Fundamentals of Radiation Radioactivity Radioactive substances have unstable

Fundamentals of Radiation

Radioactivity • Radioactive substances have unstable nuclei which go through changes by emitting particles or releasing energy to become more stable.
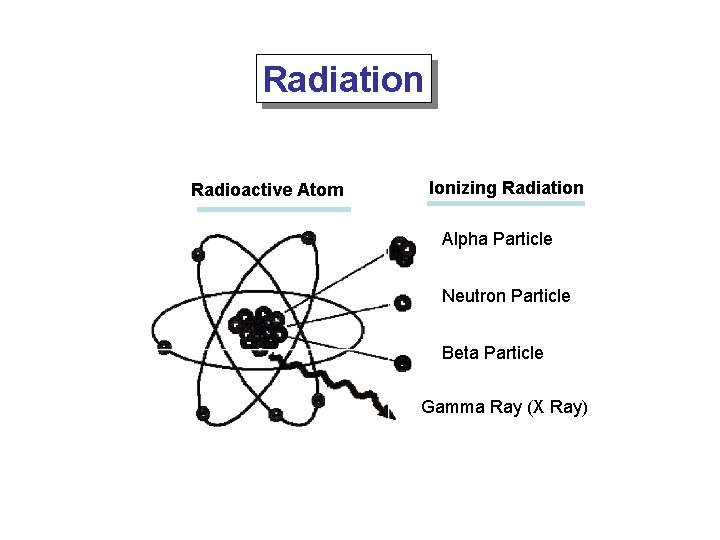
Radiation Radioactive Atom Ionizing Radiation Alpha Particle Neutron Particle Beta Particle Gamma Ray (X Ray)
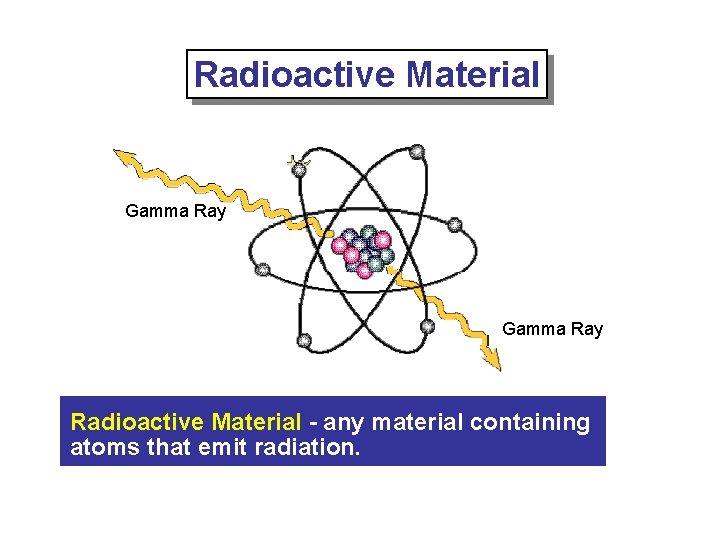
Radioactive Material Gamma Ray Radioactive Material - any material containing atoms that emit radiation.

Radioactive Contamination - is radioactive material in an unwanted place.
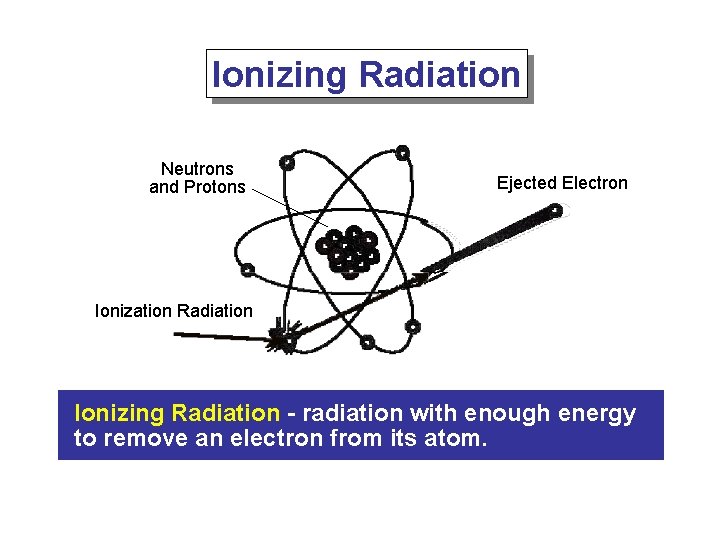
Ionizing Radiation Neutrons and Protons Ejected Electron Ionization Radiation Ionizing Radiation - radiation with enough energy to remove an electron from its atom.
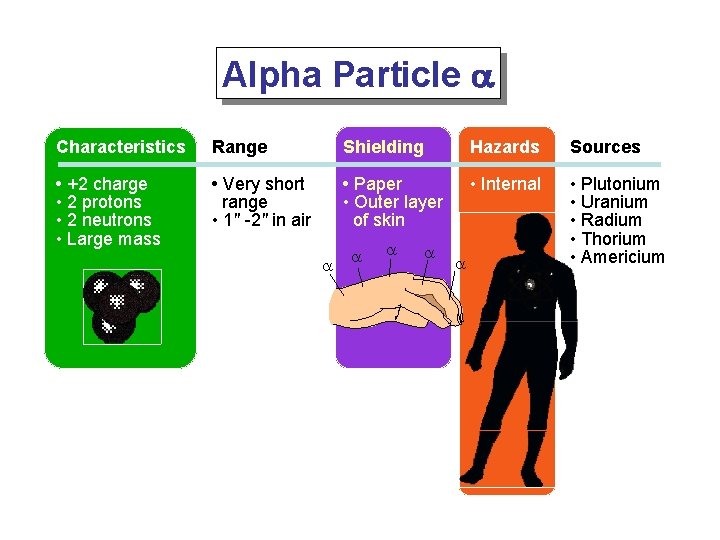
Alpha Particle a Characteristics Range Shielding Hazards Sources • +2 charge • 2 protons • 2 neutrons • Large mass • Very short range • 1" -2" in air • Paper • Outer layer of skin • Internal • Plutonium • Uranium • Radium • Thorium • Americium a a a
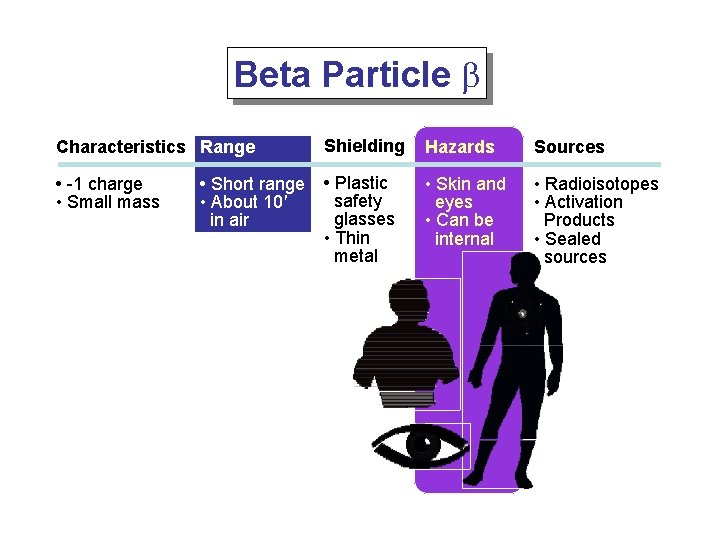
Beta Particle b Characteristics Range Shielding Hazards Sources • -1 charge • Small mass • Plastic safety glasses • Thin metal • Skin and eyes • Can be internal • Radioisotopes • Activation Products • Sealed sources • Short range • About 10' in air
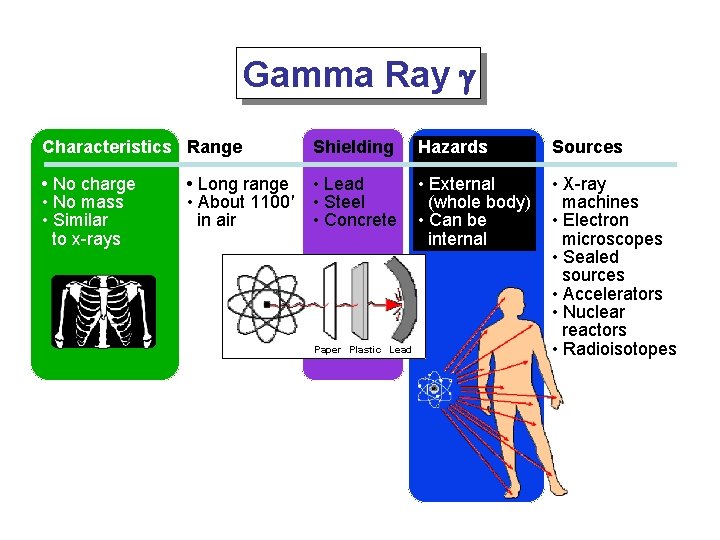
Gamma Ray g Characteristics Range Shielding Hazards Sources • No charge • No mass • Similar to x-rays • Lead • Steel • Concrete • External (whole body) • Can be internal • X-ray machines • Electron microscopes • Sealed sources • Accelerators • Nuclear reactors • Radioisotopes • Long range • About 1100' in air Paper Plastic Lead
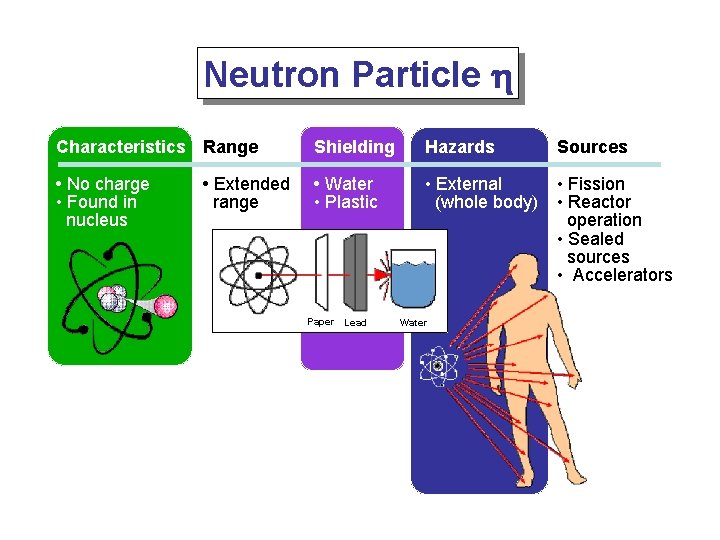
Neutron Particle h Characteristics Range Shielding Hazards Sources • No charge • Found in nucleus • Water • Plastic • External (whole body) • Fission • Reactor operation • Sealed sources • Accelerators • Extended range Paper Lead Water

Radiation Units roentgen (R) • measures exposure (ionization) of air by X-rays & gamma-rays rad (radiation absorbed dose) • measures energy deposited in any material by any type of ionizing radiation rem (Roentgen Equivalent to Man) • estimates biological damage or health risk due to absorption of ionizing radiation • unit of dose equivalent

Radioactivity Units Measure the number of nuclear transformations (disintegrations) which occur in a certain time period Curie (abbreviated, Ci) = 37, 000, 000 disintegrations per second (dps) = 2, 200, 000, 000 disintegrations per minute (dpm) 2 of Radioactive contamination measures an amount activity over a unit of surface area. e. g. 5000 dpm/100 cm 2
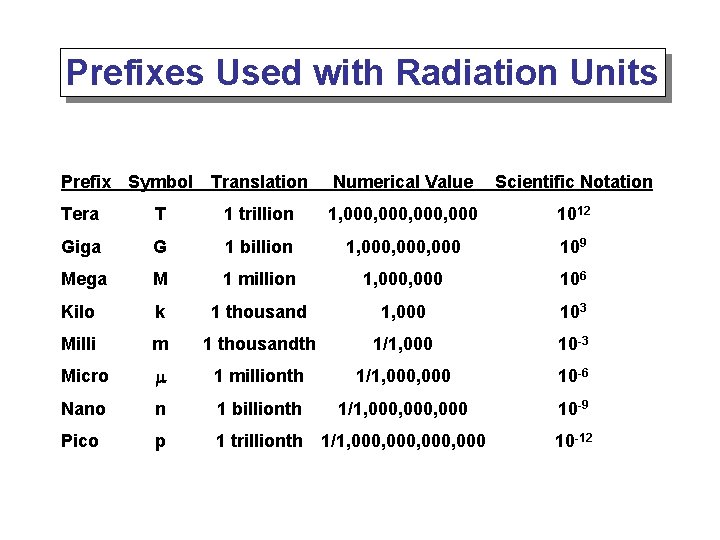
Prefixes Used with Radiation Units Prefix Symbol Translation Numerical Value Scientific Notation Tera T 1 trillion 1, 000, 000 1012 Giga G 1 billion 1, 000, 000 109 Mega M 1 million 1, 000 106 Kilo k 1 thousand 1, 000 103 Milli m 1 thousandth 1/1, 000 10 -3 Micro m 1 millionth 1/1, 000 10 -6 Nano n 1 billionth 1/1, 000, 000 10 -9 Pico p 1 trillionth 1/1, 000, 000 10 -12
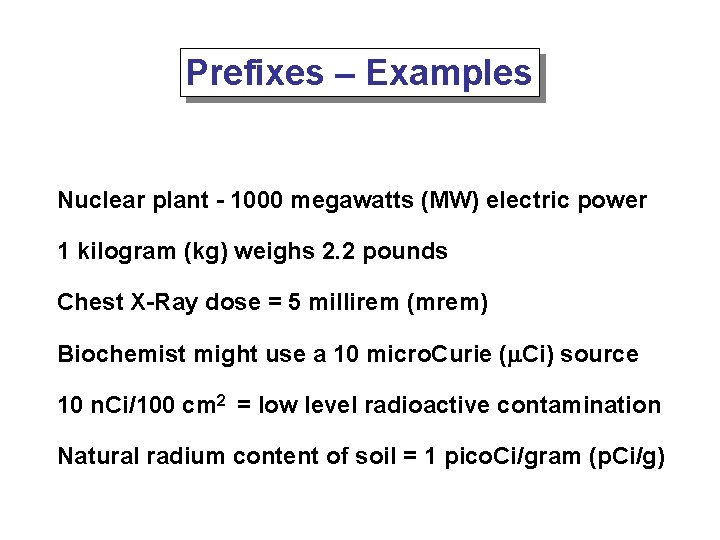
Prefixes – Examples Nuclear plant - 1000 megawatts (MW) electric power 1 kilogram (kg) weighs 2. 2 pounds Chest X-Ray dose = 5 millirem (mrem) Biochemist might use a 10 micro. Curie (m. Ci) source 10 n. Ci/100 cm 2 = low level radioactive contamination Natural radium content of soil = 1 pico. Ci/gram (p. Ci/g)
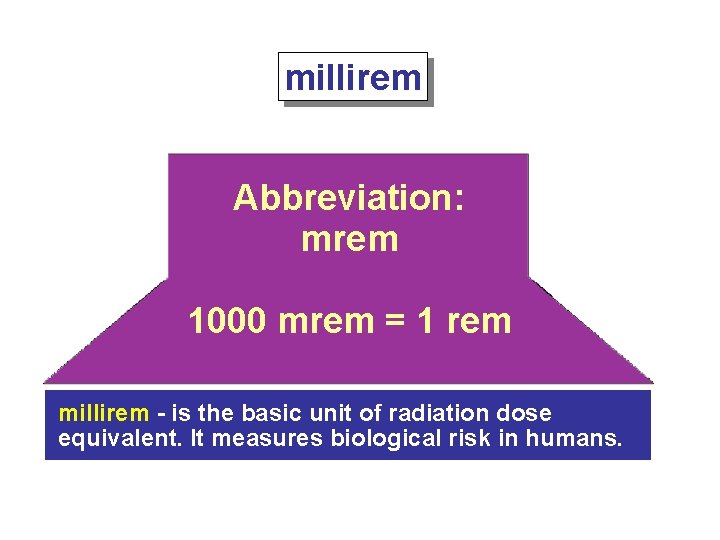
millirem Abbreviation: mrem 1000 mrem = 1 rem millirem - is the basic unit of radiation dose equivalent. It measures biological risk in humans.
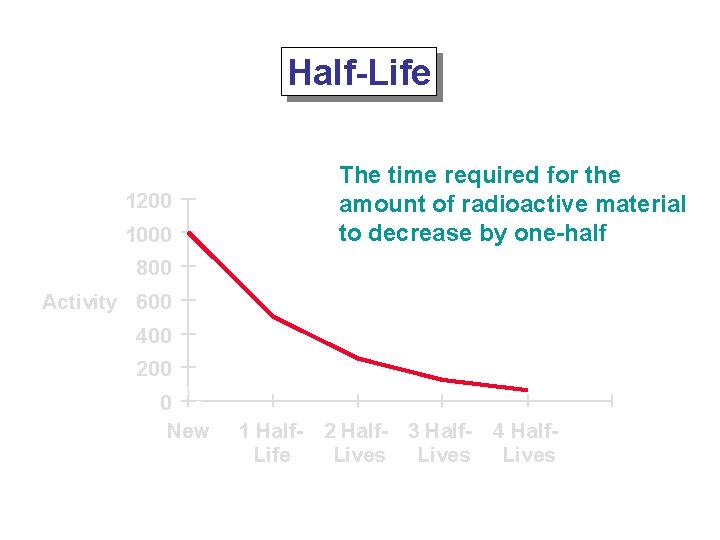
Half-Life The time required for the amount of radioactive material to decrease by one-half 1200 1000 800 Activity 600 400 200 0 New 1 Half. Life 2 Half- 3 Half. Lives 4 Half. Lives
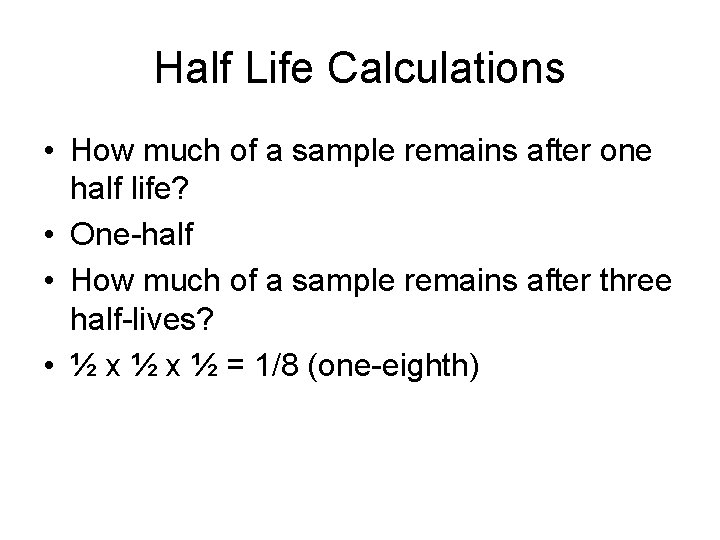
Half Life Calculations • How much of a sample remains after one half life? • One-half • How much of a sample remains after three half-lives? • ½ x ½ = 1/8 (one-eighth)
Acute Radiation Dose Acute radiation dose refers to persons who receive large amounts of radiation over a short period of time.

Chronic Radiation Dose Chronic radiation dose refers to persons who receive small amounts of radiation over a long period of time.
Chronic Radiation Dose Chronic radiation dose refers to persons who receive small amounts of radiation over a long period of time. There is a slight risk that cancer may be caused by chronic radiation doses. This risk level is very small compared to the natural occurrence rate of cancer.

LNT Assumption The previous statements assume a Linear, No-Threshold (LNT) response to radiation. There is a growing body of scientific evidence that this assumption is incorrect, and that low levels of radiation exposure are not harmful. There is also evidence that low levels of radiation exposure can have a beneficial (i. e. , hormesis) effect.
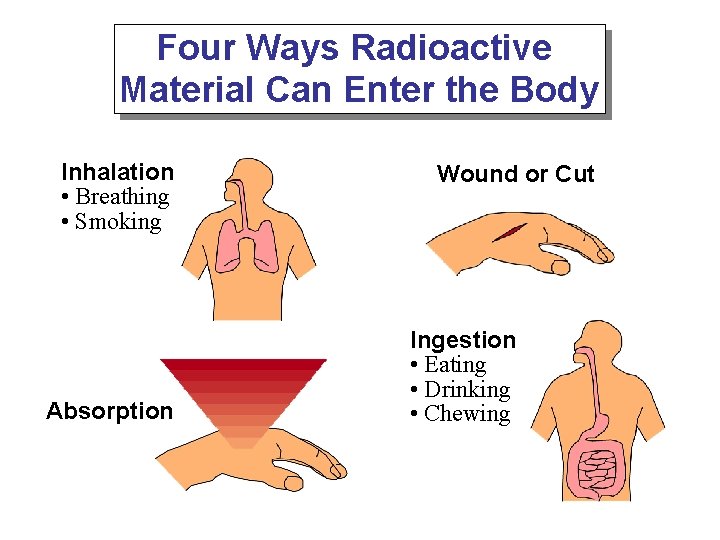
Four Ways Radioactive Material Can Enter the Body Inhalation • Breathing • Smoking Absorption Wound or Cut Ingestion • Eating • Drinking • Chewing
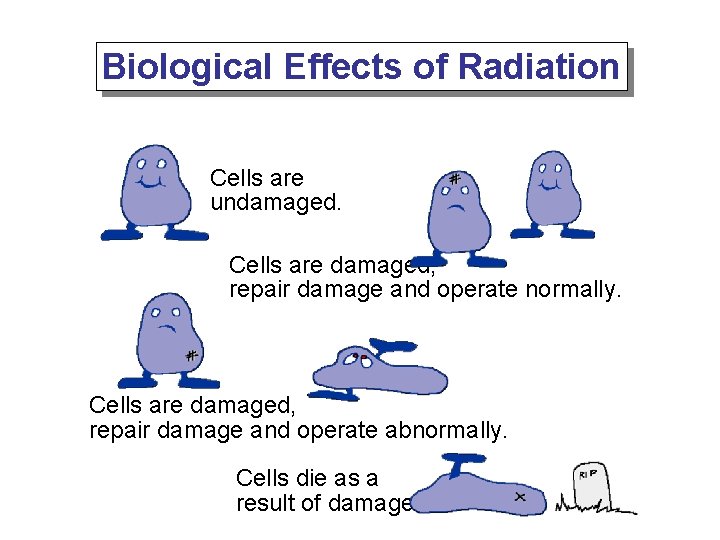
Biological Effects of Radiation Cells are undamaged. Cells are damaged, repair damage and operate normally. Cells are damaged, repair damage and operate abnormally. Cells die as a result of damage.
Health Effects • Radiation effects on cell chromosomes: Somatic Effects observed in the exposed individual Heritable (Genetic) Effects observed in future generations of exposed individual
The fetus is. RW MORE I sensitive than an adult.

No Heritable Effects from Ionizing Radiation Have Been Observed in Humans Heritable effects have been observed in laboratory animals.
The average annual dose to the general population from natural background and man-made sources is 360 mrem. Terrestrial Sources Radon Cosmic Radiation Radon Internal Sources Other
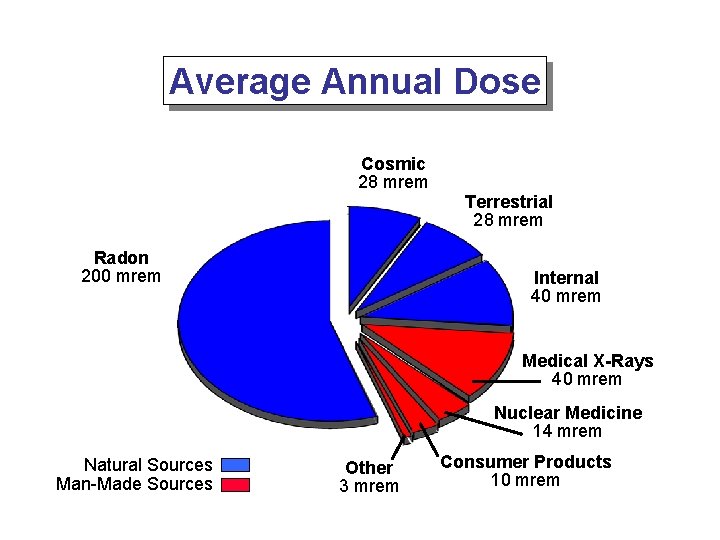
Average Annual Dose Cosmic 28 mrem Radon 200 mrem Terrestrial 28 mrem Internal 40 mrem Medical X-Rays 40 mrem Nuclear Medicine 14 mrem Natural Sources Man-Made Sources Other 3 mrem Consumer Products 10 mrem
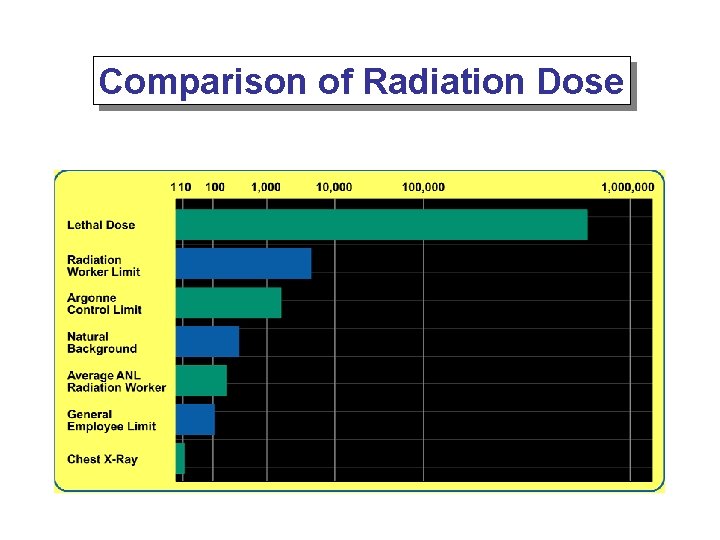
Comparison of Radiation Dose

Relative risk of RW dying: I 1 in a million odds. • Smoking 1. 4 cigarettes (lung cancer) • Eating 40 tablespoons of peanut butter • Eating 100 charcoal broiled steaks • 2 days in New York City (air pollution) • Driving 40 miles in a car (accident) • Flying 2500 miles in a jet (accident) • Canoeing for 6 miles • Receiving 10 mrem radiation dose (cancer)

Risk – Loss of Life Expectancy Days of Average Life Expectancy Lost Due to Various Causes Being an unmarried male Smoking (1 pack/day) Being an unmarried female Being a coal miner 25% overweight Alcohol abuse (U. S. average) Being a construction worker Driving a motor vehicle All industries Radiation: 100 mrem/yr x 70 years Coffee 3500 2250 1600 1100 777 365 227 207 60 10 6
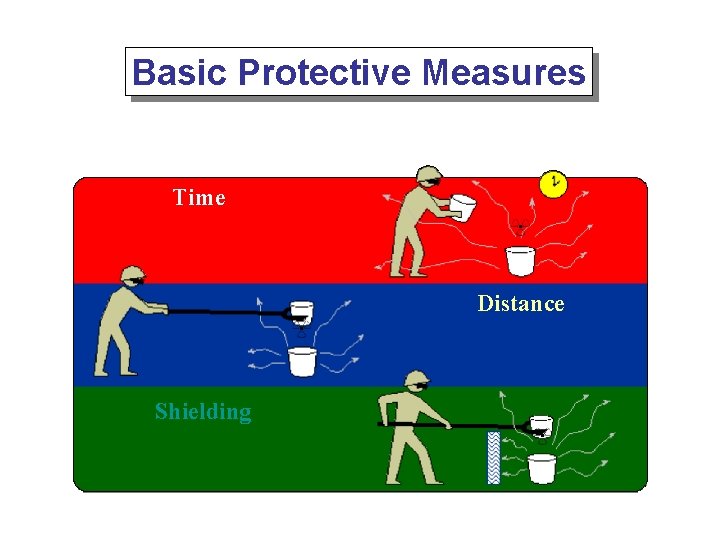
Basic Protective Measures Time Distance Shielding

Uses of Nuclear Energy other than Weapons 1. Nuclear Medicine 2. Nuclear Power Generation
Medical Science • Disease diagnosis and treatment (99 m. Tc, 60 Co, 131 I) • Testing of new pharmaceuticals • Biomedical research - human genome project, cancer cell biology, AIDS and Alzheimer’s research • Positron Emission Topography [PET] (18 F) • Magnetic Resonance Imaging [MRI] • X-rays • Sterilization of disposable medical supplies
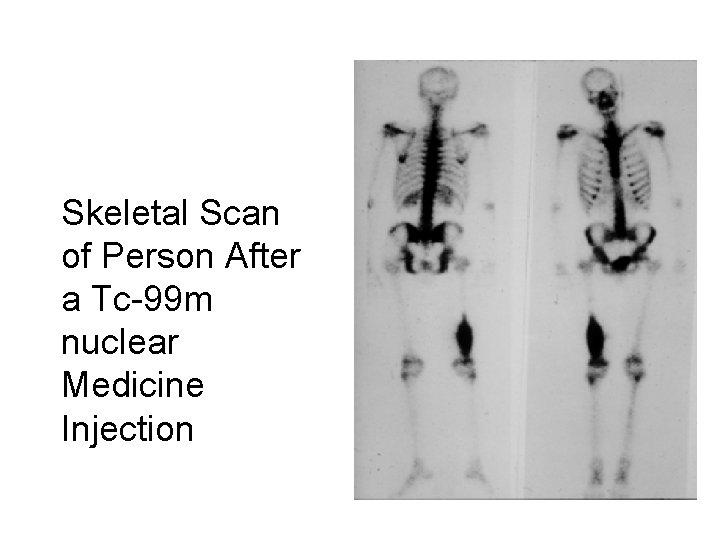
Skeletal Scan of Person After a Tc-99 m nuclear Medicine Injection
Nuclear Power Generation Fission and Fusion

Fission The process of splitting heavier nuclei into lighter nuclei Neutrons released in fission reactions can start a chain reaction This can lead to uncontrolled reactions. To control nuclear chain reactions, we use critical mass—minimum amount of a substance that can undergo a fission reaction and also sustain a a chain reaction.

Nuclear Power Plant In a nuclear power plant, fission is used to create power. The nuclear energy is transformed to heat energy which is transformed to mechanical energy which is transformed to make electricity (electrical energy). Remember energy cannot be created nor destroyed. It can only change forms.
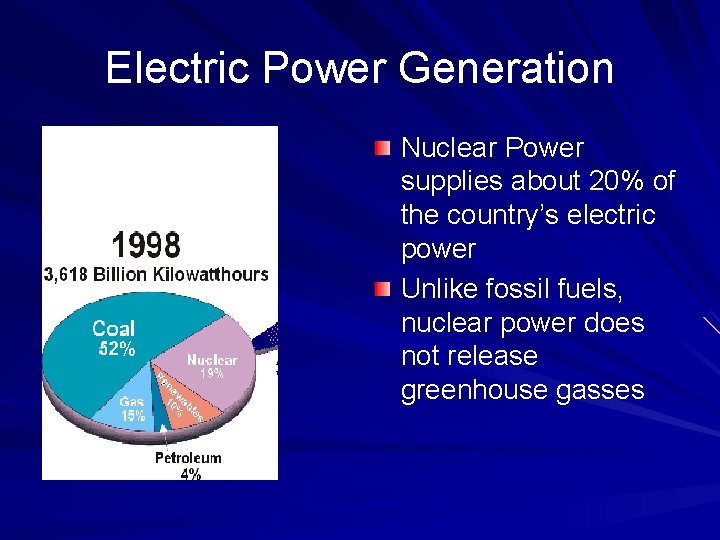
Electric Power Generation Nuclear Power supplies about 20% of the country’s electric power Unlike fossil fuels, nuclear power does not release greenhouse gasses

Power Plant generator that produces 840 Megawatts

Pipes carry steam that powers the generator

Secondary Structure protects the reactor from accidents like a jet airplane

Control Room at a Nuclear Power Plant

Chernobyl http: //www. worldnuclear. org/info/chernobyl/inf 07. htm

Fusion Energy obtained from very light nuclei combining At high temperatures like those of a star, the H nuclei combine But there is an initial outlay of a lot of energy to overcome the repelling forces of the protons of the two hydrogen atoms

Fusion In nuclear fusion four hydrogen nuclei are fused into a helium nucleus and two positrons. Positrons are positive electrons

Positives for Fusion Produces a lot of energy with a tiny bit of matter The byproducts are not nearly as toxic Less concern about radiation leakage

Negatives for Fusion Requires 100, 000 K (six times hotter than the sun’s core) The sun has this temperature and that’s where fusion occurs continually The sun is able to do this with extreme pressure due to high gravity

Fusion on Earth We can achieve this with microwaves, lasers, and ion particles To increase the pressure we use high powered magnetic fields, powerful lasers, and ion beams
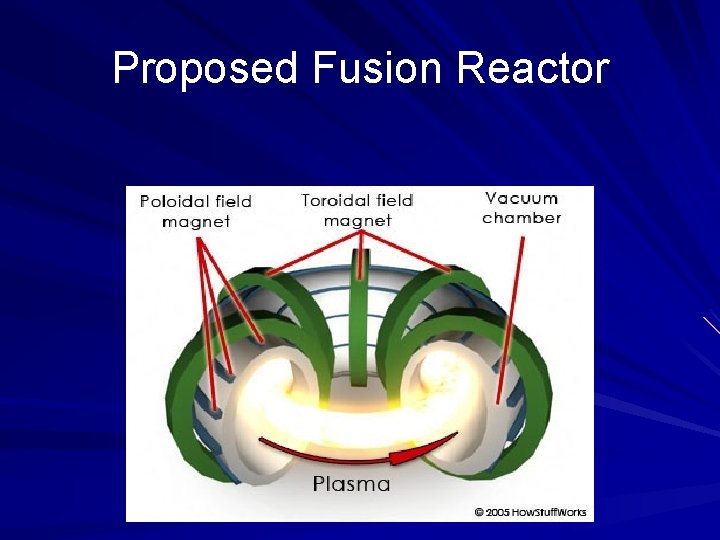
Proposed Fusion Reactor

Comparing Fission and Fusion l. Reactions l. Availability of Resources l. Safety l. Waste Material
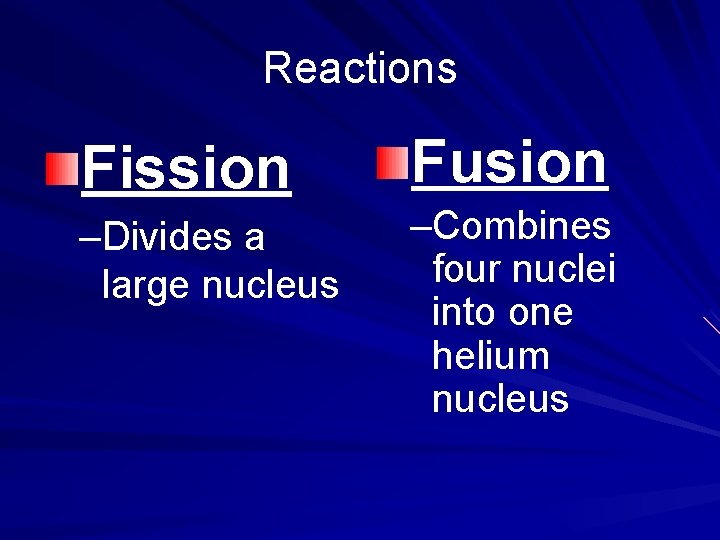
Reactions Fission –Divides a large nucleus Fusion –Combines four nuclei into one helium nucleus

Available Resource Material Fission –Requires radioactive substances like uranium Fusion – Currently requires an isotope of hydrogen called tritium which is also radioactive – Future: may use hydrogen (not radioactive)

Safety Fission – Has proven track record of safety although disasters like Chernobyl make us realize how dangerous it could be Fusion – Eventually if feasible it would be able to produce more energy than fission and have no radiation danger

Products (end result of the reaction) Fission – Although the radioactive cores last a very long time, they eventually after nearly 100 years need to be disposed of – Usually we put them in mountains to shield us from the radiation Fusion – Product is helium which is not radioactive nor dangerous.
Timeline Websites -http: //www. nuclearfiles. org/menu/timeline/flash_index. htm

National Atomic Museum Home | About Us | Membership | Museum Store | Education | New Museum | Search
Timelines
Atomic Archive
- Slides: 59