Fundamentals of Anatomy Physiology Eleventh Edition Chapter 27





















- Slides: 116

Fundamentals of Anatomy & Physiology Eleventh Edition Chapter 27 Fluid, Electrolyte, and Acid-Base Balance Lecture Presentation by Deborah A. Hutchinson Seattle University © 2018 Pearson Education, Inc.

27 -1 Fluid, Electrolyte, and Acid-Base Balance § Water – Makes up 99 percent of fluid volume outside cells • Extracellular fluid (ECF) – Essential ingredient of cytosol inside cells • Intracellular fluid (ICF) – All cellular operations rely on water • As diffusion medium for gases, nutrients, and wastes 2 © 2018 Pearson Education, Inc.

27 -1 Fluid, Electrolyte, Acid-Base Balance § Volumes, solute concentrations, and p. H of ECF and ICF – Stabilized by three interrelated processes • Fluid balance • Electrolyte balance • Acid-base balance 3 © 2018 Pearson Education, Inc.

27 -1 Fluid, Electrolyte, Acid-Base Balance § Fluid balance – Daily balance between • Amount of water gained • Amount of water lost to environment – Involves regulating content and distribution of body water in ECF and ICF – Digestive system • Primary source of water gains – Urinary system • Primary route of water loss 4 © 2018 Pearson Education, Inc.

27 -1 Fluid, Electrolyte, Acid-Base Balance § Electrolyte balance – Electrolytes • Ions released through dissociation of inorganic compounds • Can conduct electrical current in solution – Electrolyte balance • When gains and losses for every electrolyte are in balance • Primarily involves balancing rates of absorption across digestive tract with rates of loss at kidneys 5 © 2018 Pearson Education, Inc.

27 -1 Fluid, Electrolyte, Acid-Base Balance § Acid-base balance – Precisely balances production and loss of hydrogen ions (H+) – Body generates acids during normal metabolism • Reduce p. H – Kidneys • Secrete H+ into urine • Generate buffers that enter bloodstream – Lungs • Affect p. H through elimination of carbon dioxide 6 © 2018 Pearson Education, Inc.

27 -2 Fluid Compartments § Body water content – Mostly in ICF – Water accounts for roughly • 60 percent of adult male body weight • 50 percent of adult female body weight 7 © 2018 Pearson Education, Inc.

27 -2 Fluid Compartments § Fluid compartments of ECF and ICF – Intracellular fluid (ICF) • Cytosol inside cells – Extracellular fluid (ECF) • Primarily, interstitial fluid of peripheral tissues and plasma of circulating blood 8 © 2018 Pearson Education, Inc.

27 -2 Fluid Compartments § Extracellular fluid (ECF) – Minor components • Lymph • Cerebrospinal fluid (CSF) • Synovial fluid • Serous fluids (pleural, pericardial, and peritoneal) • Aqueous humor • Perilymph • Endolymph 9 © 2018 Pearson Education, Inc.

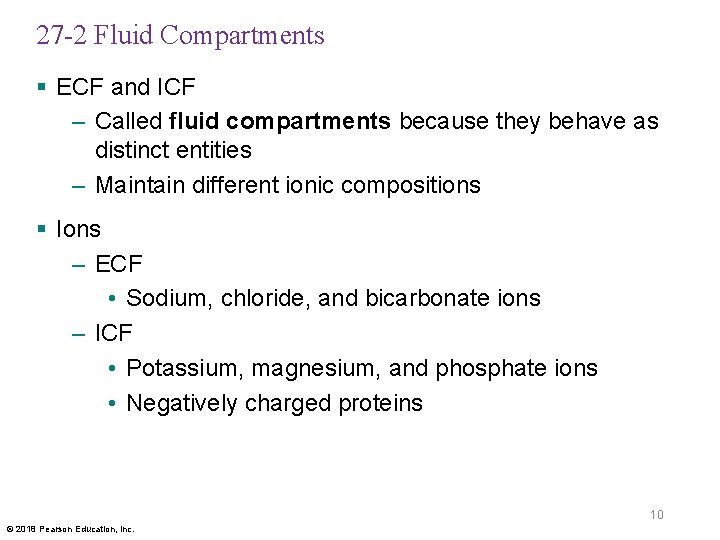
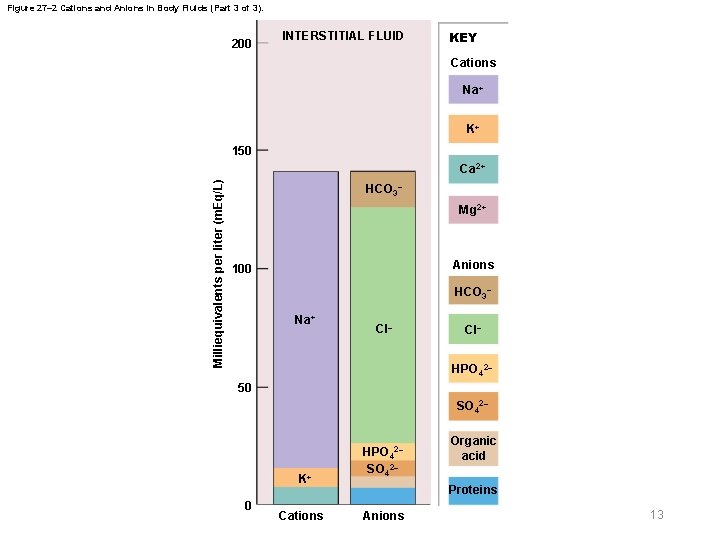
27 -2 Fluid Compartments § ECF and ICF – Called fluid compartments because they behave as distinct entities – Maintain different ionic compositions § Ions – ECF • Sodium, chloride, and bicarbonate ions – ICF • Potassium, magnesium, and phosphate ions • Negatively charged proteins 10 © 2018 Pearson Education, Inc.

Figure 27– 2 Cations and Anions in Body Fluids (Part 1 of 3). 200 INTRACELLULAR FLUID Na+ HCO 3– Cl– KEY Cations Na+ K+ 150 Milliequivalents per liter (m. Eq/L) Ca 2+ HPO 42– Mg 2+ K+ Anions 100 HCO 3– SO 42– Cl– HPO 42– 50 SO 42– Proteins Organic acid Mg 2+ Proteins 0 Cations Anions 11

Figure 27– 2 Cations and Anions in Body Fluids (Part 2 of 3). PLASMA 200 KEY Cations Na+ K+ Milliequivalents per liter (m. Eq/L) 150 HCO 3– Ca 2+ Mg 2+ Anions 100 Na+ Cl– HCO 3– Cl– HPO 42– 50 0 HPO 42– SO 42– Org. acid Organic acid K+ Ca 2+ Proteins Cations Anions Proteins 12

Figure 27– 2 Cations and Anions in Body Fluids (Part 3 of 3). 200 INTERSTITIAL FLUID KEY Cations Na+ K+ 150 Milliequivalents per liter (m. Eq/L) Ca 2+ HCO 3– Mg 2+ Anions 100 HCO 3– Na+ Cl– HPO 42– 50 SO 42– K+ 0 Cations HPO 42– SO 42– Organic acid Proteins Anions 13

27 -2 Fluid Compartments § Osmotic concentrations of ICF and ECF – Identical – Osmosis across plasma membranes eliminates minor differences § Ion exchange – Between ICF and ECF occurs across selectively permeable plasma membranes by • Osmosis • Diffusion • Carrier-mediated transport 14 © 2018 Pearson Education, Inc.

27 -2 Fluid Compartments § Exchanges among subdivisions of ECF – Occur primarily across endothelial lining of capillaries – Also from interstitial spaces to plasma • Via lymphatic vessels that drain into venous system 15 © 2018 Pearson Education, Inc.

27 -2 Fluid Compartments § Solute content of ECF – Varies regionally in types and amounts of • Electrolytes • Proteins • Nutrients • Wastes 16 © 2018 Pearson Education, Inc.

27 -2 Fluid Compartments § Regulation of fluids and electrolytes – All homeostatic mechanisms that monitor and adjust body fluid composition respond to changes in ECF, not ICF – No receptors directly monitor fluid or electrolyte balance – Cells cannot move water by active transport – Body’s water or electrolyte content will rise if dietary gains exceed environmental losses, and will fall if losses exceed gains 17 © 2018 Pearson Education, Inc.

27 -2 Fluid Compartments § Primary hormones that regulate fluid and electrolyte balance – Antidiuretic hormone – Aldosterone – Natriuretic peptides 18 © 2018 Pearson Education, Inc.

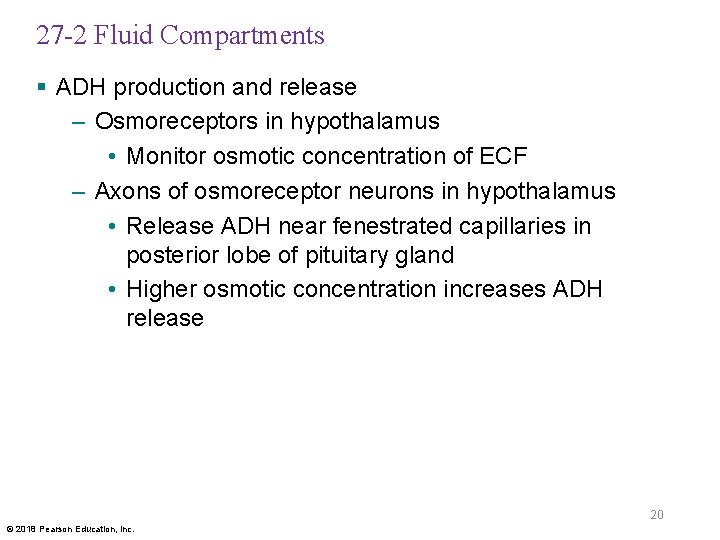
27 -2 Fluid Compartments § Antidiuretic hormone (ADH) – Stimulates water conservation at kidneys • Reducing urinary water loss • Concentrating urine – Stimulates hypothalamic thirst center • Promoting fluid intake 19 © 2018 Pearson Education, Inc.

27 -2 Fluid Compartments § ADH production and release – Osmoreceptors in hypothalamus • Monitor osmotic concentration of ECF – Axons of osmoreceptor neurons in hypothalamus • Release ADH near fenestrated capillaries in posterior lobe of pituitary gland • Higher osmotic concentration increases ADH release 20 © 2018 Pearson Education, Inc.

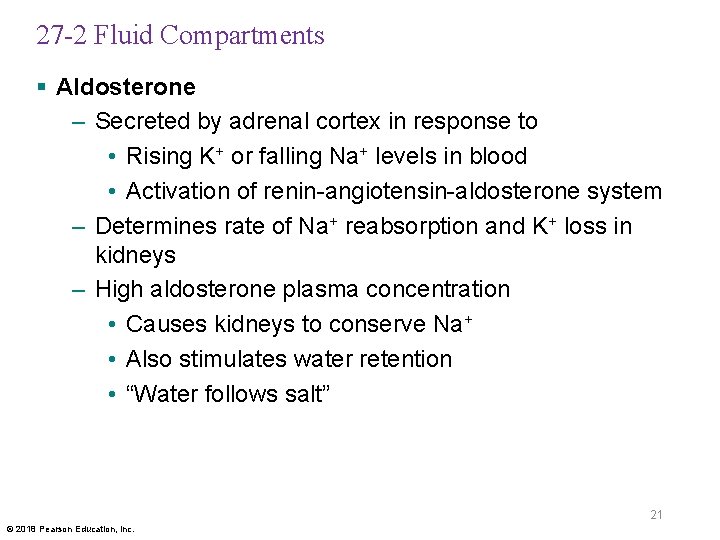
27 -2 Fluid Compartments § Aldosterone – Secreted by adrenal cortex in response to • Rising K+ or falling Na+ levels in blood • Activation of renin-angiotensin-aldosterone system – Determines rate of Na+ reabsorption and K+ loss in kidneys – High aldosterone plasma concentration • Causes kidneys to conserve Na+ • Also stimulates water retention • “Water follows salt” 21 © 2018 Pearson Education, Inc.

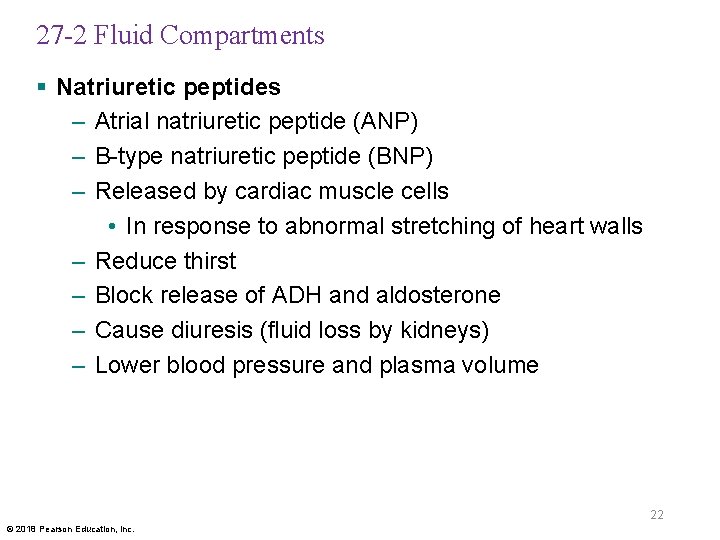
27 -2 Fluid Compartments § Natriuretic peptides – Atrial natriuretic peptide (ANP) – B-type natriuretic peptide (BNP) – Released by cardiac muscle cells • In response to abnormal stretching of heart walls – Reduce thirst – Block release of ADH and aldosterone – Cause diuresis (fluid loss by kidneys) – Lower blood pressure and plasma volume 22 © 2018 Pearson Education, Inc.

27 -3 Fluid Balance § Fluid balance – When amounts of water gained and lost each day are equal – Body’s water content and Na+ concentration of ECF are inversely correlated 23 © 2018 Pearson Education, Inc.

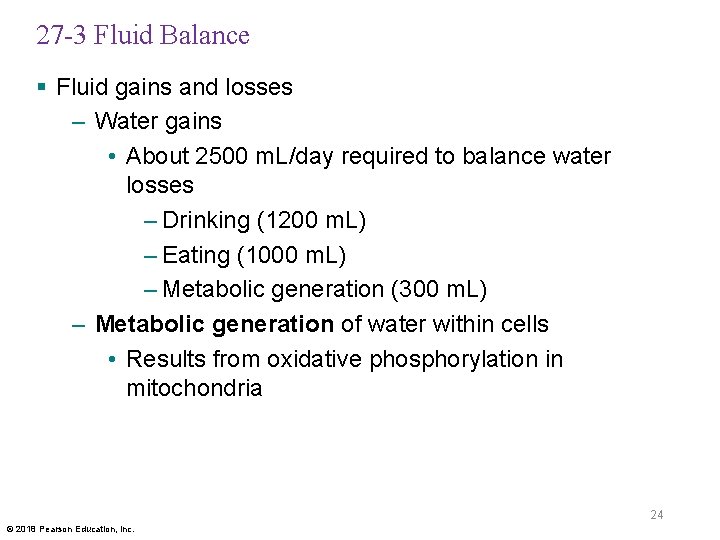
27 -3 Fluid Balance § Fluid gains and losses – Water gains • About 2500 m. L/day required to balance water losses – Drinking (1200 m. L) – Eating (1000 m. L) – Metabolic generation (300 m. L) – Metabolic generation of water within cells • Results from oxidative phosphorylation in mitochondria 24 © 2018 Pearson Education, Inc.

27 -3 Fluid Balance § Fluid gains and losses – Water losses • About 2500 m. L each day – Urine – Feces – Insensible perspiration • Sensible perspiration (sweat) varies with activities – Can cause significant water loss (4 L/hr) • Fever can increase water losses 25 © 2018 Pearson Education, Inc.

27 -3 Fluid Balance § Water can move between ECF and ICF – Normally in osmotic equilibrium • No large-scale circulation between them 26 © 2018 Pearson Education, Inc.

27 -3 Fluid Balance § Fluid movement within ECF – Water circulates freely – Net hydrostatic pressure • Pushes water out of plasma • Into interstitial fluid – Net colloid osmotic pressure • Draws water out of interstitial fluid • Into plasma – Interaction between opposing forces • Results in continuous filtration of fluid 27 © 2018 Pearson Education, Inc.

27 -3 Fluid Balance § Fluid movement within ECF – ECF fluid volume is redistributed • From lymphatic vessels to venous circulation – ECF volume • 80 percent is in interstitial fluid and minor fluid compartments • 20 percent in plasma 28 © 2018 Pearson Education, Inc.

27 -3 Fluid Balance § Edema (swelling) – Movement of abnormal amounts of water from plasma into interstitial fluid § Lymphedema – Edema caused by blockage of lymphatic drainage 29 © 2018 Pearson Education, Inc.

27 -3 Fluid Balance § Fluid shifts – Rapid water movements between ECF and ICF • In response to an osmotic gradient – If osmotic concentration of ECF increases • Fluid becomes hypertonic to ICF • Water moves from cells to ECF – If osmotic concentration of ECF decreases • Fluid becomes hypotonic to ICF • Water moves from ECF into cells 30 © 2018 Pearson Education, Inc.

27 -3 Fluid Balance § Fluid shifts – ICF volume is much greater than ECF volume • ICF acts as water reserve • Prevents large osmotic changes in ECF 31 © 2018 Pearson Education, Inc.

27 -3 Fluid Balance § Dehydration (water depletion) – Develops when water losses are greater than gains – If water is lost, but electrolytes retained • ECF osmotic concentration rises • Water moves from ICF to ECF • Because of large volume, net change in ICF is small 32 © 2018 Pearson Education, Inc.

27 -3 Fluid Balance § Severe water loss – May result from • Excessive perspiration • Inadequate water consumption • Repeated vomiting • Diarrhea – Homeostatic responses • Physiological mechanisms (ADH and renin secretion) • Behavioral changes (increasing fluid intake) 33 © 2018 Pearson Education, Inc.

27 -3 Fluid Balance § Distribution of water gains – If water is gained, but electrolytes are not • ECF volume increases • ECF becomes hypotonic to ICF • Fluid shifts from ECF to ICF • May result in hyperhydration (water excess) – Occurs when excess water shifts into ICF – Distorting cells – Changing solute concentrations around enzymes – Disrupting normal cell functions 34 © 2018 Pearson Education, Inc.

27 -3 Fluid Balance § Causes of hyperhydration – Ingestion of a large volume of water – Injection of hypotonic solution into bloodstream – Inability to eliminate excess water in urine • Chronic renal failure • Heart failure • Cirrhosis – Endocrine disorders • Excessive ADH production 35 © 2018 Pearson Education, Inc.

27 -3 Fluid Balance § Signs of hyperhydration – Abnormally low Na+ concentrations (hyponatremia) – Effects on CNS function (water intoxication) 36 © 2018 Pearson Education, Inc.

27 -4 Electrolyte Balance § Electrolyte balance – When gains and losses are equal for each electrolyte in body • Total electrolyte concentrations directly affect water balance • Concentrations of individual electrolytes affect cell functions 37 © 2018 Pearson Education, Inc.

27 -4 Electrolyte Balance § Sodium – Dominant cation in ECF – Sodium salts provide 90 percent of ECF osmotic concentration • Sodium chloride (Na. Cl) • Sodium bicarbonate (Na. HCO 3) 38 © 2018 Pearson Education, Inc.

27 -4 Electrolyte Balance § General rules of electrolyte balance – Most common problems with electrolyte balance are caused by imbalance between gains and losses of Na+ – Problems with K+ balance are less common but more dangerous than Na+ imbalance 39 © 2018 Pearson Education, Inc.

27 -4 Electrolyte Balance § Sodium balance – Total amount of Na+ in ECF represents a balance between two factors • Na+ uptake across digestive epithelium • Na+ excretion in urine and perspiration 40 © 2018 Pearson Education, Inc.

27 -4 Electrolyte Balance § Sodium balance – Typical Na+ gains and losses • 48– 144 m. Eq (1. 1– 3. 3 g) per day – If gains exceed losses • Total Na+ content of ECF rises – If losses exceed gains • Na+ content of ECF declines 41 © 2018 Pearson Education, Inc.

27 -4 Electrolyte Balance § Sodium balance and ECF volume – Na+ regulatory mechanism changes ECF volume • Keeps Na+ concentration fairly stable – When Na+ gains exceed losses • Plasma Na+ concentration increases temporarily • Fluid exits ICF in fluid shift, increasing ECF volume • Normal concentration of Na+ is maintained – When Na+ losses exceed gains • ECF volume and osmotic concentration decrease • Water loss at kidneys regains osmotic balance 42 © 2018 Pearson Education, Inc.

27 -4 Electrolyte Balance § Large changes in ECF volume – Corrected by homeostatic mechanisms that regulate blood volume and pressure • If ECF volume rises, blood volume goes up • If ECF volume drops, blood volume goes down • A rise in blood volume elevates blood pressure • A drop in blood volume lowers blood pressure – ECF volume can be monitored indirectly as baroreceptors monitor blood pressure 43 © 2018 Pearson Education, Inc.

27 -4 Electrolyte Balance § Disorders of sodium balance – Hyponatremia • Body water content rises (hyperhydration) • Na+ concentration of ECF <135 m. Eq/L – Hypernatremia • Body water content declines (dehydration) • Na+ concentration of ECF >145 m. Eq/L 44 © 2018 Pearson Education, Inc.

27 -4 Electrolyte Balance § If ECF volume is inadequate – Blood volume and blood pressure decline – Renin-angiotensin-aldosterone system is activated – Water and Na+ losses are reduced – Water and Na+ gains are increased – ECF volume increases 45 © 2018 Pearson Education, Inc.

27 -4 Electrolyte Balance § If plasma volume is too large – Venous return increases • Stimulating release of natriuretic peptides – Reduce thirst – Block secretion of ADH and aldosterone – Salt and water loss at kidneys increases – ECF volume decreases 46 © 2018 Pearson Education, Inc.

27 -4 Electrolyte Balance § Potassium balance – 98 percent of potassium in body is in ICF – Cells expend energy to recover K+ that diffused from cytoplasm into ECF – Concentration of K+ in ECF is a balance between • Rate of gain across digestive epithelium • Rate of loss in urine 47 © 2018 Pearson Education, Inc.

27 -4 Electrolyte Balance § Potassium losses in urine – Regulated by activities of ion pumps • Along distal convoluted tubule and collecting system • Na+ from tubular fluid are exchanged for K+ (typically) in peritubular fluid – Limited to amount gained by absorption across digestive epithelium • About 50– 150 m. Eq (1. 9– 5. 8 g) per day 48 © 2018 Pearson Education, Inc.

27 -4 Electrolyte Balance § Factors affecting rate of K+ secretion into urine – Changes in K+ concentration of ECF – Changes in p. H – Aldosterone levels 49 © 2018 Pearson Education, Inc.

27 -4 Electrolyte Balance § Changes in K+ concentration of ECF – Higher K+ concentration in ECF leads to higher rate of secretion § Changes in p. H – Low p. H of ECF lowers p. H of peritubular fluid – H+ rather than K+ are exchanged for Na+ in tubular fluid – Rate of K+ secretion declines 50 © 2018 Pearson Education, Inc.

27 -4 Electrolyte Balance § Aldosterone levels – Affect K+ loss in urine – Ion pumps sensitive to aldosterone reabsorb Na+ from tubular fluid in exchange for K+ – High plasma K+ concentration also stimulates aldosterone secretion directly 51 © 2018 Pearson Education, Inc.

27 -4 Electrolyte Balance § Disorders of K+ imbalance – Hypokalemia • Deficiency of K+ in bloodstream – Hyperkalemia • Elevated level of K+ in bloodstream 52 © 2018 Pearson Education, Inc.

Figure 27– 7 Major Factors Involved in Disturbances of Potassium Ion Balance. When the blood concentration of K+ falls below 2 m. Eq/L, extensive muscular weakness develops, followed by eventual paralysis. Severe hypokalemia is potentially lethal due to its effects on the heart. Factors Promoting Hypokalemia Several diuretics can produce hypokalemia by increasing the volume of urine produced. The endocrine disorder called aldosteronism, characterized by excessive aldosterone secretion, results in hypokalemia by overstimulating Na+ retention and K+ loss. Normal potassium range in blood: (3. 5– 5. 0 m. Eq/L) High K+ concentration in the blood produces an equally dangerous condition known as hyperkalemia. Severe hyperkalemia occurs when the K+ concentration exceeds 7 m. Eq/L, which results in cardiac arrhythmias. Factors Promoting Hyperkalemia Chronically low blood p. H promotes hyperkalemia by interfering with K+ excretion by the kidneys. Kidney failure due to damage or disease will prevent normal K+ secretion and thereby produce hyperkalemia. Several drugs promote diuresis by blocking Na+ reabsorption by the kidneys. When Na+ reabsorption slows down, so does K+ secretion, and hyperkalemia can result. 53

27 -4 Electrolyte Balance § Calcium balance – Calcium is most abundant mineral in body – A typical person has 1– 2 kg • 99 percent of which is deposited in skeleton – Calcium ions (Ca 2+) function • In muscular and neural activities • In blood clotting • As cofactors for enzymatic reactions • As second messengers 54 © 2018 Pearson Education, Inc.

27 -4 Electrolyte Balance § Hormones and calcium homeostasis – Parathyroid hormone (PTH) and calcitriol • Raise Ca 2+ concentrations in ECF – Calcitonin • Opposes PTH and calcitriol 55 © 2018 Pearson Education, Inc.

27 -4 Electrolyte Balance § Ca 2+ gains – Absorption at digestive tract – Reabsorption in kidneys – Both stimulated by PTH and calcitriol § Ca 2+ losses – In bile, urine, or feces – Very small (0. 8– 1. 2 g/day) • Represents about 0. 03 percent of calcium reserve in skeleton 56 © 2018 Pearson Education, Inc.

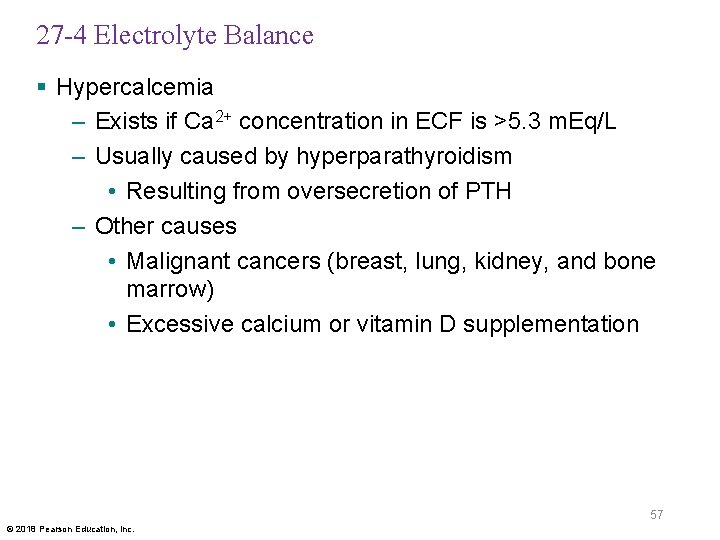
27 -4 Electrolyte Balance § Hypercalcemia – Exists if Ca 2+ concentration in ECF is >5. 3 m. Eq/L – Usually caused by hyperparathyroidism • Resulting from oversecretion of PTH – Other causes • Malignant cancers (breast, lung, kidney, and bone marrow) • Excessive calcium or vitamin D supplementation 57 © 2018 Pearson Education, Inc.

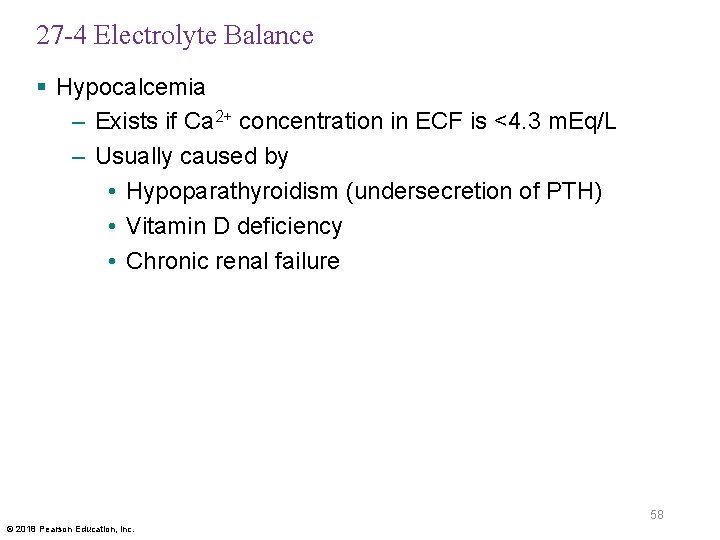
27 -4 Electrolyte Balance § Hypocalcemia – Exists if Ca 2+ concentration in ECF is <4. 3 m. Eq/L – Usually caused by • Hypoparathyroidism (undersecretion of PTH) • Vitamin D deficiency • Chronic renal failure 58 © 2018 Pearson Education, Inc.

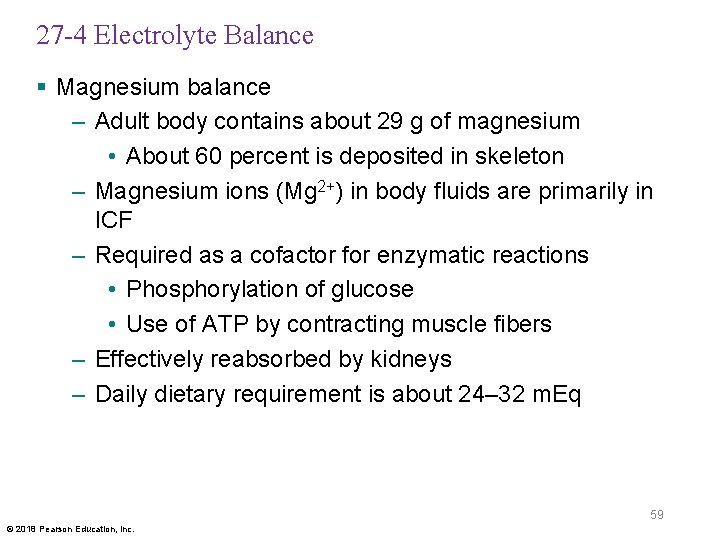
27 -4 Electrolyte Balance § Magnesium balance – Adult body contains about 29 g of magnesium • About 60 percent is deposited in skeleton – Magnesium ions (Mg 2+) in body fluids are primarily in ICF – Required as a cofactor for enzymatic reactions • Phosphorylation of glucose • Use of ATP by contracting muscle fibers – Effectively reabsorbed by kidneys – Daily dietary requirement is about 24– 32 m. Eq 59 © 2018 Pearson Education, Inc.

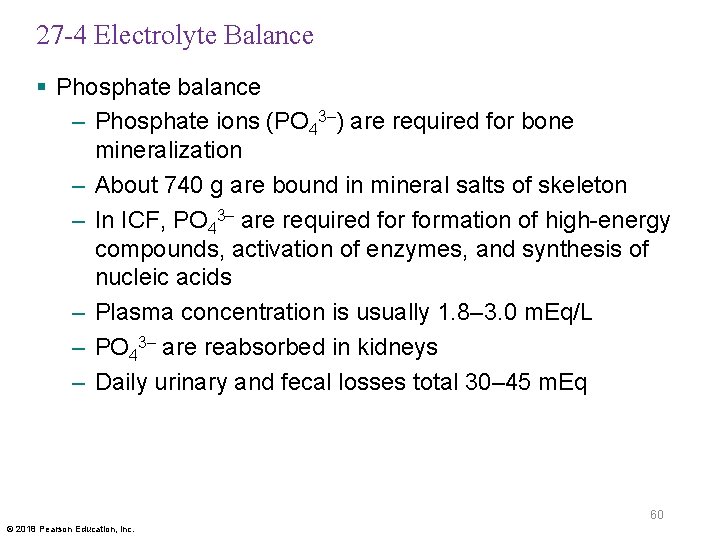
27 -4 Electrolyte Balance § Phosphate balance – Phosphate ions (PO 43–) are required for bone mineralization – About 740 g are bound in mineral salts of skeleton – In ICF, PO 43– are required formation of high-energy compounds, activation of enzymes, and synthesis of nucleic acids – Plasma concentration is usually 1. 8– 3. 0 m. Eq/L – PO 43– are reabsorbed in kidneys – Daily urinary and fecal losses total 30– 45 m. Eq 60 © 2018 Pearson Education, Inc.

27 -4 Electrolyte Balance § Chloride balance – Chloride ions are most abundant anions in ECF – Plasma concentration is 100– 108 m. Eq/L – ICF concentrations are usually low (3 m. Eq/L) – Cl–are absorbed across digestive tract with Na+ • And reabsorbed with Na+ by carrier proteins along renal tubules – Daily loss is only 48– 146 m. Eq (1. 7– 5. 1 g) 61 © 2018 Pearson Education, Inc.

27 -5 Acid-Base Balance § p. H – A measure of acidity or alkalinity of a solution – p. H of body fluids depends on dissolved • Acids • Bases • Salts – p. H of body fluids is altered by addition of acids or bases – p. H of ECF • Narrowly limited, usually 7. 35– 7. 45 62 © 2018 Pearson Education, Inc.

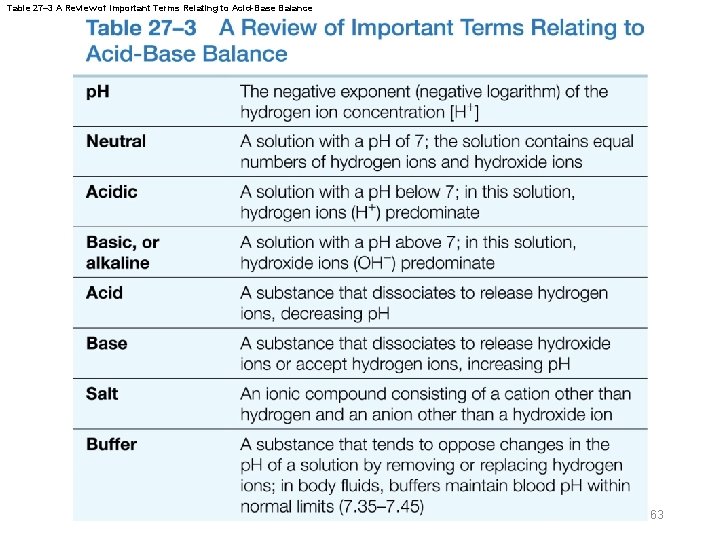
Table 27– 3 A Review of Important Terms Relating to Acid-Base Balance 63

27 -5 Acid-Base Balance § Acidosis – Physiological state resulting from abnormally low blood p. H – Acidemia is when blood p. H <7. 35 § Alkalosis – Physiological state resulting from abnormally high blood p. H – Alkalemia is when blood p. H >7. 45 64 © 2018 Pearson Education, Inc.

27 -5 Acid-Base Balance § Acidosis and alkalosis – Affect virtually all body systems • Particularly nervous and cardiovascular systems – Both are dangerous • But acidosis is more common • Because normal cellular activities generate acids § Acid-base balance – Control of p. H – Homeostatic process of great physiological and clinical significance 65 © 2018 Pearson Education, Inc.

27 -5 Acid-Base Balance § Types of acids in body – Fixed acids – Metabolic acids – Volatile acids 66 © 2018 Pearson Education, Inc.

27 -5 Acid-Base Balance § Fixed acids – Acids that do not leave solution – Once produced they remain in body fluids • Until eliminated by kidneys – Sulfuric acid and phosphoric acid • Most important fixed acids in body • Generated during catabolism of – Amino acids – Phospholipids – Nucleic acids 67 © 2018 Pearson Education, Inc.

27 -5 Acid-Base Balance § Metabolic acids – Participants in, or by-products of, cellular metabolism • Pyruvic acid • Lactic acid • Ketone bodies – Metabolized rapidly under normal conditions • Significant accumulations do not occur 68 © 2018 Pearson Education, Inc.

27 -5 Acid-Base Balance § Volatile acids – Can leave body by entering atmosphere at lungs – Carbonic acid (H 2 CO 3) breaks down into carbon dioxide and water • CO 2 diffuses from bloodstream into alveoli 69 © 2018 Pearson Education, Inc.

27 -5 Acid-Base Balance § Carbonic anhydrase – Enzyme that catalyzes formation of carbonic acid from carbon dioxide and water – Found in • Cytoplasm of red blood cells • Liver and kidney cells • Parietal cells of stomach • Many other cells 70 © 2018 Pearson Education, Inc.

27 -5 Acid-Base Balance § CO 2 and p. H – Most CO 2 in solution converts to carbonic acid – Most carbonic acid dissociates into • Hydrogen ions (H+) • Bicarbonate ions (HCO 3–) – Partial pressure of CO 2 (PCO 2) • The most important factor affecting blood p. H 71 © 2018 Pearson Education, Inc.

27 -5 Acid-Base Balance § PCO and p. H are inversely related 2 – When CO 2 levels rise • H+ and HCO 3– are released • p. H decreases – At alveoli • CO 2 diffuses into atmosphere • H+ and HCO 3– in alveolar capillaries decrease • Blood p. H rises 72 © 2018 Pearson Education, Inc.

27 -5 Acid-Base Balance § Mechanisms of p. H control – To maintain homeostasis, the body balances • Gains and losses of hydrogen ions 73 © 2018 Pearson Education, Inc.

27 -5 Acid-Base Balance § Hydrogen ions (H+) – Gained • At digestive tract • Through cellular metabolic activities – Eliminated • At kidneys • At lungs – Must be neutralized to avoid tissue damage – Acids produced in normal metabolic activity • Temporarily neutralized by buffers in body fluids 74 © 2018 Pearson Education, Inc.

27 -5 Acid-Base Balance § Acids and bases may be strong or weak – Strong acids and strong bases • Dissociate completely in solution • Example: hydrochloric acid (HCl) – Weak acids or weak bases • Do not dissociate completely in solution • Some molecules remain intact • Weak acid releases fewer H+ than does a strong acid, and thus has less effect on p. H of solution 75 © 2018 Pearson Education, Inc.

27 -5 Acid-Base Balance § Carbonic acid – Weak acid – In ECF at normal p. H • Equilibrium state exists: H 2 CO 3 ↔ H+ + HCO 3– 76 © 2018 Pearson Education, Inc.

27 -5 Acid-Base Balance § Buffers – Dissolved compounds that stabilize p. H of solution • By adding or removing H+ – Weak acids • Can donate H+ – Weak bases • Can absorb H+ 77 © 2018 Pearson Education, Inc.

27 -5 Acid-Base Balance § Buffer system – Consists of a combination of • A weak acid • An anion released by its dissociation – Anion functions as a weak base – In solution, molecules of the weak acid exist in equilibrium with its dissociation products 78 © 2018 Pearson Education, Inc.

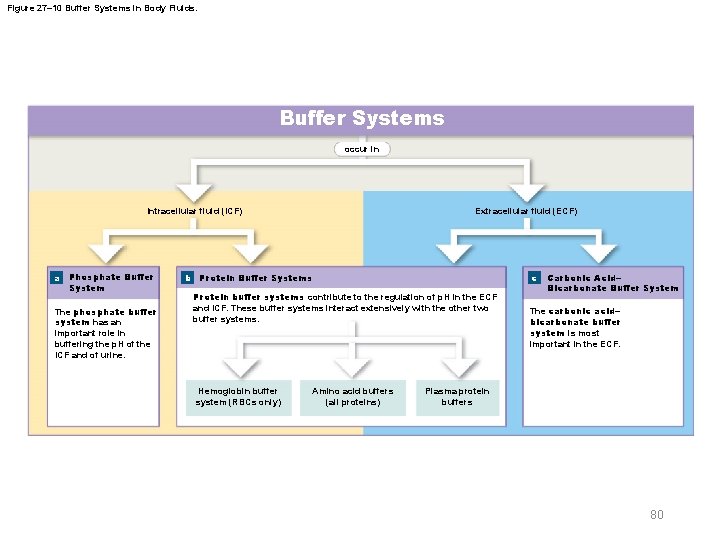
27 -5 Acid-Base Balance § Three major buffer systems – Phosphate buffer system – Protein buffer systems – Carbonic acid–bicarbonate buffer system 79 © 2018 Pearson Education, Inc.

Figure 27– 10 Buffer Systems in Body Fluids. Buffer Systems occur in Intracellular fluid (ICF) a Phosphate Buffer System The phosphate buffer system has an important role in buffering the p. H of the ICF and of urine. Extracellular fluid (ECF) b Protein Buffer Systems c Protein buffer systems contribute to the regulation of p. H in the ECF and ICF. These buffer systems interact extensively with the other two buffer systems. Hemoglobin buffer system (RBCs only) Amino acid buffers (all proteins) Carbonic Acid– Bicarbonate Buffer System The carbonic acid– bicarbonate buffer system is most important in the ECF. Plasma protein buffers 80

27 -5 Acid-Base Balance § Phosphate buffer system – Consists of H 2 PO 4– (a weak acid) and its anion (HPO 42–) – Cells contain a weak base (Na 2 HPO 4) • Provides additional HPO 42– for this buffer system – Important in buffering p. H of ICF and urine 81 © 2018 Pearson Education, Inc.

27 -5 Acid-Base Balance § Protein buffer systems – Rely on amino acids • Respond to p. H changes by accepting or releasing H+ – Important in ECF and ICF – If p. H rises • Carboxyl group of amino acid dissociates • Releasing a hydrogen ion, acting as weak acid • Carboxyl group becomes carboxylate ion 82 © 2018 Pearson Education, Inc.

27 -5 Acid-Base Balance § Protein buffer systems – At normal p. H (7. 35– 7. 45) • Carboxyl groups of most amino acids have already given up their H+ – If p. H decreases • Carboxylate ion and amino group act as weak bases • Accepting H+ • Forming carboxyl group and amino ion 83 © 2018 Pearson Education, Inc.

27 -5 Acid-Base Balance § Protein buffer systems – Carboxyl and amino groups in peptide bonds cannot function as buffers – Most buffering capacity of proteins is provided by R groups of amino acids 84 © 2018 Pearson Education, Inc.

27 -5 Acid-Base Balance § Hemoglobin buffer system – CO 2 in plasma rapidly diffuses into red blood cells • And is converted to carbonic acid – As carbonic acid dissociates • Bicarbonate ions diffuse into plasma • In exchange for chloride ions (chloride shift) • Hydrogen ions are buffered by hemoglobin molecules 85 © 2018 Pearson Education, Inc.

27 -5 Acid-Base Balance § Hemoglobin buffer system – The only intracellular buffer system with an immediate effect on p. H of ECF – Helps prevent major changes in p. H when plasma PCO 2 is rising or falling 86 © 2018 Pearson Education, Inc.

27 -5 Acid-Base Balance § Carbonic acid–bicarbonate buffer system – Carbon dioxide • Constantly generated by body cells • Most is converted to carbonic acid – This buffer system involves carbonic acid and its dissociation products • H+ and bicarbonate ions – Prevents changes in p. H caused by metabolic acids and fixed acids in ECF 87 © 2018 Pearson Education, Inc.

27 -5 Acid-Base Balance § Limitations of carbonic acid–bicarbonate buffer system – Cannot protect ECF from changes in p. H that result from increased or decreased levels of CO 2 – Functions only when respiratory system and respiratory control centers are working normally – Ability to buffer acids is limited by availability of bicarbonate ions 88 © 2018 Pearson Education, Inc.

27 -5 Acid-Base Balance § Bicarbonate ion shortage is rare because – Body fluids contain large reserve of sodium bicarbonate • Called bicarbonate reserve – Additional HCO 3– can be generated by kidneys 89 © 2018 Pearson Education, Inc.

27 -5 Acid-Base Balance § Limitations of buffer systems – Provide only temporary solution to acid-base imbalance – Do not eliminate H+ – Supply of buffer molecules is limited 90 © 2018 Pearson Education, Inc.

27 -5 Acid-Base Balance § Regulation of acid-base balance – To preserve homeostasis, captured H+ must be • Permanently tied up in water molecules through CO 2 removal at lungs • Removed from body fluids by secretion at kidneys – Requires balancing H+ gains and losses – To maintain body p. H within narrow limits, buffer systems coordinate with • Respiratory mechanisms • Renal mechanisms 91 © 2018 Pearson Education, Inc.

27 -5 Acid-Base Balance § Respiratory and renal mechanisms – Support buffer systems by • Secreting or absorbing H+ • Controlling excretion of acids and bases • Generating additional buffers 92 © 2018 Pearson Education, Inc.

27 -5 Acid-Base Balance § Respiratory compensation – Change in respiratory rate • Helps stabilize p. H of ECF – Occurs whenever body p. H moves outside normal limits – Directly affects carbonic acid–bicarbonate buffer system 93 © 2018 Pearson Education, Inc.

27 -5 Acid-Base Balance § Respiratory compensation – Increasing or decreasing rate of respiration alters p. H by lowering or raising PCO 2 – When PCO rises 2 • p. H falls • Addition of CO 2 drives buffer system to right – When PCO falls 2 • p. H rises • Removal of CO 2 drives buffer system to left 94 © 2018 Pearson Education, Inc.

27 -5 Acid-Base Balance § Renal compensation – Change in rates of H+ and HCO 3– secretion or reabsorption by kidneys • In response to changes in plasma p. H – Body normally generates enough metabolic and fixed acids each day to add 100 m. Eq of H+ to ECF – H+ are excreted in urine – Kidneys assist lungs by eliminating any CO 2 that • Enters renal tubules during filtration • Diffuses into tubular fluid en route to renal pelvis 95 © 2018 Pearson Education, Inc.

27 -5 Acid-Base Balance § Hydrogen ions – Secreted into tubular fluid along • Proximal convoluted tubule (PCT) • Distal convoluted tubule (DCT) • Collecting system 96 © 2018 Pearson Education, Inc.

27 -5 Acid-Base Balance § Buffers in urine – Required to eliminate large numbers of H+ – Glomerular filtration provides components of • Carbonic acid–bicarbonate buffer system • Phosphate buffer system – Tubule cells of PCT • Generate ammonia • Ammonia buffer system 97 © 2018 Pearson Education, Inc.

27 -5 Acid-Base Balance § Renal responses to acidosis – Secretion of H+ – Activity of buffers in tubular fluid – Removal of CO 2 – Reabsorption of Na. HCO 3 98 © 2018 Pearson Education, Inc.

27 -5 Acid-Base Balance § Renal responses to alkalosis – Rate of H+ secretion at kidneys declines – Tubule cells do not reclaim bicarbonate ions in tubular fluid – Collecting system transports HCO 3– into tubular fluid while releasing H+ and Cl– into peritubular fluid 99 © 2018 Pearson Education, Inc.

27 -6 Disorders of Acid-Base Balance § Serious and prolonged disturbances of acid-base balance can result in – Disorders affecting circulating buffers, respiratory performance, or renal function – Cardiovascular conditions such as heart failure or hypotension – Conditions affecting CNS • Neural damage or disease can affect reflexes essential to p. H regulation 100 © 2018 Pearson Education, Inc.

27 -6 Disorders of Acid-Base Balance § Serious abnormalities in acid-base balance – Acute phase • Initial phase • p. H moves rapidly out of normal range – Compensated phase • When condition persists • Physiological adjustments occur 101 © 2018 Pearson Education, Inc.

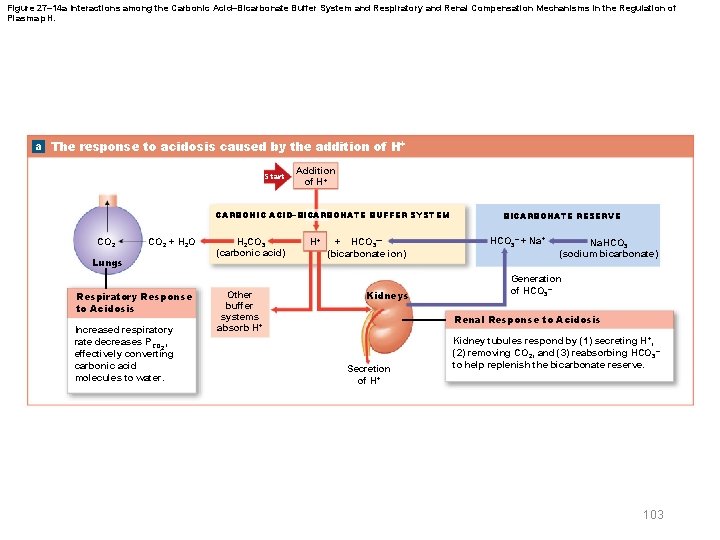
27 -6 Disorders of Acid-Base Balance § Respiratory acid-base disorders – Result from imbalance between • CO 2 generation in peripheral tissues • CO 2 excretion at lungs – Cause abnormal CO 2 levels in ECF § Metabolic acid-base disorders – Result from • Generation of metabolic or fixed acids • Conditions affecting HCO 3– concentration in ECF 102 © 2018 Pearson Education, Inc.

Figure 27– 14 a Interactions among the Carbonic Acid–Bicarbonate Buffer System and Respiratory and Renal Compensation Mechanisms in the Regulation of Plasma p. H. a The response to acidosis caused by the addition of H+ Start Addition of H+ CARBONIC ACID–BICARBONATE BUFFER SYSTEM CO 2 + H 2 O Lungs Respiratory Response to Acidosis Increased respiratory rate decreases PCO 2, effectively converting carbonic acid molecules to water. H 2 CO 3 (carbonic acid) Other buffer systems absorb H+ H+ + HCO 3— (bicarbonate ion) Kidneys BICARBONATE RESERVE HCO 3– + Na. HCO 3 (sodium bicarbonate) Generation of HCO 3– Renal Response to Acidosis Secretion of H+ Kidney tubules respond by (1) secreting H+, (2) removing CO 2, and (3) reabsorbing HCO 3– to help replenish the bicarbonate reserve. 103

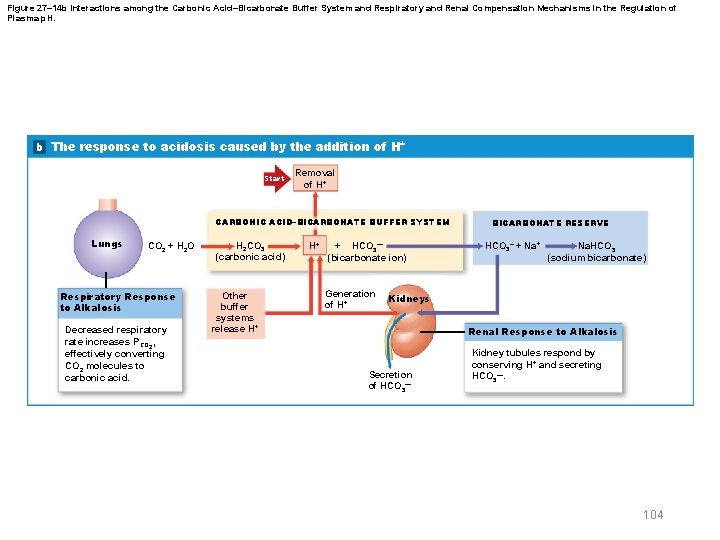
Figure 27– 14 b Interactions among the Carbonic Acid–Bicarbonate Buffer System and Respiratory and Renal Compensation Mechanisms in the Regulation of Plasma p. H. b The response to acidosis caused by the addition of H+ Start Removal of H+ CARBONIC ACID–BICARBONATE BUFFER SYSTEM Lungs CO 2 + H 2 O Respiratory Response to Alkalosis Decreased respiratory rate increases PCO 2, effectively converting CO 2 molecules to carbonic acid. H 2 CO 3 (carbonic acid) Other buffer systems release H+ H+ + HCO 3— (bicarbonate ion) Generation of H+ BICARBONATE RESERVE HCO 3– + Na. HCO 3 (sodium bicarbonate) Kidneys Renal Response to Alkalosis Secretion of HCO 3— Kidney tubules respond by conserving H+ and secreting HCO 3—. 104

27 -6 Disorders of Acid-Base Balance § Respiratory acidosis – Develops when respiratory system cannot eliminate all CO 2 generated by peripheral tissues – Primary sign • Low blood p. H due to hypercapnia (high blood PCO 2) – Primary cause • Hypoventilation (abnormally low respiratory rate) 105 © 2018 Pearson Education, Inc.

27 -6 Disorders of Acid-Base Balance § Respiratory acidosis – Acute respiratory acidosis • Develops if decline in p. H is severe • Immediate, life-threatening condition – Chronic respiratory acidosis • Develops when normal respiratory function has been compromised • Compensatory mechanisms have not failed completely 106 © 2018 Pearson Education, Inc.

27 -6 Disorders of Acid-Base Balance § Respiratory alkalosis – Primary sign • High blood p. H due to hypocapnia (low blood PCO ) 2 – Primary cause • Hyperventilation (abnormally high respiratory rate) 107 © 2018 Pearson Education, Inc.

27 -6 Disorders of Acid-Base Balance § Metabolic acidosis – Three major causes • High production of fixed or metabolic acids – H+ overload buffer system – Lactic acidosis • After strenuous exercise or hypoxia – Ketoacidosis • In starvation and diabetes mellitus • Impaired H+ excretion at kidneys • Severe bicarbonate ion loss 108 © 2018 Pearson Education, Inc.

27 -6 Disorders of Acid-Base Balance § Metabolic alkalosis – Caused by elevated HCO 3– concentrations – Bicarbonate ions interact with H+ in solution • Forming H 2 CO 3 – Decreased H+ concentration causes alkalosis 109 © 2018 Pearson Education, Inc.

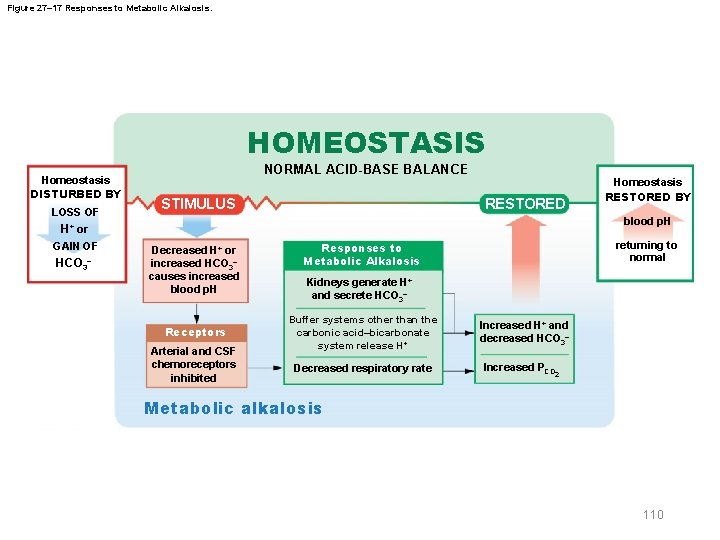
Figure 27– 17 Responses to Metabolic Alkalosis. HOMEOSTASIS Homeostasis DISTURBED BY LOSS OF NORMAL ACID-BASE BALANCE RESTORED STIMULUS blood p. H H+ or GAIN OF HCO 3– Homeostasis RESTORED BY Decreased H+ or increased HCO 3– causes increased blood p. H Receptors Arterial and CSF chemoreceptors inhibited returning to normal Responses to Metabolic Alkalosis Kidneys generate H+ and secrete HCO 3– Buffer systems other than the carbonic acid–bicarbonate system release H + Decreased respiratory rate Increased H+ and decreased HCO 3– Increased PCO 2 Metabolic alkalosis 110

27 -6 Disorders of Acid-Base Balance § Respiratory and metabolic acidosis – Typically linked • Low O 2 results in generation of lactic acid • Hypoventilation leads to low arterial PO 2 § Detection of acidosis and alkalosis – Includes measuring p. H, PCO , PO , and HCO 3– levels in 2 2 blood – After diagnosis of acidosis or alkalosis, • Condition is classified as respiratory or metabolic 111 © 2018 Pearson Education, Inc.

27 -7 Effects of Aging § Fluid, electrolyte, and acid-base balance – Fetal p. H control • Buffers in fetal bloodstream provide short-term p. H control • Maternal kidneys eliminate H+ – Newborn • Body water content is high (75 percent of body weight) • Basic aspects of electrolyte balance are the same as in adult 112 © 2018 Pearson Education, Inc.

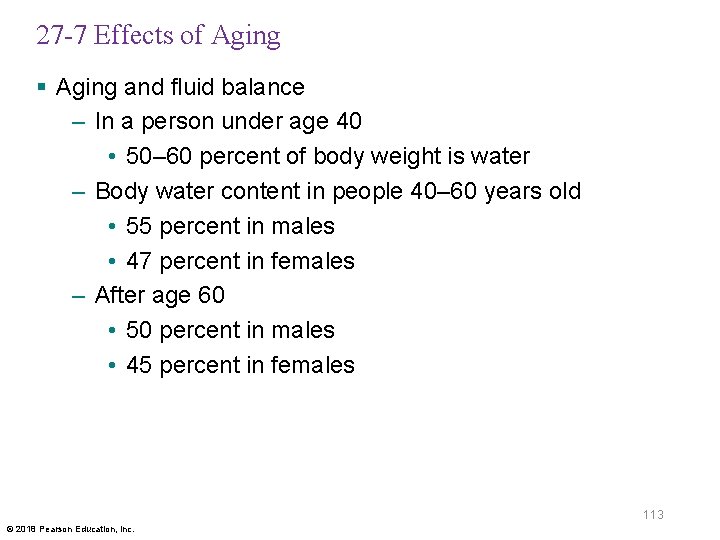
27 -7 Effects of Aging § Aging and fluid balance – In a person under age 40 • 50– 60 percent of body weight is water – Body water content in people 40– 60 years old • 55 percent in males • 47 percent in females – After age 60 • 50 percent in males • 45 percent in females 113 © 2018 Pearson Education, Inc.

27 -7 Effects of Aging § Aging and fluid balance – Decreased body water content reduces dilution of wastes, toxins, and drugs – Reduction in glomerular filtration rate and number of functional nephrons • Reduces p. H regulation by renal compensation – Ability to concentrate urine declines • More water is lost in urine – Insensible perspiration increases as skin becomes thinner 114 © 2018 Pearson Education, Inc.

27 -7 Effects of Aging § Aging and fluid balance – Maintaining fluid balance requires higher daily water intake – Reduction in ADH and aldosterone sensitivity • Reduces ability to conserve body water when losses exceed gains – Muscle mass and skeletal mass decrease • Causing net loss in body mineral content • Loss can be prevented in part by exercise and increased dietary mineral supply 115 © 2018 Pearson Education, Inc.

27 -7 Effects of Aging § Aging and acid-base balance – Reduction in vital capacity • Reduces respiratory compensation • Increases risk of respiratory acidosis • Aggravated by arthritis and emphysema – Disorders affecting major systems become more common with increasing age 116 © 2018 Pearson Education, Inc.
 Management eleventh edition
Management eleventh edition Management 11th edition by stephen p robbins
Management 11th edition by stephen p robbins Management eleventh edition
Management eleventh edition Management eleventh edition
Management eleventh edition Human anatomy and physiology seventh edition marieb
Human anatomy and physiology seventh edition marieb Anatomy and physiology edition 9
Anatomy and physiology edition 9 Human anatomy and physiology 10th edition
Human anatomy and physiology 10th edition Chapter 14 anatomy and physiology
Chapter 14 anatomy and physiology Chapter 1 introduction to human anatomy and physiology
Chapter 1 introduction to human anatomy and physiology Anatomy and physiology chapter 8 special senses
Anatomy and physiology chapter 8 special senses Chapter 13 anatomy and physiology of pregnancy
Chapter 13 anatomy and physiology of pregnancy Chapter 2 basic chemistry anatomy and physiology
Chapter 2 basic chemistry anatomy and physiology Heat and cold
Heat and cold Chapter 14 the digestive system and body metabolism
Chapter 14 the digestive system and body metabolism Chapter 10 blood anatomy and physiology
Chapter 10 blood anatomy and physiology Anatomy and physiology chapter 15
Anatomy and physiology chapter 15 Anatomy and physiology chapter 1
Anatomy and physiology chapter 1 Holes anatomy and physiology chapter 1
Holes anatomy and physiology chapter 1 Gi tract histology
Gi tract histology Distal and proximal
Distal and proximal Chapter 2 human reproductive anatomy and physiology
Chapter 2 human reproductive anatomy and physiology Appendicular skeleton pectoral girdle
Appendicular skeleton pectoral girdle Chapter 6 general anatomy and physiology
Chapter 6 general anatomy and physiology Cranial cephalic
Cranial cephalic Bhore committee
Bhore committee Eleventh 5 year plan
Eleventh 5 year plan 11th five year plan
11th five year plan For his eleventh birthday elvis presley
For his eleventh birthday elvis presley Physiology of sport and exercise 5th edition
Physiology of sport and exercise 5th edition Respiratory physiology
Respiratory physiology Tattoo anatomy and physiology
Tattoo anatomy and physiology International anatomy olympiad
International anatomy olympiad External parts of a leaf
External parts of a leaf Bone anatomy and physiology
Bone anatomy and physiology Anatomy of an ulcer
Anatomy of an ulcer Liver hilus
Liver hilus Hypogastric region
Hypogastric region Hypogastric region
Hypogastric region Google image
Google image Http://anatomy and physiology
Http://anatomy and physiology Appendix physiology
Appendix physiology Aohs foundations of anatomy and physiology 1
Aohs foundations of anatomy and physiology 1 Aohs foundations of anatomy and physiology 1
Aohs foundations of anatomy and physiology 1 Anatomical planes
Anatomical planes Unit 26 agriscience
Unit 26 agriscience Science olympiad anatomy and physiology 2020 cheat sheet
Science olympiad anatomy and physiology 2020 cheat sheet Degluttination
Degluttination Physiology
Physiology Aohs foundations of anatomy and physiology 1
Aohs foundations of anatomy and physiology 1 Aohs foundations of anatomy and physiology 1
Aohs foundations of anatomy and physiology 1 What produces bile
What produces bile Cornell notes for anatomy and physiology
Cornell notes for anatomy and physiology Holes essential of human anatomy and physiology
Holes essential of human anatomy and physiology Anatomy and physiology unit 7 cardiovascular system
Anatomy and physiology unit 7 cardiovascular system Anatomy and physiology
Anatomy and physiology Aohs foundations of anatomy and physiology 1
Aohs foundations of anatomy and physiology 1 Aohs foundations of anatomy and physiology 1
Aohs foundations of anatomy and physiology 1 Animal physiology exam 1
Animal physiology exam 1 Welcome to anatomy and physiology
Welcome to anatomy and physiology Physiology of the foot and ankle
Physiology of the foot and ankle Anatomy and physiology of psoriasis
Anatomy and physiology of psoriasis Physiology vs anatomy
Physiology vs anatomy Pancreas histology slide
Pancreas histology slide Anatomy and physiology vocabulary
Anatomy and physiology vocabulary Anatomy and physiology
Anatomy and physiology Biceps muscle names
Biceps muscle names Anatomy and physiology
Anatomy and physiology Body landmarks diagram
Body landmarks diagram Anatomy and physiology
Anatomy and physiology Anatomy and physiology
Anatomy and physiology Anatomy and physiology
Anatomy and physiology Dorsifelxion
Dorsifelxion Figure 11-8 arteries
Figure 11-8 arteries Anatomy and physiology
Anatomy and physiology Anatomy and physiology
Anatomy and physiology Figure 10-1 blood
Figure 10-1 blood Sheep brain
Sheep brain Anatomy and physiology of the eye
Anatomy and physiology of the eye Principles of anatomy and physiology tortora
Principles of anatomy and physiology tortora Irn.org anatomy and physiology
Irn.org anatomy and physiology Anatomy and physiology body parts
Anatomy and physiology body parts Unit 26 animal anatomy physiology and nutrition
Unit 26 animal anatomy physiology and nutrition The digestive system and body metabolism
The digestive system and body metabolism Anatomy and physiology of the retina
Anatomy and physiology of the retina Anatomy and physiology
Anatomy and physiology Anatomy and physiology of meningitis ppt
Anatomy and physiology of meningitis ppt Jeopardy anatomy and physiology game
Jeopardy anatomy and physiology game Homeostasis definition
Homeostasis definition Anatomy and physiology
Anatomy and physiology Respiratory system of human
Respiratory system of human Physiology trivia
Physiology trivia Fish anatomy and physiology
Fish anatomy and physiology Anatomy of brain
Anatomy of brain Anatomy and physiology
Anatomy and physiology Long axis of body
Long axis of body 2012 pearson education inc anatomy and physiology
2012 pearson education inc anatomy and physiology Unit 5 anatomy and physiology
Unit 5 anatomy and physiology Fish anatomy and physiology
Fish anatomy and physiology Cengage anatomy and physiology
Cengage anatomy and physiology Fundamentals of information systems 9th edition
Fundamentals of information systems 9th edition Fundamentals of information systems 9th edition
Fundamentals of information systems 9th edition Fluid mechanics fundamentals and applications 3rd edition
Fluid mechanics fundamentals and applications 3rd edition Digital fundamentals 10th edition
Digital fundamentals 10th edition Machining fundamentals 10th edition
Machining fundamentals 10th edition Fundamentals of organizational communication
Fundamentals of organizational communication Fundamentals of organizational communication 9th edition
Fundamentals of organizational communication 9th edition Fundamentals of corporate finance 3rd canadian edition
Fundamentals of corporate finance 3rd canadian edition Digital fundamentals by floyd 10th edition
Digital fundamentals by floyd 10th edition Floyd digital fundamentals 10th edition pdf
Floyd digital fundamentals 10th edition pdf Dc/ac fundamentals a systems approach
Dc/ac fundamentals a systems approach Computer security fundamentals 4th edition
Computer security fundamentals 4th edition Management fundamentals 8th edition
Management fundamentals 8th edition Fundamentals of information systems
Fundamentals of information systems Fundamentals of corporate finance third canadian edition
Fundamentals of corporate finance third canadian edition Fundamentals of corporate finance fifth edition
Fundamentals of corporate finance fifth edition Corporate finance 6th edition
Corporate finance 6th edition Fundamentals of abnormal psychology ninth edition
Fundamentals of abnormal psychology ninth edition