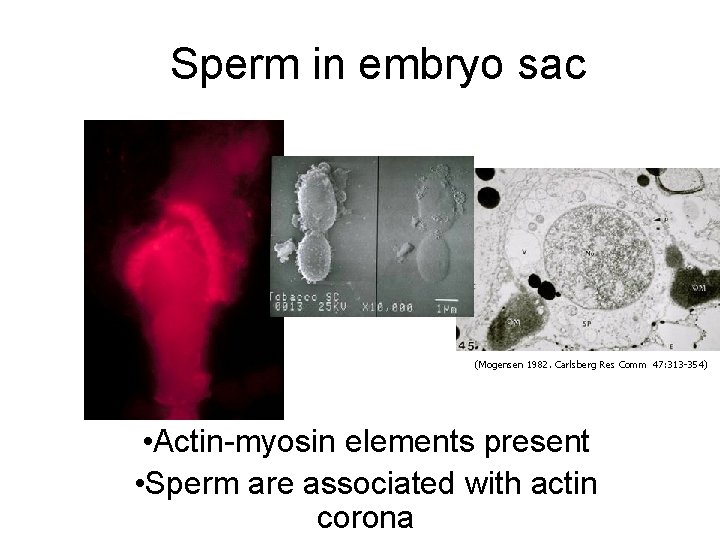
Sperm in embryo sac (Mogensen 1982. Carlsberg Res Comm 47: 313 -354) • Actin-myosin elements present • Sperm are associated with actin corona

Double Fertilization • Two sperm cells deposited in the embryo sac. • One sperm cell aligns & fuses with egg: – Sperm and egg nuclei fuse, forming zygote – Zygote forms embryo and subsequently seedling • Other sperm cell aligns & fuses with central cell & polar nuclei: – Sperm and polar nuclei fuse, forming primary endosperm nucleus (PEN) – Nutritive endosperm forms, provides food source for seedling

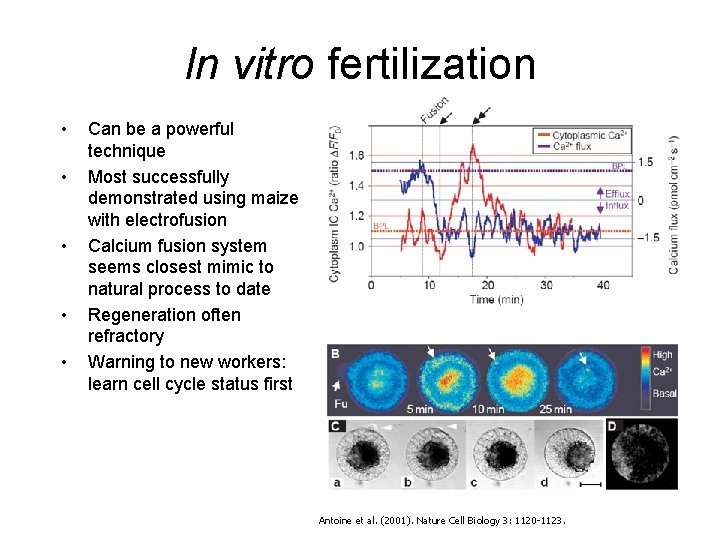
In vitro fertilization • • • Can be a powerful technique Most successfully demonstrated using maize with electrofusion Calcium fusion system seems closest mimic to natural process to date Regeneration often refractory Warning to new workers: learn cell cycle status first Antoine et al. (2001). Nature Cell Biology 3: 1120 -1123.

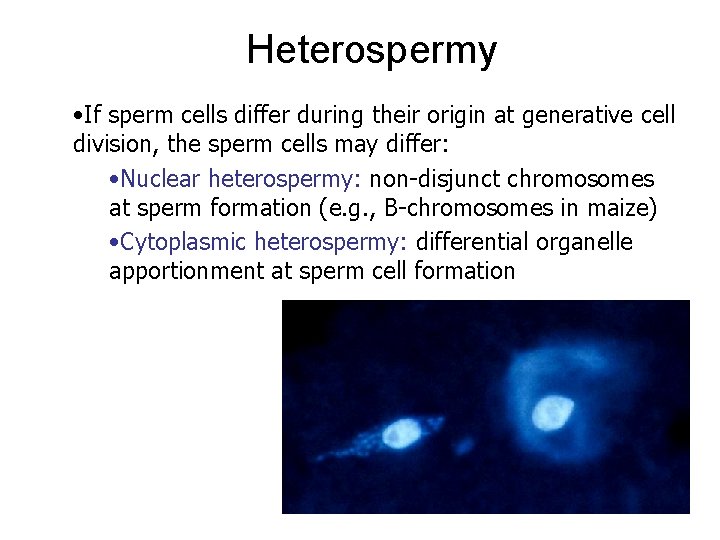
Heterospermy • If sperm cells differ during their origin at generative cell division, the sperm cells may differ: • Nuclear heterospermy: non-disjunct chromosomes at sperm formation (e. g. , B-chromosomes in maize) • Cytoplasmic heterospermy: differential organelle apportionment at sperm cell formation

Sperm Cell Dimorphism

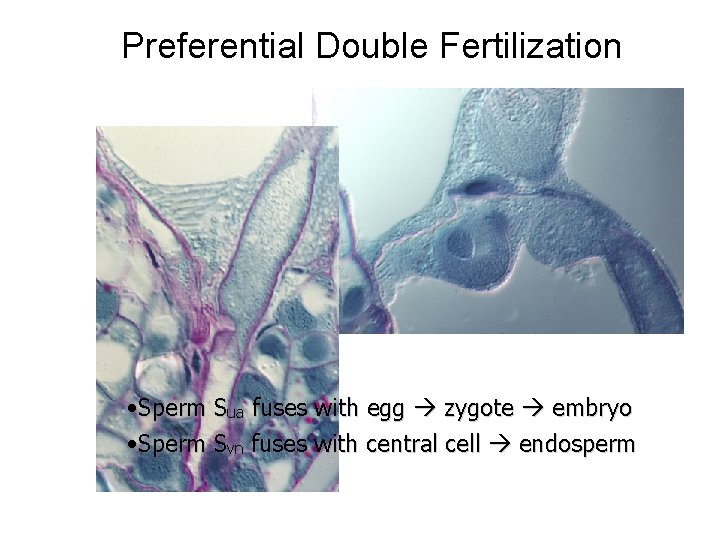
Preferential Double Fertilization • Sperm Sua fuses with egg zygote embryo • Sperm Svn fuses with central cell endosperm