Fundamentals of Anatomy Physiology Eleventh Edition Chapter 2









































- Slides: 115

Fundamentals of Anatomy & Physiology Eleventh Edition Chapter 2 The Chemical Level of Organization Lecture Presentation by Deborah A. Hutchinson Seattle University

Learning Outcomes 2 -1 Describe an atom and how atomic structure affects interactions between atoms. 2 -2 Compare the ways in which atoms combine to form molecules and compounds. 2 -3 Distinguish among the major types of chemical reactions that are important for studying physiology. 2 -4 Describe the crucial role of enzymes in metabolism. 2 -5 Distinguish between inorganic compounds and organic compounds. 2 -6 Explain how the chemical properties of water make life possible. 2

Learning Outcomes 2 -7 Explain what p. H is and discuss its importance. 2 -8 Describe the physiological roles of acids, bases, and salts and the role of buffers in body fluids. 2 -9 Describe monomers and polymers, and the importance of functional groups in organic compounds. 2 -10 Discuss the structures and functions of carbohydrates. 2 -11 Discuss the structures and functions of lipids. 2 -12 Discuss the structures and functions of proteins. 2 -13 Discuss the structures and functions of nucleic acids. 2 -14 Discuss the structures and functions of high-energy compounds. 3

An Introduction to the Chemical Level § Chemistry – Science that deals with the structure of matter – Including • Structure of atoms • Basic chemical building blocks • How atoms combine to form increasingly complex structures 4

2 -1 Atoms and Atomic Structure § Matter – Anything that takes up space and has mass – Made up of atoms • Atoms join together to form chemicals with different characteristics • Chemical characteristics determine physiology at molecular and cellular levels 5

2 -1 Atoms and Atomic Structure § Subatomic particles – Protons • Positive charge, 1 mass unit – Neutrons • Neutral, 1 mass unit – Electrons • Negative charge, low mass 6

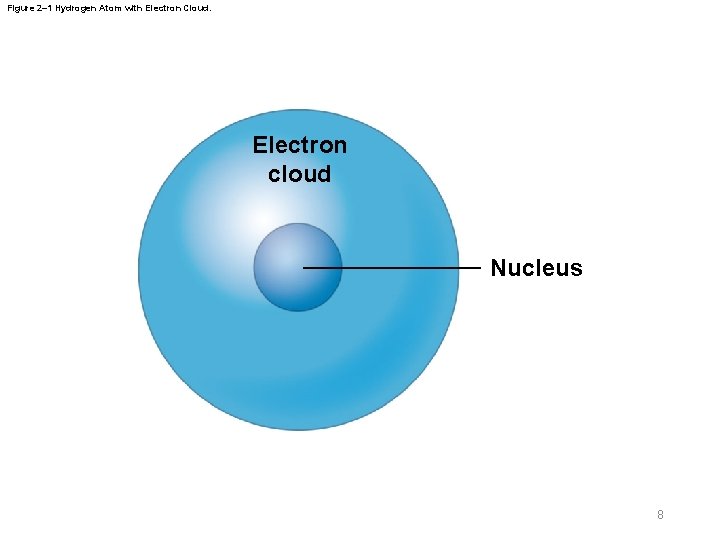
2 -1 Atoms and Atomic Structure § Atomic structure – Atomic number • Number of protons – Nucleus • Contains protons and neutrons – Electron cloud • Spherical area that contains electrons – Electron shell • Two-dimensional representation of electron cloud 7

Figure 2– 1 Hydrogen Atom with Electron Cloud. Electron cloud Nucleus 8

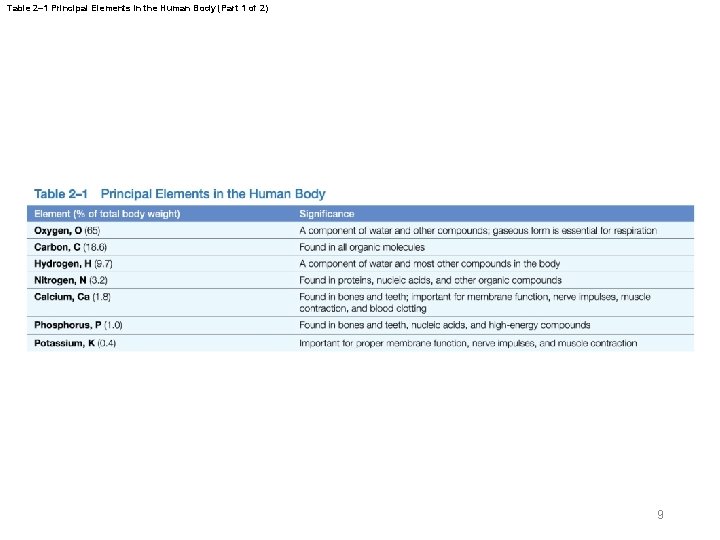
Table 2– 1 Principal Elements in the Human Body (Part 1 of 2) 9

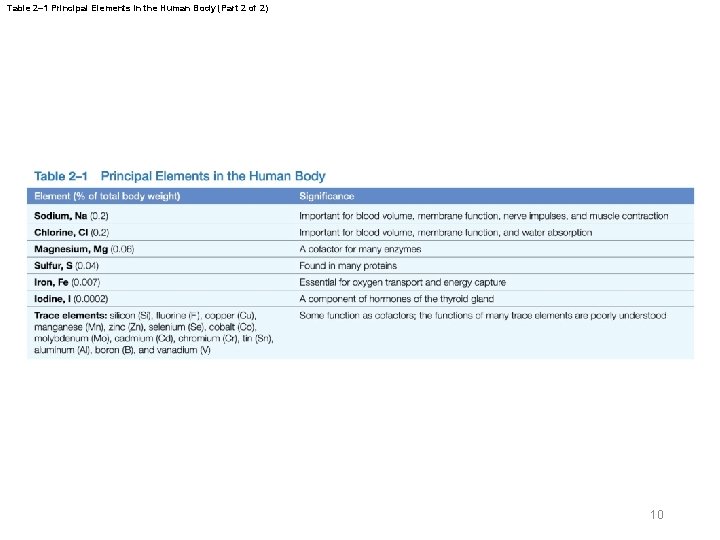
Table 2– 1 Principal Elements in the Human Body (Part 2 of 2) 10

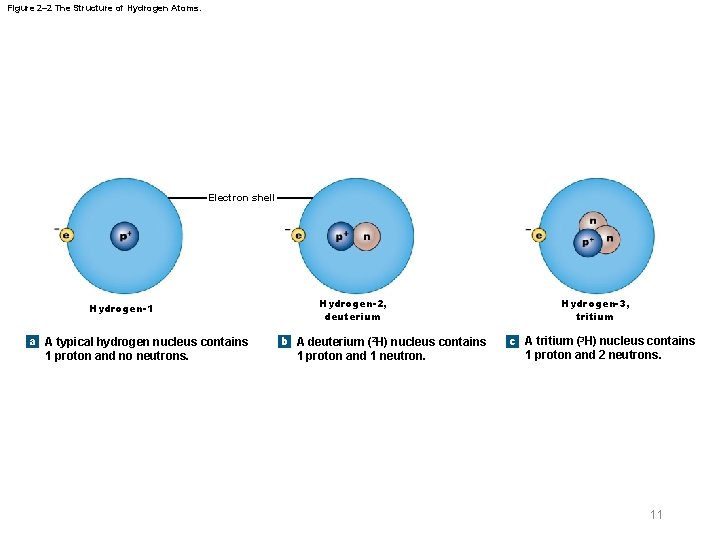
Figure 2– 2 The Structure of Hydrogen Atoms. Electron shell Hydrogen-1 a A typical hydrogen nucleus contains 1 proton and no neutrons. Hydrogen-2, deuterium b A deuterium (2 H) nucleus contains 1 proton and 1 neutron. Hydrogen-3, tritium c A tritium (3 H) nucleus contains 1 proton and 2 neutrons. 11

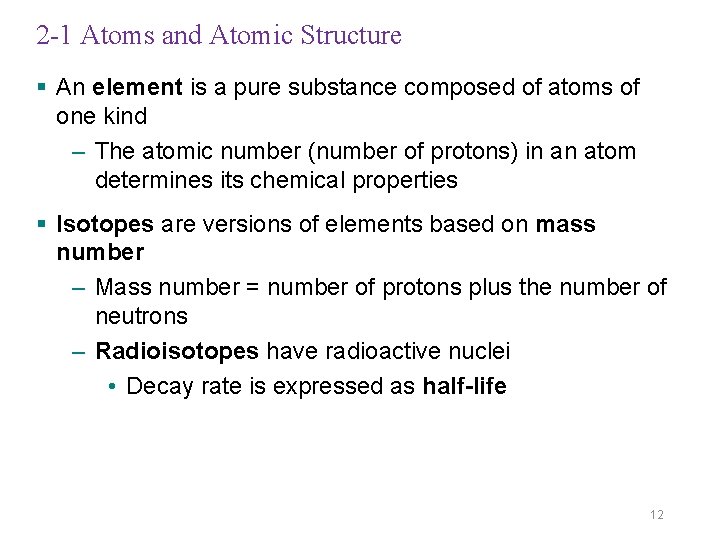
2 -1 Atoms and Atomic Structure § An element is a pure substance composed of atoms of one kind – The atomic number (number of protons) in an atom determines its chemical properties § Isotopes are versions of elements based on mass number – Mass number = number of protons plus the number of neutrons – Radioisotopes have radioactive nuclei • Decay rate is expressed as half-life 12

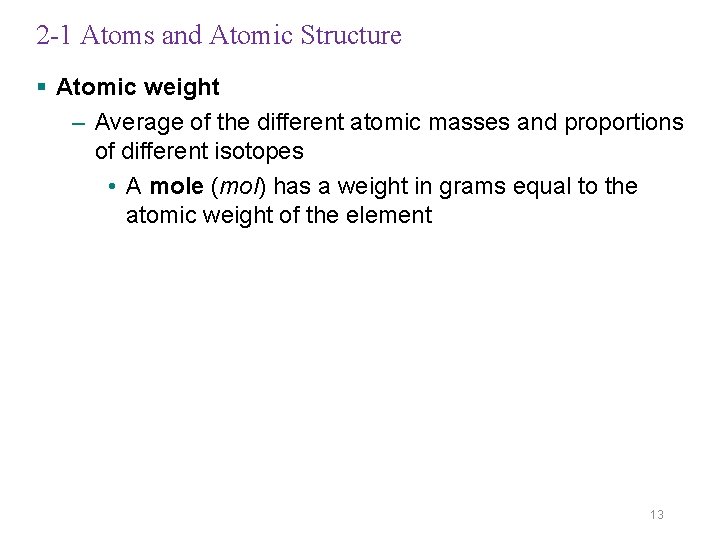
2 -1 Atoms and Atomic Structure § Atomic weight – Average of the different atomic masses and proportions of different isotopes • A mole (mol) has a weight in grams equal to the atomic weight of the element 13

2 -1 Atoms and Atomic Structure § Electrons and energy levels – Electrons in the electron cloud determine the reactivity of an atom – Electron cloud contains shells, or energy levels, that can hold a limited number of electrons • Lower shells fill first • Outermost shell is the valence shell, and it determines bonding 14

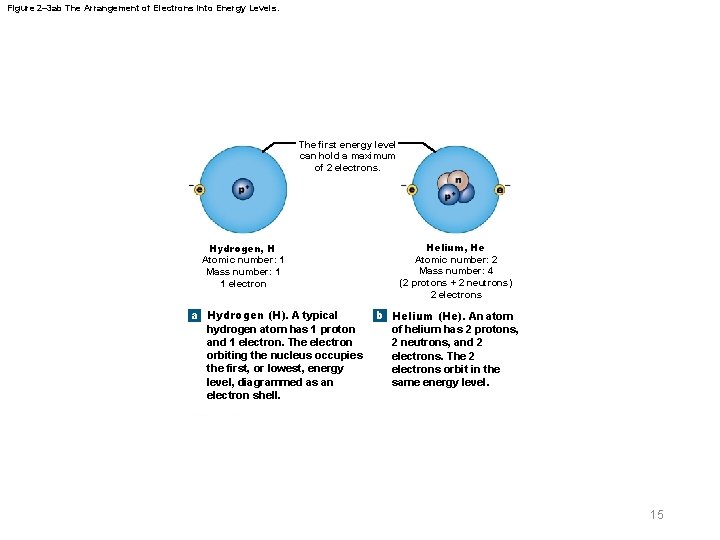
Figure 2– 3 ab The Arrangement of Electrons into Energy Levels. The first energy level can hold a maximum of 2 electrons. Hydrogen, H Atomic number: 1 Mass number: 1 1 electron a Hydrogen (H). A typical hydrogen atom has 1 proton and 1 electron. The electron orbiting the nucleus occupies the first, or lowest, energy level, diagrammed as an electron shell. Helium, He Atomic number: 2 Mass number: 4 (2 protons + 2 neutrons) 2 electrons b Helium (He). An atom of helium has 2 protons, 2 neutrons, and 2 electrons. The 2 electrons orbit in the same energy level. 15

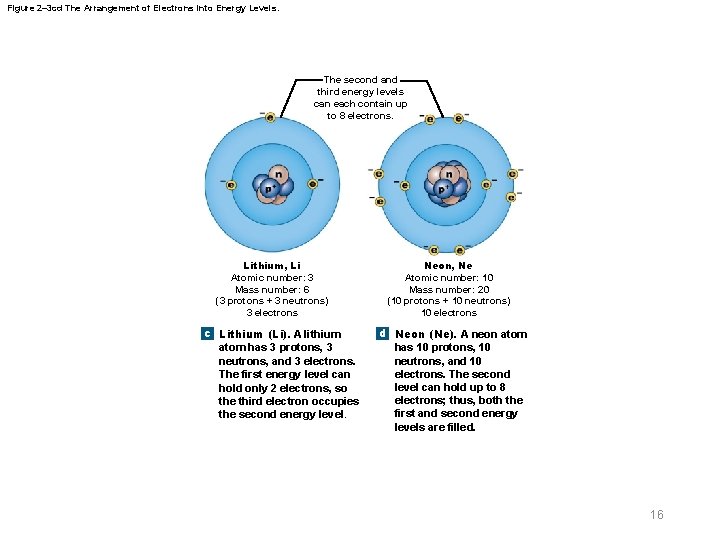
Figure 2– 3 cd The Arrangement of Electrons into Energy Levels. The second and third energy levels can each contain up to 8 electrons. Lithium, Li Atomic number: 3 Mass number: 6 (3 protons + 3 neutrons) 3 electrons c Lithium (Li). A lithium atom has 3 protons, 3 neutrons, and 3 electrons. The first energy level can hold only 2 electrons, so the third electron occupies the second energy level. Neon, Ne Atomic number: 10 Mass number: 20 (10 protons + 10 neutrons) 10 electrons d Neon (Ne). A neon atom has 10 protons, 10 neutrons, and 10 electrons. The second level can hold up to 8 electrons; thus, both the first and second energy levels are filled. 16

2 -2 Molecules and Compounds § Chemical bonds form molecules and compounds – Molecule • Two or more atoms joined by strong bonds – Compound • Two or more atoms of different elements joined by strong or weak bonds – Not all molecules are compounds and not all compounds consist of molecules – Molecular weight of a molecule or compound is the sum of the atomic weights of its atoms 17

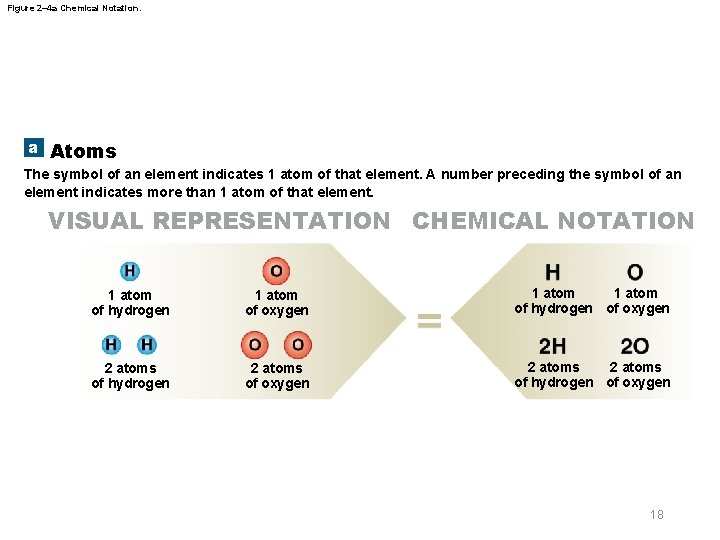
Figure 2– 4 a Chemical Notation. a Atoms The symbol of an element indicates 1 atom of that element. A number preceding the symbol of an element indicates more than 1 atom of that element. VISUAL REPRESENTATION CHEMICAL NOTATION 1 atom of hydrogen 1 atom of oxygen 1 atom of hydrogen of oxygen 2 atoms of hydrogen 2 atoms of oxygen 2 atoms of hydrogen of oxygen 18

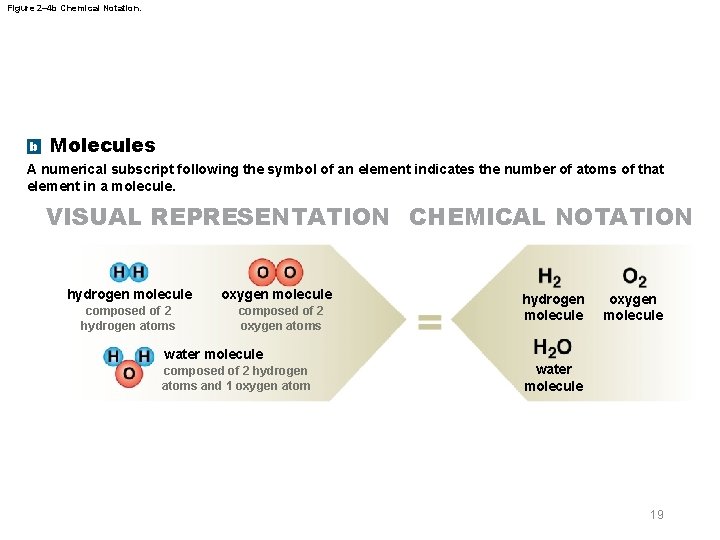
Figure 2– 4 b Chemical Notation. b Molecules A numerical subscript following the symbol of an element indicates the number of atoms of that element in a molecule. VISUAL REPRESENTATION CHEMICAL NOTATION hydrogen molecule composed of 2 hydrogen atoms oxygen molecule composed of 2 oxygen atoms hydrogen molecule oxygen molecule water molecule composed of 2 hydrogen atoms and 1 oxygen atom water molecule 19

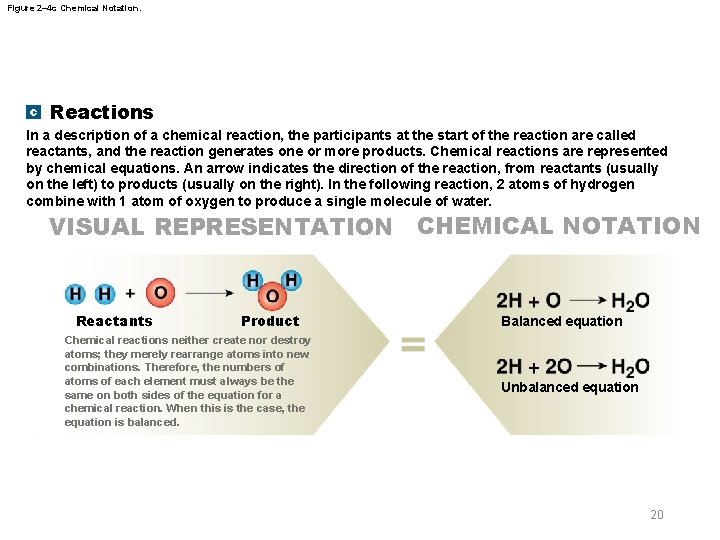
Figure 2– 4 c Chemical Notation. c Reactions In a description of a chemical reaction, the participants at the start of the reaction are called reactants, and the reaction generates one or more products. Chemical reactions are represented by chemical equations. An arrow indicates the direction of the reaction, from reactants (usually on the left) to products (usually on the right). In the following reaction, 2 atoms of hydrogen combine with 1 atom of oxygen to produce a single molecule of water. VISUAL REPRESENTATION CHEMICAL NOTATION Reactants Product Chemical reactions neither create nor destroy atoms; they merely rearrange atoms into new combinations. Therefore, the numbers of atoms of each element must always be the same on both sides of the equation for a chemical reaction. When this is the case, the equation is balanced. Balanced equation Unbalanced equation 20

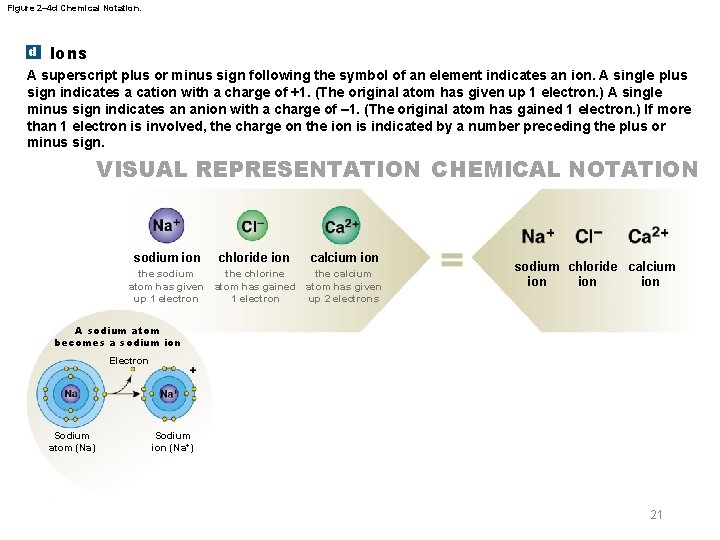
Figure 2– 4 d Chemical Notation. d Ions A superscript plus or minus sign following the symbol of an element indicates an ion. A single plus sign indicates a cation with a charge of +1. (The original atom has given up 1 electron. ) A single minus sign indicates an anion with a charge of – 1. (The original atom has gained 1 electron. ) If more than 1 electron is involved, the charge on the ion is indicated by a number preceding the plus or minus sign. VISUAL REPRESENTATION CHEMICAL NOTATION sodium ion the sodium atom has given up 1 electron chloride ion calcium ion the chlorine the calcium atom has gained atom has given 1 electron up 2 electrons sodium chloride calcium ion ion A sodium atom becomes a sodium ion Electron Sodium atom (Na) Sodium ion (Na+) 21

2 -2 Molecules and Compounds § Chemical bonds – Involve sharing, gaining, and losing electrons – Three major types of chemical bonds 1. Ionic bonds 2. Covalent bonds 3. Hydrogen bonds 22

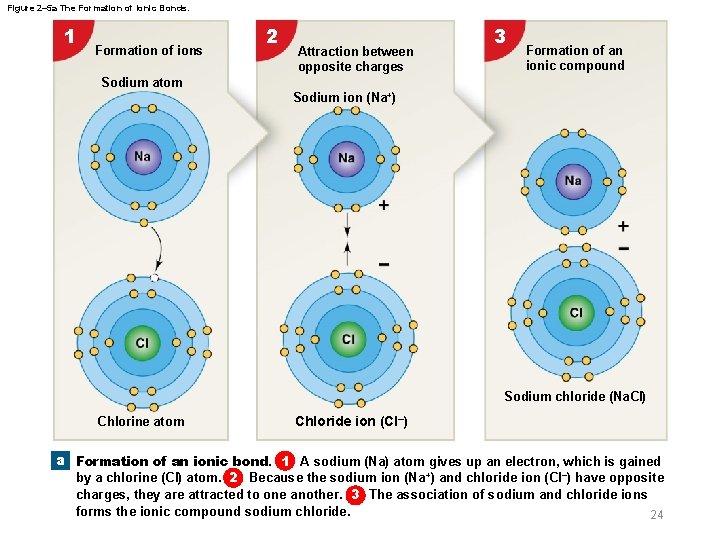
2 -2 Molecules and Compounds § Ionic bonds – An ion is an atom with an electric charge – One atom—the electron donor—loses one or more electrons and becomes a cation – Another atom—the electron acceptor—gains those same electrons and becomes an anion – Ionic bonds are attractions between cations (positive ions) and anions (negative ions) 23

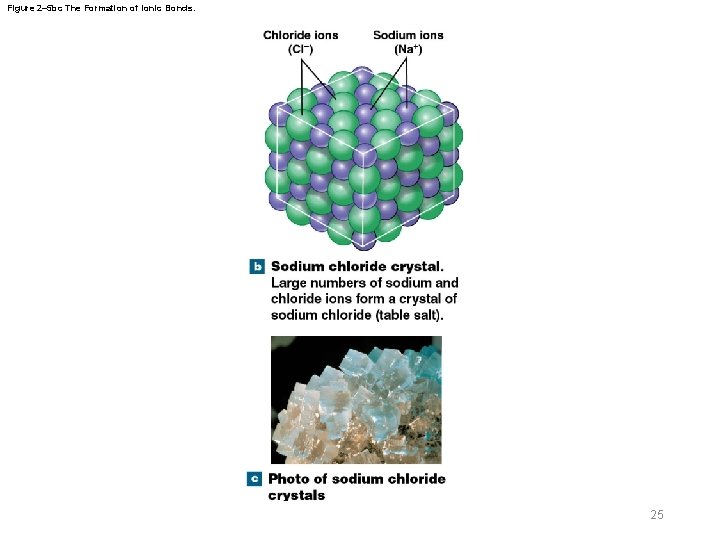
Figure 2– 5 a The Formation of Ionic Bonds. 1 Formation of ions 2 Attraction between opposite charges 3 Formation of an ionic compound Sodium atom Sodium ion (Na+) Sodium chloride (Na. Cl) Chlorine atom Chloride ion (Cl–) a Formation of an ionic bond. 1 A sodium (Na) atom gives up an electron, which is gained by a chlorine (Cl) atom. 2 Because the sodium ion (Na +) and chloride ion (Cl–) have opposite charges, they are attracted to one another. 3 The association of sodium and chloride ions forms the ionic compound sodium chloride. 24

Figure 2– 5 bc The Formation of Ionic Bonds. 25

2 -2 Molecules and Compounds § Covalent bonds – Strong bonds involving shared electrons • One electron is donated by each atom to make the pair of electrons • Sharing one pair of electrons is a single covalent bond • Sharing two pairs of electrons is a double covalent bond • Sharing three pairs of electrons is a triple covalent bond 26

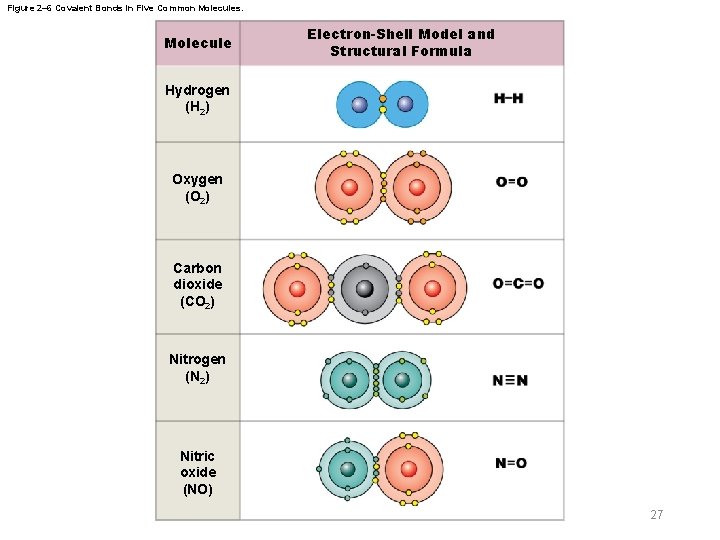
Figure 2– 6 Covalent Bonds in Five Common Molecules. Molecule Electron-Shell Model and Structural Formula Hydrogen (H 2) Oxygen (O 2) Carbon dioxide (CO 2) Nitrogen (N 2) Nitric oxide (NO) 27

2 -2 Molecules and Compounds § Covalent bonds – Nonpolar covalent bonds • Equal sharing of electrons between atoms that have equal pull on the electrons – Polar covalent bonds • Unequal sharing of electrons because one atom has a disproportionately strong pull on the electrons • Form polar molecules—like water 28

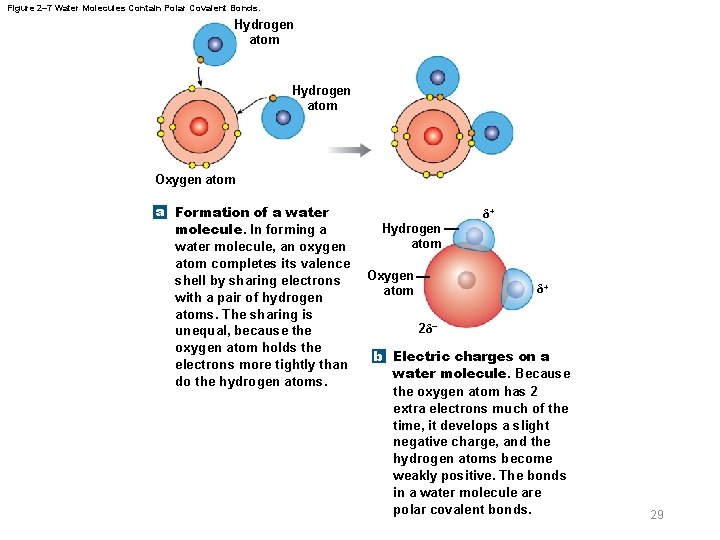
Figure 2– 7 Water Molecules Contain Polar Covalent Bonds. Hydrogen atom Oxygen atom a Formation of a water molecule. In forming a water molecule, an oxygen atom completes its valence shell by sharing electrons with a pair of hydrogen atoms. The sharing is unequal, because the oxygen atom holds the electrons more tightly than do the hydrogen atoms. δ+ Hydrogen atom Oxygen atom δ+ 2δ– b Electric charges on a water molecule. Because the oxygen atom has 2 extra electrons much of the time, it develops a slight negative charge, and the hydrogen atoms become weakly positive. The bonds in a water molecule are polar covalent bonds. 29

2 -2 Molecules and Compounds § Hydrogen bonds – Weak polar bonds between adjacent molecules based on electrical attractions – Involve attractions between a slight positive charge and a slight negative charge – Hydrogen bonds between H 2 O molecules cause surface tension 30

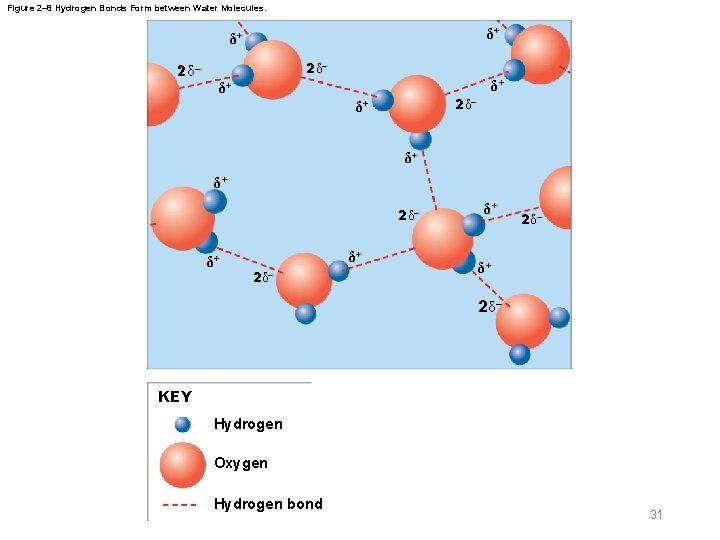
Figure 2– 8 Hydrogen Bonds Form between Water Molecules. δ+ δ+ 2 δ– 2δ– δ+ δ+ 2δ– δ+ 2δ– KEY Hydrogen Oxygen Hydrogen bond 31

2 -2 Molecules and Compounds § States of matter – Solid • Constant volume and shape – Liquid • Constant volume but changes shape – Gas • Changes volume and shape 32

2 -3 Chemical Reactions § In a chemical reaction – Either new bonds are formed or existing bonds are broken • Reactants – Materials going into a reaction • Products – Materials coming out of a reaction • Metabolism – All of the reactions that are occurring at one time 33

2 -3 Chemical Reactions § Energy – The capacity to do work § Work – Movement of an object or change in matter § Kinetic energy – Energy of motion § Potential energy – Stored energy § Chemical energy – Potential energy stored in chemical bonds 34

2 -3 Chemical Reactions § Types of chemical reactions 1. Decomposition 2. Synthesis 3. Exchange 4. Reversible 35

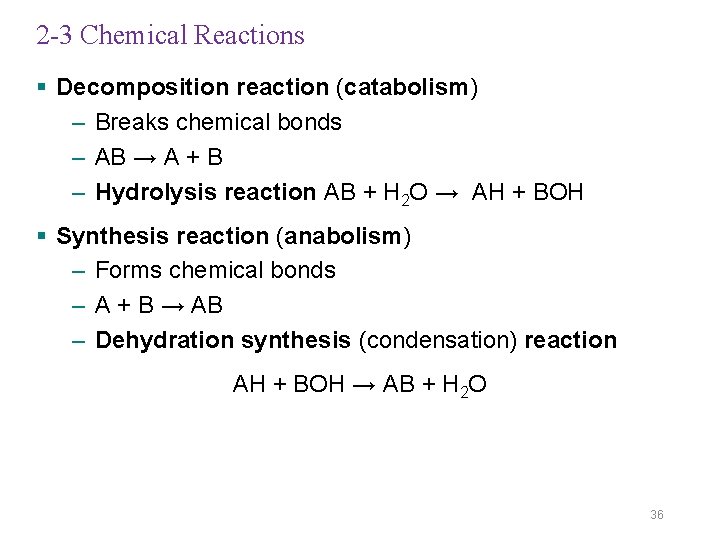
2 -3 Chemical Reactions § Decomposition reaction (catabolism) – Breaks chemical bonds – AB → A + B – Hydrolysis reaction AB + H 2 O → AH + BOH § Synthesis reaction (anabolism) – Forms chemical bonds – A + B → AB – Dehydration synthesis (condensation) reaction AH + BOH → AB + H 2 O 36

2 -3 Chemical Reactions § Exchange reaction – Involves decomposition first, then synthesis – AB + CD → AD + CB 37

2 -3 Chemical Reactions § Reversible reactions – A + B ↔ AB – At equilibrium, the amounts of chemicals do not change even though the reactions are still occurring • Reversible reactions seek equilibrium, balancing opposing reaction rates • When reactants are added or removed, reaction rates adjust to reach a new equilibrium 38

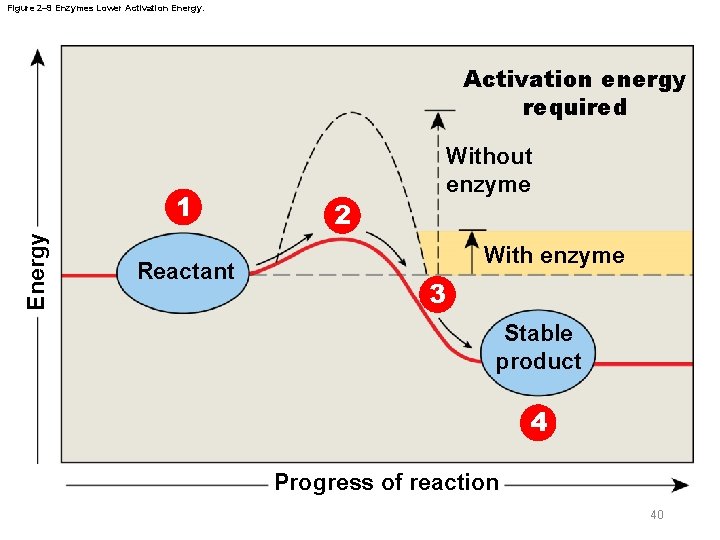
2 -4 Enzymes § Biochemical reactions – In cells, do not occur spontaneously • Activation energy is the amount of energy needed to start a reaction • Enzymes are protein catalysts that lower the activation energy of reactions 39

Figure 2– 9 Enzymes Lower Activation Energy. Activation energy required Energy 1 Reactant 2 Without enzyme With enzyme 3 Stable product 4 Progress of reaction 40

2 -4 Enzymes § Exergonic reactions – Release energy § Endergonic reactions – Absorb energy 41

2 -5 Inorganic and Organic Compounds § Nutrients – Essential molecules obtained from food § Metabolites – Molecules made or broken down in the body § Inorganic compounds – Carbon dioxide, oxygen, water, and inorganic acids, bases, and salts § Organic compounds – Molecules containing carbon and hydrogen – Carbohydrates, proteins, lipids, and nucleic acids 42

2 -6 Properties of Water § Water (H 2 O) – Accounts for up to two-thirds of total body weight – Produces solutions—uniform mixtures of two or more substances • A solution consists of a solvent, or liquid, and solutes • Solutes are the dissolved substances 43

2 -6 Properties of Water § Universal solvent – Many molecules are water soluble § Reactivity – Water serves as a reactant in some reactions § High heat capacity – Heat capacity is the heat required to raise the temperature of a unit mass of a substance 1ºC § Lubrication – To moisten and reduce friction 44

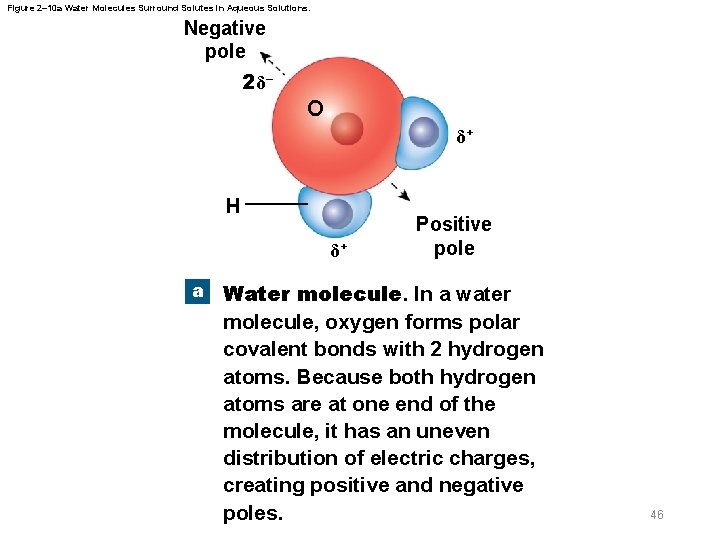
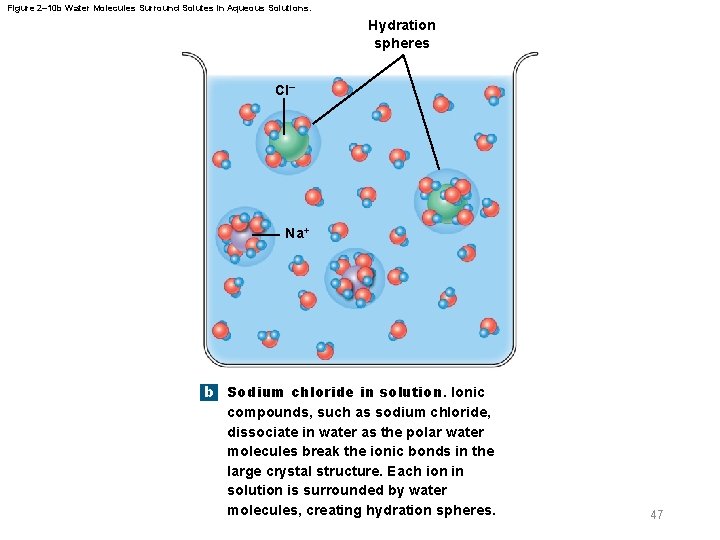
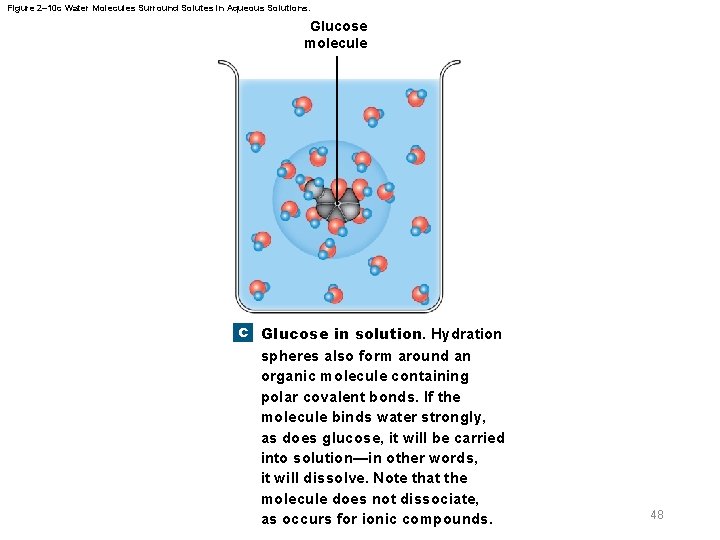
2 -6 Properties of Water § Properties of aqueous solutions – Water is a polar molecule – Many inorganic compounds split into smaller molecules via dissociation in water – Ionization is dissociation into ions – Polar water molecules form hydration spheres around ions and small polar molecules that keep them in solution 45

Figure 2– 10 a Water Molecules Surround Solutes in Aqueous Solutions. Negative pole 2 δ– O δ+ H δ+ a Positive pole Water molecule. In a water molecule, oxygen forms polar covalent bonds with 2 hydrogen atoms. Because both hydrogen atoms are at one end of the molecule, it has an uneven distribution of electric charges, creating positive and negative poles. 46

Figure 2– 10 b Water Molecules Surround Solutes in Aqueous Solutions. Hydration spheres Cl– Na+ b Sodium chloride in solution. Ionic compounds, such as sodium chloride, dissociate in water as the polar water molecules break the ionic bonds in the large crystal structure. Each ion in solution is surrounded by water molecules, creating hydration spheres. 47

Figure 2– 10 c Water Molecules Surround Solutes in Aqueous Solutions. Glucose molecule c Glucose in solution. Hydration spheres also form around an organic molecule containing polar covalent bonds. If the molecule binds water strongly, as does glucose, it will be carried into solution—in other words, it will dissolve. Note that the molecule does not dissociate, as occurs for ionic compounds. 48

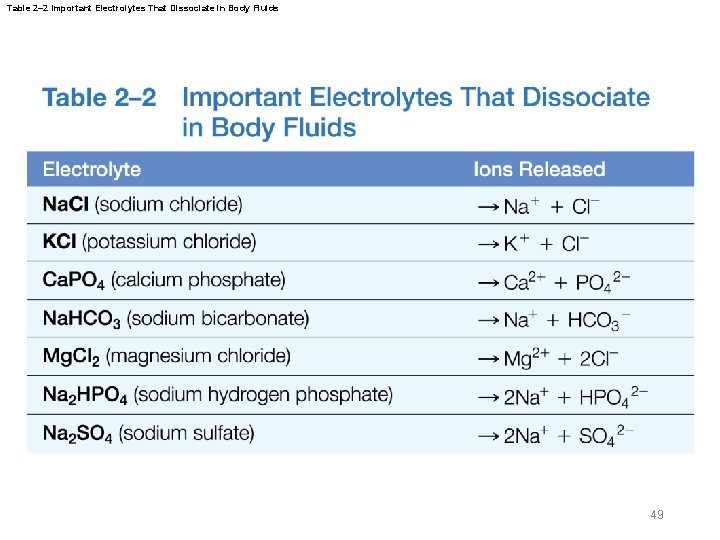
Table 2– 2 Important Electrolytes That Dissociate in Body Fluids 49

2 -6 Properties of Water § Electrolytes and body fluids – Electrolytes are inorganic ions that conduct electricity in solution – Electrolyte imbalance seriously disturbs vital body functions 50

2 -6 Properties of Water § Hydrophilic and hydrophobic compounds – Hydrophilic • hydro- = water, philos = loving • Includes ions and polar molecules • Interact with water – Hydrophobic • phobos = fear • Includes nonpolar molecules, fats, and oils • Do not interact with water 51

2 -6 Properties of Water § Colloids and suspensions – Colloid • A solution containing dispersed proteins or other large molecules • Example: blood plasma – Suspension • Contains large particles that settle out of solution • Example: whole blood 52

2 -7 p. H and Homeostasis § p. H – The negative logarithm of the hydrogen ion concentration of a solution in moles per liter § Neutral p. H – A balance of H+ and OH– – Pure water = 7. 0 53

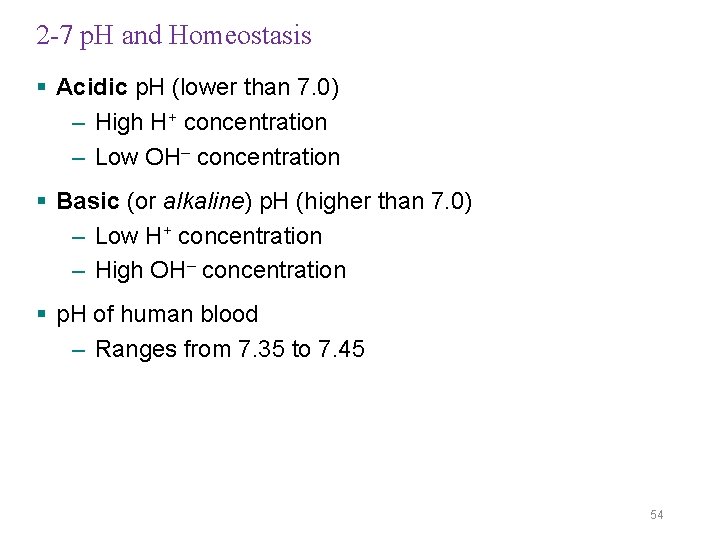
2 -7 p. H and Homeostasis § Acidic p. H (lower than 7. 0) – High H+ concentration – Low OH– concentration § Basic (or alkaline) p. H (higher than 7. 0) – Low H+ concentration – High OH– concentration § p. H of human blood – Ranges from 7. 35 to 7. 45 54

2 -7 p. H and Homeostasis § p. H scale – Has an inverse relationship with H+ concentration • More H+ ions means lower p. H, fewer H+ ions means higher p. H 55

Figure 2– 11 The p. H Scale Indicates Hydrogen Ion Concentration (Part 1 of 2). 1 mol/L hydrochloric acid Beer, vinegar, wine, Tomatoes, pickles grapes Stomach acid Extremely acidic p. H 0 [H+] 100 (mol/L) 1 10− 1 Urine Saliva, milk Increasing concentration of H+ 2 10− 2 3 10− 3 4 10− 4 5 10− 5 6 10− 6 Pure water Neutral 7 10− 7 56

2 -8 Acids, Bases, and Salts § Acid (proton donor) – A solute that adds hydrogen ions to a solution – Strong acids dissociate completely in solution § Base (proton acceptor) – A solute that removes hydrogen ions from a solution – Strong bases dissociate completely in solution § Weak acids and weak bases – Fail to dissociate completely – Help to balance the p. H 57

2 -8 Acids, Bases, and Salts § Salt – Solute that dissociates into cations and anions other than hydrogen ions and hydroxide ions 58

2 -8 Acids, Bases, and Salts § Buffers and p. H control – Buffers stabilize p. H of solutions • Buffer systems often involve a weak acid and its related salt (weak base) • Neutralize strong acids or strong bases • Carbonic acid–bicarbonate buffer system is very important in humans – Antacids • Use sodium bicarbonate to neutralize hydrochloric acid in the stomach 59

2 -9 Monomers and Polymers § Each macromolecule of life is made up of monomer subunits – Identical monomers (molecules) join together to form a polymer 60

2 -10 Carbohydrates § Organic molecules – Contain H, C, and usually O – Are covalently bonded – Contain functional groups that determine their chemistry – Include carbohydrates, lipids, proteins, and nucleic acids 61

2 -10 Carbohydrates § Carbohydrates – Contain carbon, hydrogen, and oxygen in a 1: 2: 1 ratio • Some are isomers—molecules with the same molecular formula but different structures § Monosaccharides – Simple sugars with three to seven carbon atoms – Glucose, fructose, galactose 62

2 -10 Carbohydrates § Disaccharides – Two monosaccharides condensed by dehydration synthesis – Sucrose, maltose § Polysaccharides – Polymers of many sugars condensed by dehydration synthesis – Glycogen, starch, cellulose 63

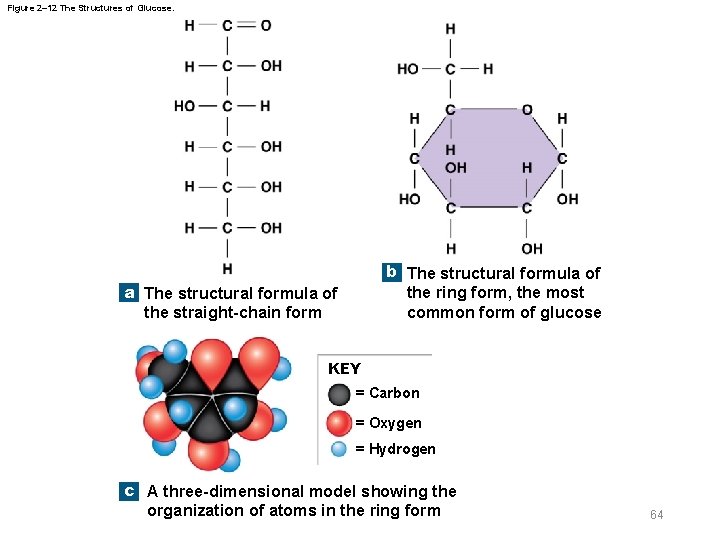
Figure 2– 12 The Structures of Glucose. b The structural formula of the ring form, the most common form of glucose a The structural formula of the straight-chain form KEY = Carbon = Oxygen = Hydrogen c A three-dimensional model showing the organization of atoms in the ring form 64

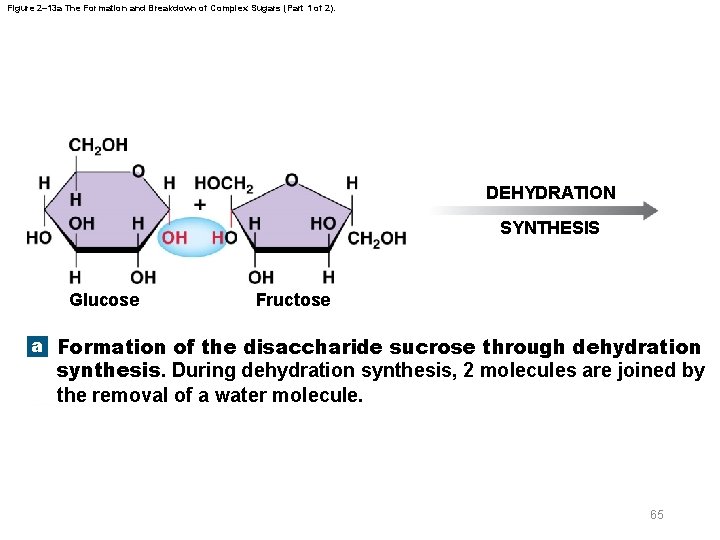
Figure 2– 13 a The Formation and Breakdown of Complex Sugars (Part 1 of 2). DEHYDRATION SYNTHESIS Glucose Fructose a Formation of the disaccharide sucrose through dehydration synthesis. During dehydration synthesis, 2 molecules are joined by the removal of a water molecule. 65

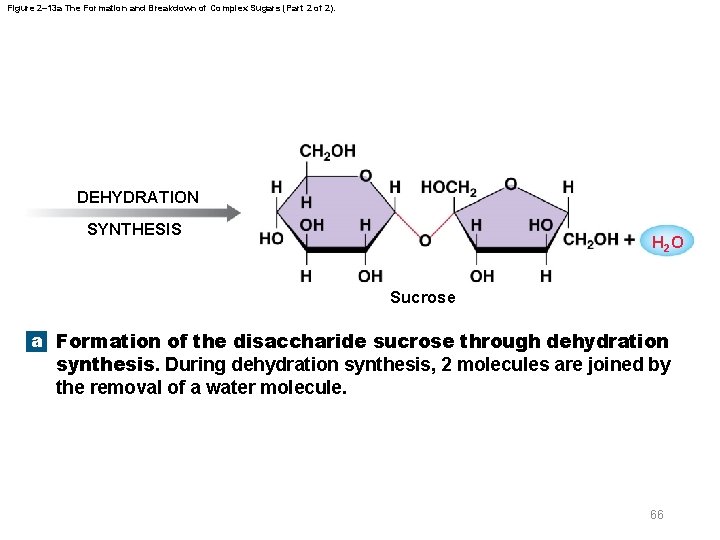
Figure 2– 13 a The Formation and Breakdown of Complex Sugars (Part 2 of 2). DEHYDRATION SYNTHESIS H 2 O Sucrose a Formation of the disaccharide sucrose through dehydration synthesis. During dehydration synthesis, 2 molecules are joined by the removal of a water molecule. 66

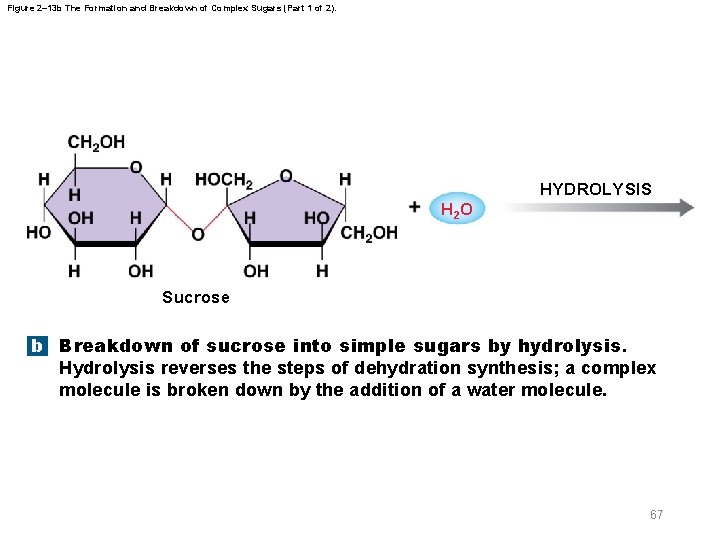
Figure 2– 13 b The Formation and Breakdown of Complex Sugars (Part 1 of 2). HYDROLYSIS H 2 O Sucrose b Breakdown of sucrose into simple sugars by hydrolysis. Hydrolysis reverses the steps of dehydration synthesis; a complex molecule is broken down by the addition of a water molecule. 67

Figure 2– 13 b The Formation and Breakdown of Complex Sugars (Part 2 of 2). HYDROLYSIS Glucose Fructose b Breakdown of sucrose into simple sugars by hydrolysis. Hydrolysis reverses the steps of dehydration synthesis; a complex molecule is broken down by the addition of a water molecule. 68

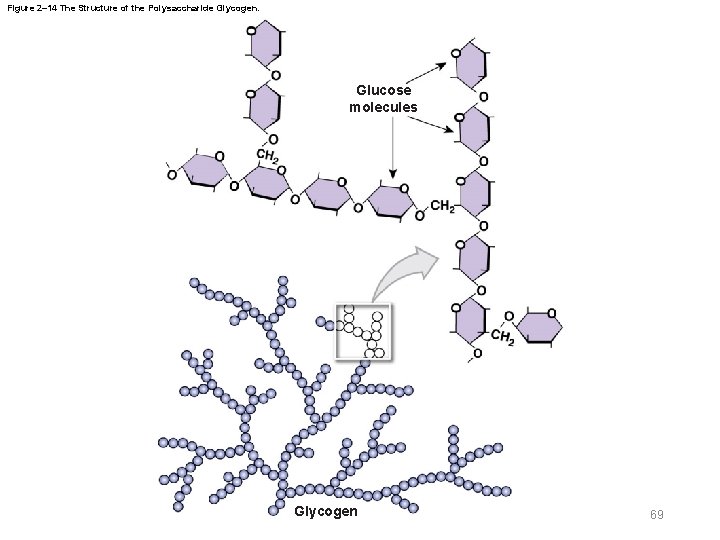
Figure 2– 14 The Structure of the Polysaccharide Glycogen. Glucose molecules Glycogen 69

2 -11 Lipids § Lipids – Mainly hydrophobic molecules such as fats, oils, and waxes – Made mostly of carbon and hydrogen atoms – Include • Fatty acids • Eicosanoids • Glycerides • Steroids • Phospholipids and glycolipids 70

2 -11 Lipids § Fatty acids – Long chains of carbon and hydrogen with a carboxyl group (COOH) at one end – Relatively nonpolar, except the carboxyl group – Fatty acids may be • Saturated with hydrogen – No double bonds in the hydrocarbon tail • Unsaturated (one or more double bonds in tail) – Monounsaturated = one double bond – Polyunsaturated = two or more double bonds 71

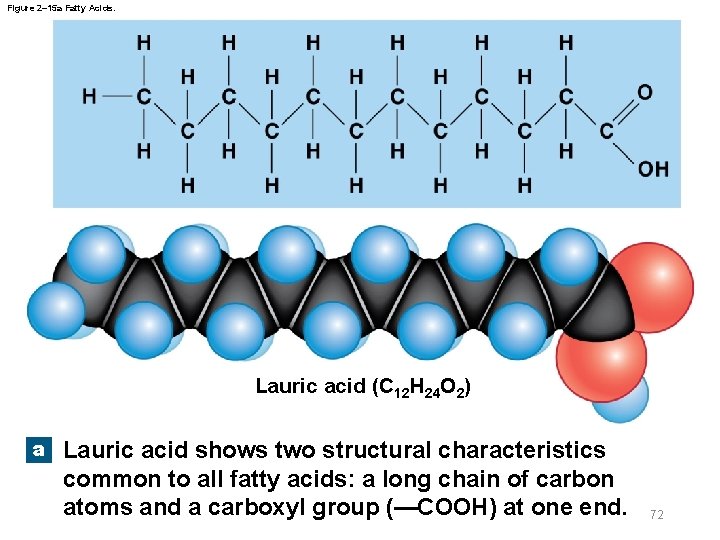
Figure 2– 15 a Fatty Acids. Lauric acid (C 12 H 24 O 2) a Lauric acid shows two structural characteristics common to all fatty acids: a long chain of carbon atoms and a carboxyl group (—COOH) at one end. 72

Figure 2– 15 b Fatty Acids. Saturated Unsaturated b A fatty acid is either saturated (has single covalent bonds only) or unsaturated (has one or more double covalent bonds). The presence of a double bond causes a sharp bend in the molecule. 73

2 -11 Lipids § Eicosanoids – Cannot be synthesized, so must be obtained from the diet – Derived from a fatty acid called arachidonic acid – Leukotrienes • Active in immune system – Prostaglandins • Short-chain fatty acids • Local hormones 74

Figure 2– 16 Prostaglandins. 75

2 -11 Lipids § Glycerides – Fatty acids attached to a glycerol molecule – Monoglyceride—glycerol plus one fatty acid – Diglyceride—glycerol plus two fatty acids – Triglycerides—glycerol plus three fatty acids • Also called triacylglycerols or neutral fats • Have three important functions 1. Energy source 2. Insulation 3. Protection 76

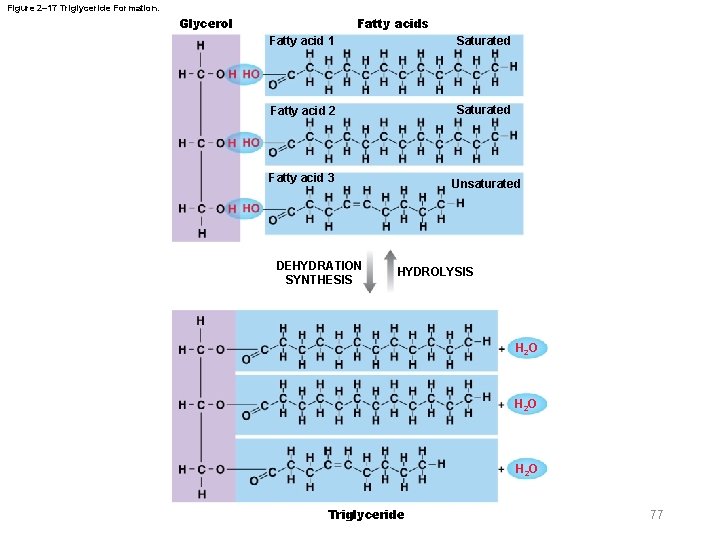
Figure 2– 17 Triglyceride Formation. Glycerol Fatty acids Fatty acid 1 Saturated Fatty acid 2 Saturated Fatty acid 3 DEHYDRATION SYNTHESIS Unsaturated HYDROLYSIS H 2 O Triglyceride 77

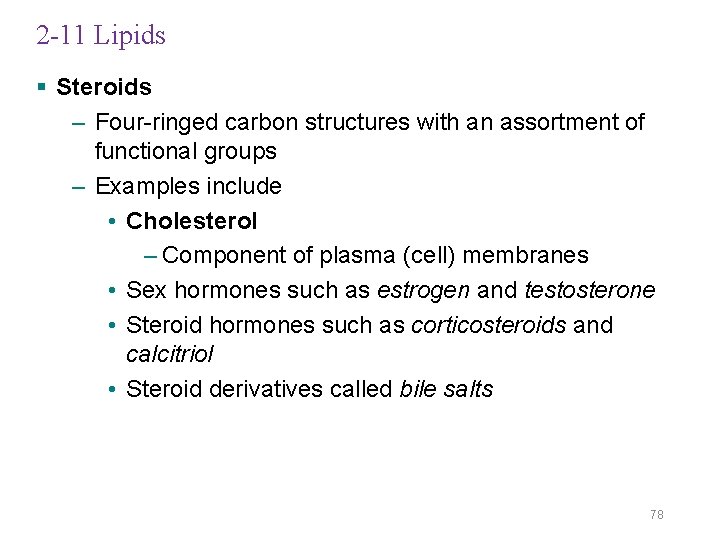
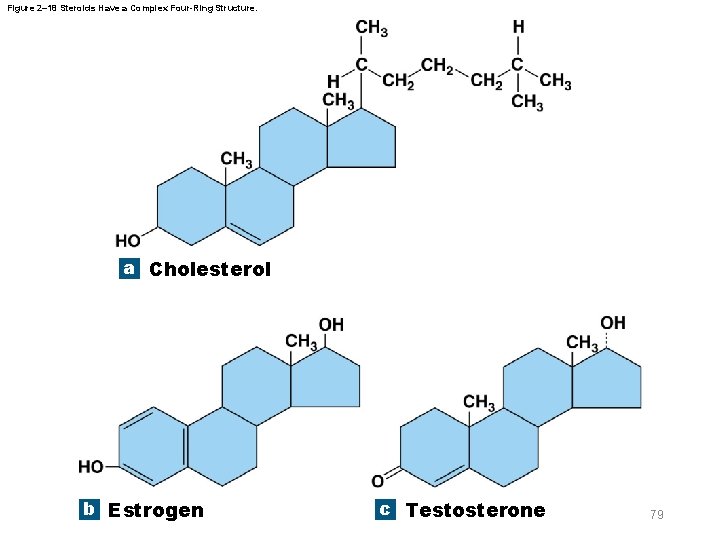
2 -11 Lipids § Steroids – Four-ringed carbon structures with an assortment of functional groups – Examples include • Cholesterol – Component of plasma (cell) membranes • Sex hormones such as estrogen and testosterone • Steroid hormones such as corticosteroids and calcitriol • Steroid derivatives called bile salts 78

Figure 2– 18 Steroids Have a Complex Four-Ring Structure. a Cholesterol b Estrogen c Testosterone 79

2 -11 Lipids § Phospholipids and glycolipids – Both can be synthesized by our cells – Contain a diglyceride attached to either a phosphate group (phospholipid) or a sugar (glycolipid) – Generally, both have hydrophilic heads and hydrophobic tails – Structural lipids—components of plasma membranes 80

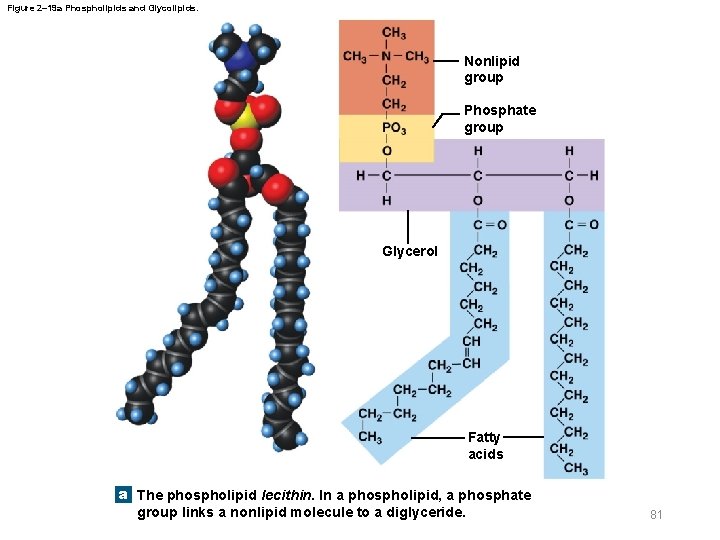
Figure 2– 19 a Phospholipids and Glycolipids. Nonlipid group Phosphate group Glycerol Fatty acids a The phospholipid lecithin. In a phospholipid, a phosphate group links a nonlipid molecule to a diglyceride. 81

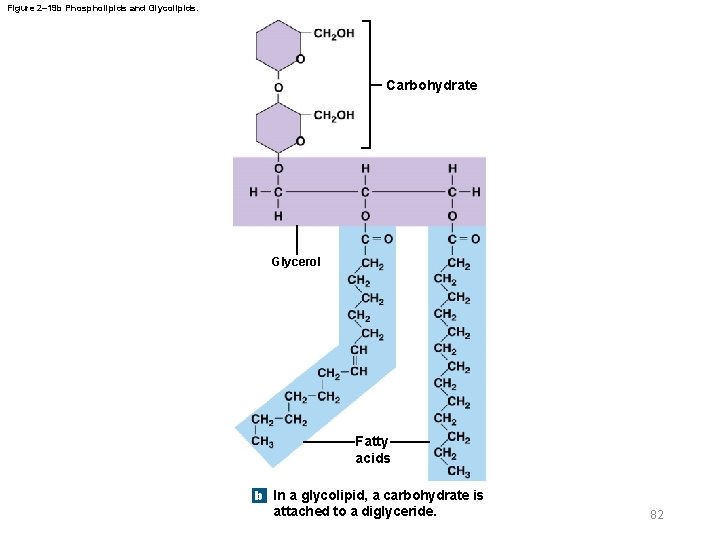
Figure 2– 19 b Phospholipids and Glycolipids. Carbohydrate Glycerol Fatty acids b In a glycolipid, a carbohydrate is attached to a diglyceride. 82

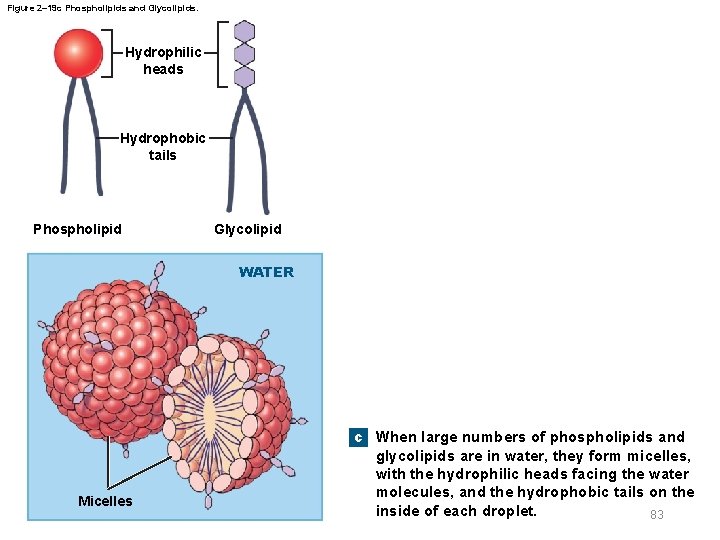
Figure 2– 19 c Phospholipids and Glycolipids. Hydrophilic heads Hydrophobic tails Phospholipid Glycolipid WATER c When large numbers of phospholipids and Micelles glycolipids are in water, they form micelles, with the hydrophilic heads facing the water molecules, and the hydrophobic tails on the inside of each droplet. 83

2 -12 Proteins § Proteins – Are the most abundant and important organic molecules – Contain basic elements • Carbon (C), hydrogen (H), oxygen (O), and nitrogen (N) – 20 amino acids are monomers that combine to form proteins (polymers) 84

2 -12 Proteins § Seven major protein functions 1. Support • Structural proteins 2. Movement • Contractile proteins 3. Transport • Transport (carrier) proteins 4. Buffering • Regulation of p. H 5. Metabolic regulation • Enzymes 6. Coordination and control • Hormones 7. Defense • Antibodies 85

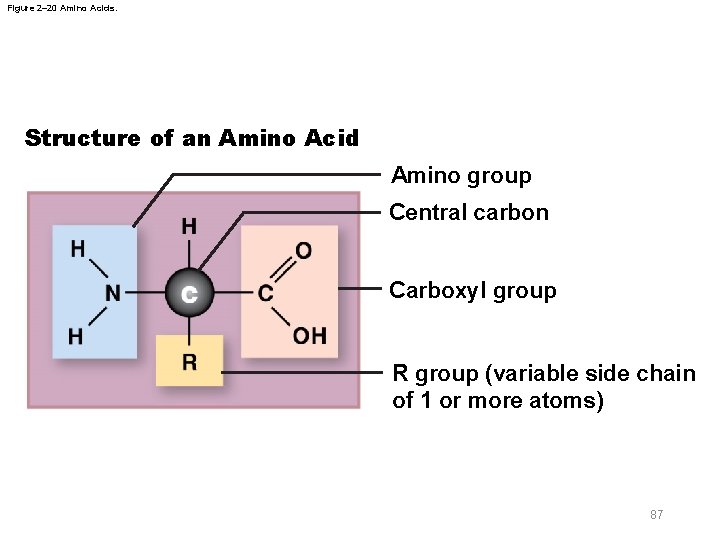
2 -12 Proteins § Protein structure – Long chains of amino acids – Each amino acid consists of 1. Central carbon atom 2. Hydrogen atom 3. Amino group (—NH 2) 4. Carboxyl group (—COOH) 5. Variable side chain, or R group 86

Figure 2– 20 Amino Acids. Structure of an Amino Acid Amino group Central carbon Carboxyl group R group (variable side chain of 1 or more atoms) 87

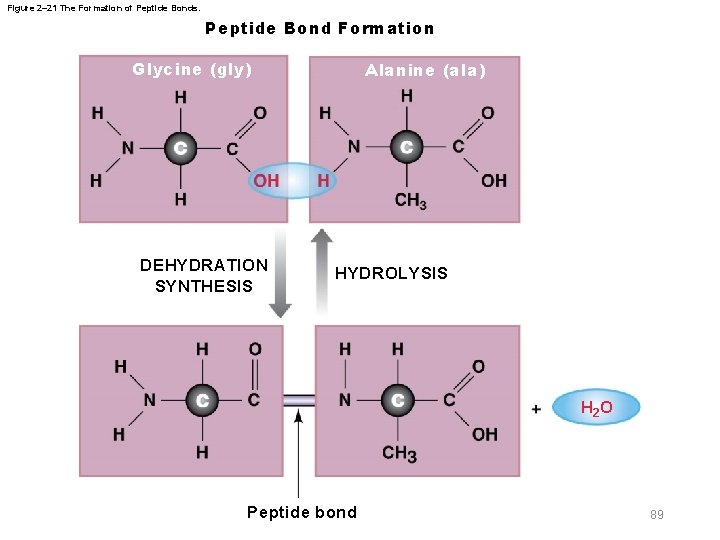
2 -12 Proteins § Linking two amino acids together – Requires dehydration synthesis between • Amino group of one amino acid and the carboxyl group of another amino acid • Forms a peptide bond – Resulting molecule is a peptide – Polypeptides are tripeptides and larger peptides 88

Figure 2– 21 The Formation of Peptide Bonds. Peptide Bond Formation Glycine (gly) DEHYDRATION SYNTHESIS Alanine (ala) HYDROLYSIS H 2 O Peptide bond 89

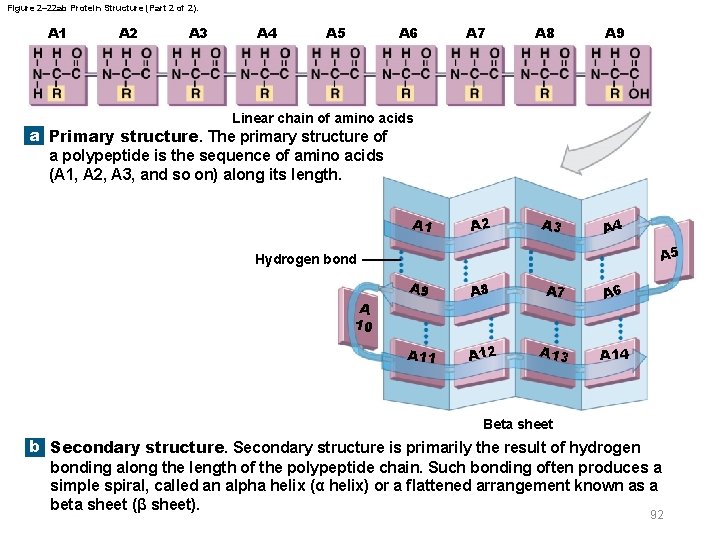
2 -12 Proteins § Protein shape – Primary structure • The sequence of amino acids along a polypeptide – Secondary structure • Hydrogen bonds form spirals or pleats – Tertiary structure • Coiling and folding produce three-dimensional shape – Quaternary structure • Final protein complex produced by interacting polypeptide chains 90

Figure 2– 22 ab Protein Structure (Part 1 of 2). A 1 A 2 A 3 A 4 A 5 A 6 A 7 A 8 A 9 Linear chain of amino acids a Primary structure. The primary structure of a polypeptide is the sequence of amino acids (A 1, A 2, A 3, and so on) along its length. Hydrogen bond A 2 A 1 A 6 A 3 A 5 A 7 A 9 Alpha helix b Secondary structure is primarily the result of hydrogen bonding along the length of the polypeptide chain. Such bonding often produces a simple spiral, called an alpha helix (α helix) or a flattened arrangement known as a beta sheet (β sheet). 91

Figure 2– 22 ab Protein Structure (Part 2 of 2). A 1 A 2 A 3 A 4 A 5 A 6 A 7 A 8 A 9 Linear chain of amino acids a Primary structure. The primary structure of a polypeptide is the sequence of amino acids (A 1, A 2, A 3, and so on) along its length. A 1 A 2 A 3 A 4 A 5 Hydrogen bond A 10 A 9 A 8 A 7 A 6 A 11 A 12 A 13 A 14 Beta sheet b Secondary structure is primarily the result of hydrogen bonding along the length of the polypeptide chain. Such bonding often produces a simple spiral, called an alpha helix (α helix) or a flattened arrangement known as a beta sheet (β sheet). 92

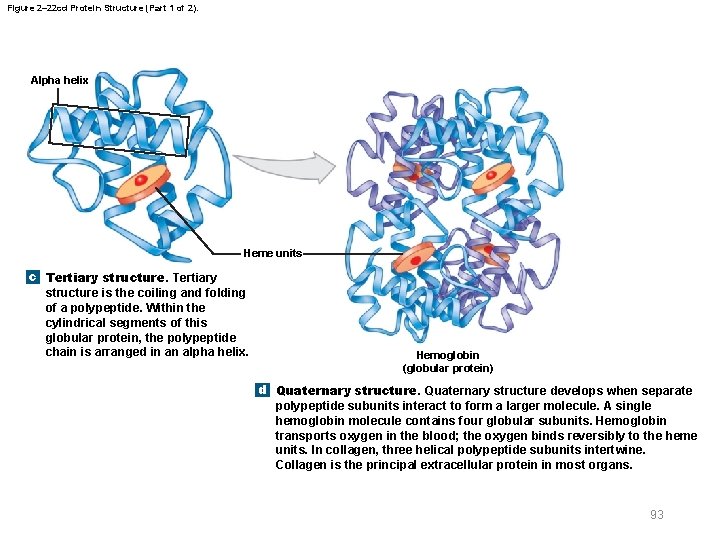
Figure 2– 22 cd Protein Structure (Part 1 of 2). Alpha helix Heme units c Tertiary structure is the coiling and folding of a polypeptide. Within the cylindrical segments of this globular protein, the polypeptide chain is arranged in an alpha helix. Hemoglobin (globular protein) d Quaternary structure develops when separate polypeptide subunits interact to form a larger molecule. A single hemoglobin molecule contains four globular subunits. Hemoglobin transports oxygen in the blood; the oxygen binds reversibly to the heme units. In collagen, three helical polypeptide subunits intertwine. Collagen is the principal extracellular protein in most organs. 93

Figure 2– 22 cd Protein Structure (Part 2 of 2). Alpha helix Heme units c Tertiary structure is the coiling and folding of a polypeptide. Within the cylindrical segments of this globular protein, the polypeptide chain is arranged in an alpha helix. Collagen (fibrous protein) d Quaternary structure develops when separate polypeptide subunits interact to form a larger molecule. A single hemoglobin molecule contains four globular subunits. Hemoglobin transports oxygen in the blood; the oxygen binds reversibly to the heme units. In collagen, three helical polypeptide subunits intertwine. Collagen is the principal extracellular protein in most organs. 94

2 -12 Proteins § Fibrous and globular proteins – Globular proteins • Soluble spheres with active functions • Shape is based on tertiary structure – Fibrous proteins • Structural sheets or strands • Shape is based on secondary or quaternary structures 95

2 -12 Proteins § Enzymes are catalysts – Proteins that lower the activation energy of a chemical reaction – Not changed or used up in the reaction – Substrates (reactants) bind to an active site on an enzyme – Enzymes exhibit 1. Specificity—catalyze only one type of reaction 2. Saturation limits—enzymes become saturated 3. Regulation—by other cellular chemicals 96

Figure 2– 23 A Simplified View of Enzyme Structure and Function (Part 1 of 4). 1 Substrates bind to active site of enzyme S 2 S 1 Substrates ENZYM E Active site 97

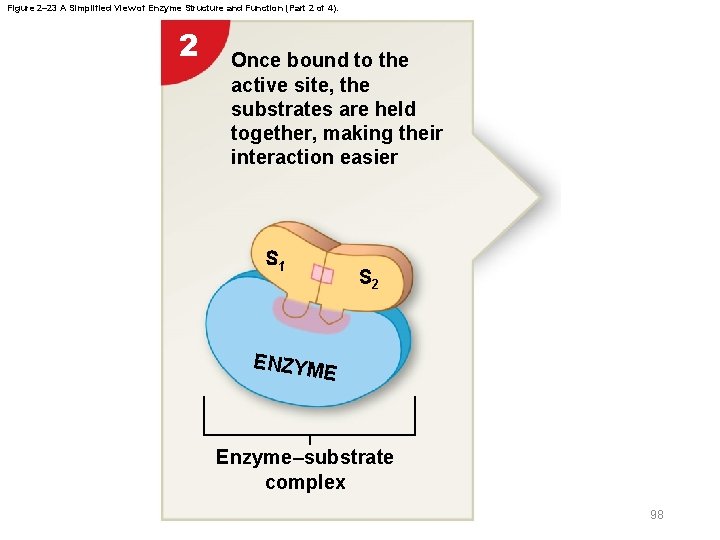
Figure 2– 23 A Simplified View of Enzyme Structure and Function (Part 2 of 4). 2 Once bound to the active site, the substrates are held together, making their interaction easier S 1 S 2 ENZYM E Enzyme–substrate complex 98

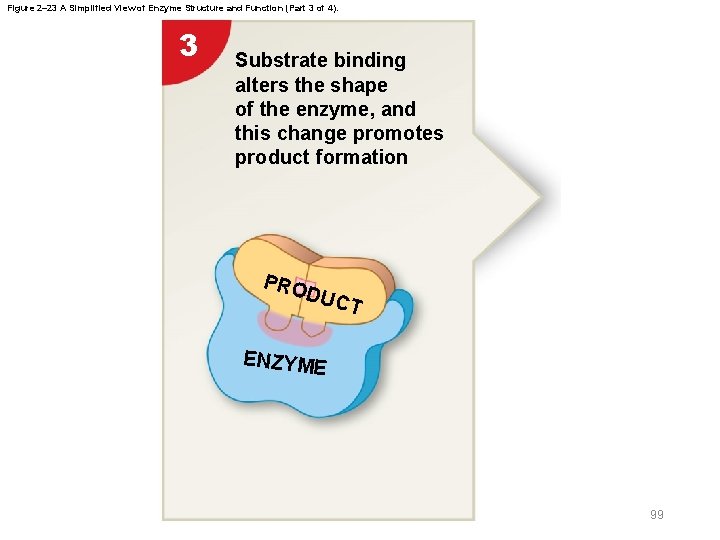
Figure 2– 23 A Simplified View of Enzyme Structure and Function (Part 3 of 4). 3 Substrate binding alters the shape of the enzyme, and this change promotes product formation PRO DUC T ENZYME 99

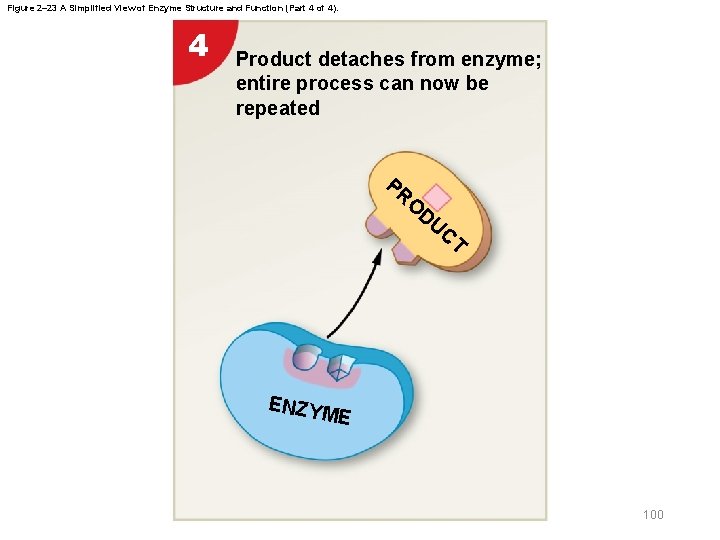
Figure 2– 23 A Simplified View of Enzyme Structure and Function (Part 4 of 4). 4 Product detaches from enzyme; entire process can now be repeated PR O DU CT ENZY ME 100

2 -12 Proteins § Cofactors and enzyme function – Cofactor • An ion or molecule that binds to an enzyme before substrates can bind – Coenzymes • Nonprotein organic cofactors (vitamins) 101

2 -12 Proteins § Temperature and p. H affect enzyme function – Denaturation • Change in shape and loss of function due to heat or p. H 102

2 -12 Proteins § Glycoproteins and proteoglycans – Glycoproteins • Large proteins + small carbohydrates • Include enzymes, antibodies, hormones, and components of plasma membranes • Mucus production – Proteoglycans • Large polysaccharides + polypeptides • Increase viscosity of fluids 103

2 -13 Nucleic Acids § Nucleic acids – Large organic molecules found in the nucleus – Store and process information – Deoxyribonucleic acid (DNA) • Determines inherited characteristics • Directs protein synthesis • Controls enzyme production • Controls metabolism – Ribonucleic acid (RNA) • Controls intermediate steps in protein synthesis 104

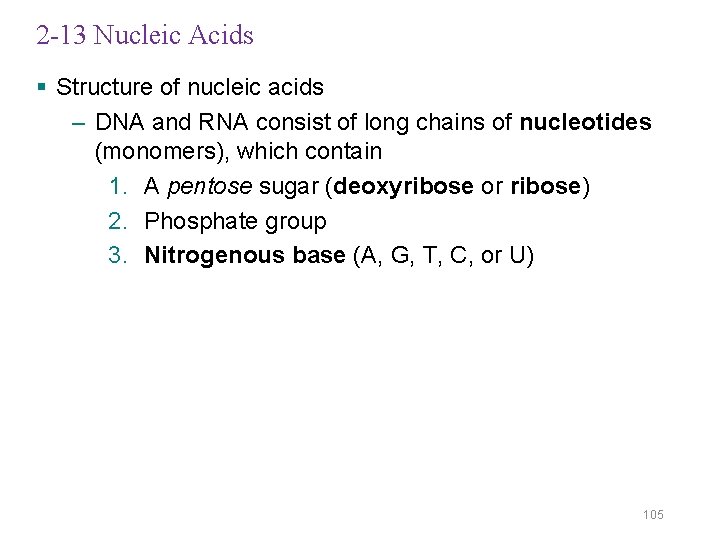
2 -13 Nucleic Acids § Structure of nucleic acids – DNA and RNA consist of long chains of nucleotides (monomers), which contain 1. A pentose sugar (deoxyribose or ribose) 2. Phosphate group 3. Nitrogenous base (A, G, T, C, or U) 105

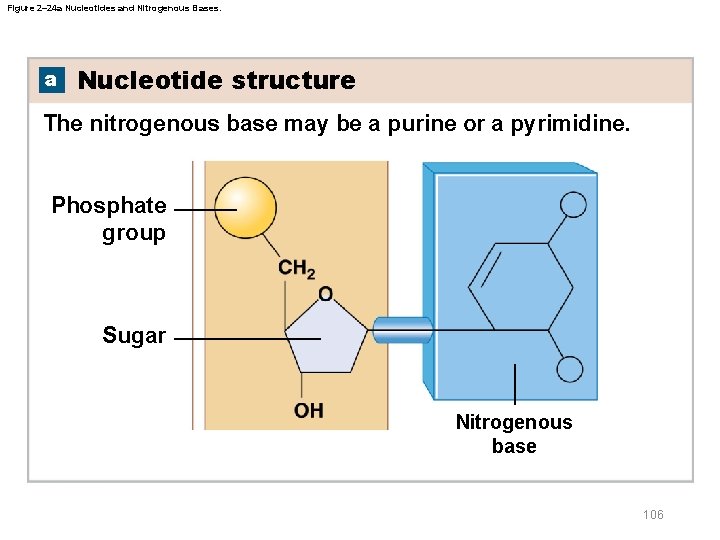
Figure 2– 24 a Nucleotides and Nitrogenous Bases. a Nucleotide structure The nitrogenous base may be a purine or a pyrimidine. Phosphate group Sugar Nitrogenous base 106

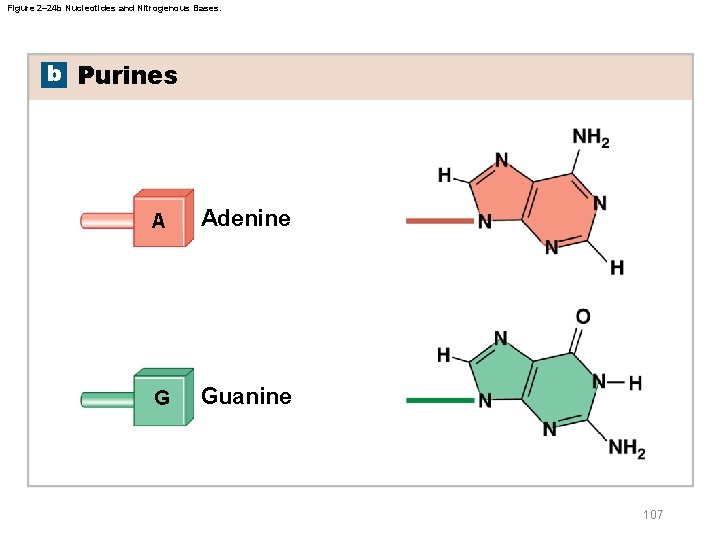
Figure 2– 24 b Nucleotides and Nitrogenous Bases. b Purines A Adenine G Guanine 107

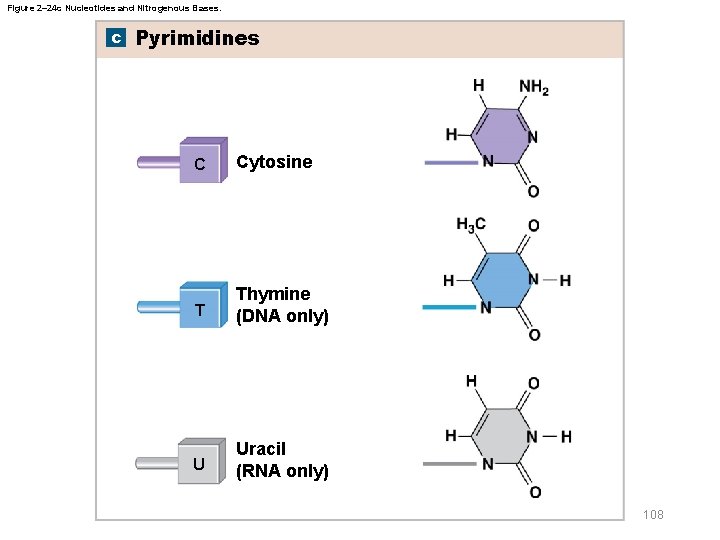
Figure 2– 24 c Nucleotides and Nitrogenous Bases. c Pyrimidines C Cytosine T Thymine (DNA only) U Uracil (RNA only) 108

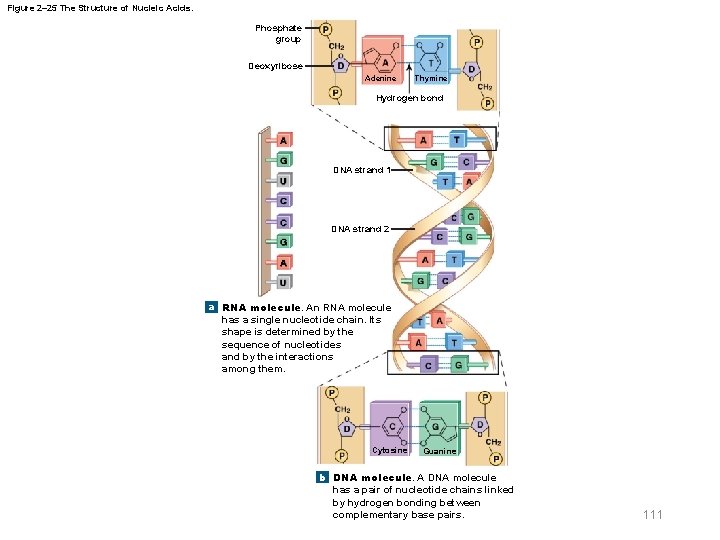
2 -13 Nucleic Acids § DNA and RNA – DNA consists of a pair of nucleotide chains • Called complementary strands • Hydrogen bonds between opposing nitrogenous bases hold the strands together • Forms a twisting double helix – RNA consists of a single chain of nucleotides • Messenger RNA (m. RNA) • Transfer RNA (t. RNA) • Ribosomal RNA (r. RNA) 109

2 -13 Nucleic Acids § Complementary base pairs – Purines pair with pyrimidines • DNA – Adenine (A) bonds to thymine (T) – Cytosine (C) bonds to guanine (G) • RNA – Uracil (U) replaces thymine (T) 110

Figure 2– 25 The Structure of Nucleic Acids. Phosphate group Deoxyribose Adenine Thymine Hydrogen bond A G DNA strand 1 U C C DNA strand 2 G A U a RNA molecule. An RNA molecule has a single nucleotide chain. Its shape is determined by the sequence of nucleotides and by the interactions among them. Cytosine Guanine b DNA molecule. A DNA molecule has a pair of nucleotide chains linked by hydrogen bonding between complementary base pairs. 111

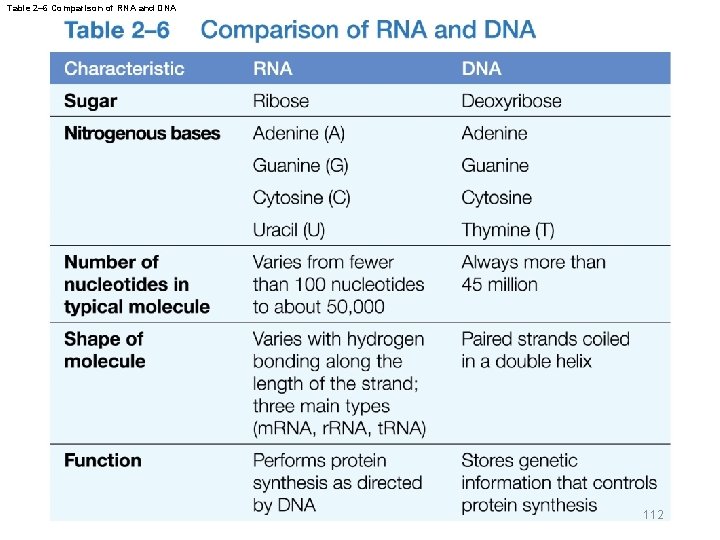
Table 2– 6 Comparison of RNA and DNA 112

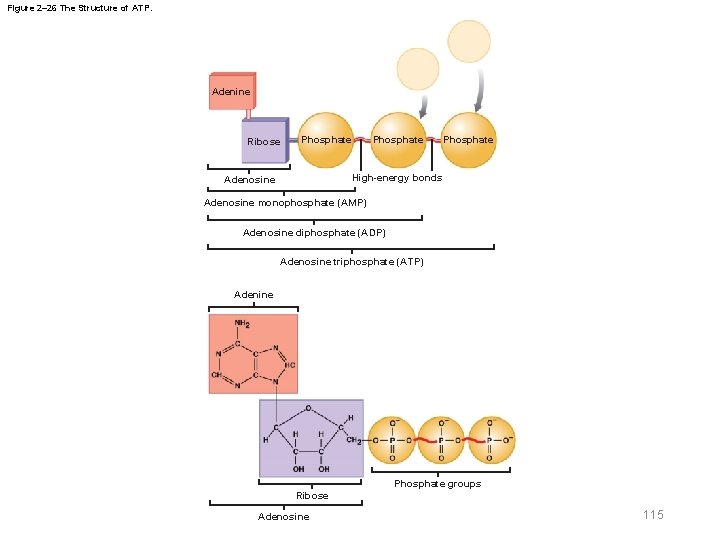
2 -14 High-Energy Compounds § Most high-energy compounds are derived from nucleotides § Phosphorylation – The process of adding a phosphate group to another molecule – Produces a high-energy bond 113

2 -14 High-Energy Compounds § Adenosine monophosphate (AMP) – Nucleotide that contains one phosphate group § Adenosine diphosphate (ADP) – Contains two phosphate groups § Adenosine triphosphate (ATP) – High-energy compound containing three phosphate groups § Adenosine triphosphatase (ATPase) – Enzyme that catalyzes the conversion of ATP to ADP 114

Figure 2– 26 The Structure of ATP. Adenine Ribose Phosphate High-energy bonds Adenosine monophosphate (AMP) Adenosine diphosphate (ADP) Adenosine triphosphate (ATP) Adenine Phosphate groups Ribose Adenosine 115
 Management eleventh edition stephen p robbins
Management eleventh edition stephen p robbins Management stephen p robbins 11th edition
Management stephen p robbins 11th edition Management eleventh edition
Management eleventh edition Management eleventh edition
Management eleventh edition Endomysium
Endomysium Anatomy and physiology edition 9
Anatomy and physiology edition 9 Human anatomy and physiology 10th edition
Human anatomy and physiology 10th edition The central sulcus divides which two lobes? (figure 14-13)
The central sulcus divides which two lobes? (figure 14-13) Waistline
Waistline Anatomy and physiology chapter 8 special senses
Anatomy and physiology chapter 8 special senses Chapter 13 anatomy and physiology of pregnancy
Chapter 13 anatomy and physiology of pregnancy Chapter 2 basic chemistry anatomy and physiology
Chapter 2 basic chemistry anatomy and physiology Chapter 7:9 lymphatic system
Chapter 7:9 lymphatic system Chapter 14 the digestive system and body metabolism
Chapter 14 the digestive system and body metabolism Chapter 10 blood anatomy and physiology
Chapter 10 blood anatomy and physiology Anatomy and physiology chapter 15
Anatomy and physiology chapter 15 Necessary life functions anatomy and physiology
Necessary life functions anatomy and physiology Holes anatomy and physiology chapter 1
Holes anatomy and physiology chapter 1 Anatomy and physiology chapter 15
Anatomy and physiology chapter 15 Distal and proximal
Distal and proximal Chapter 2 human reproductive anatomy and physiology
Chapter 2 human reproductive anatomy and physiology Anterior surface of scapula
Anterior surface of scapula Chapter 6 general anatomy and physiology
Chapter 6 general anatomy and physiology 2012 pearson education inc anatomy and physiology
2012 pearson education inc anatomy and physiology Eleventh 5 year plan
Eleventh 5 year plan Eleventh 5 year plan
Eleventh 5 year plan Thfive
Thfive For his eleventh birthday elvis presley
For his eleventh birthday elvis presley Physiology of sport and exercise 5th edition
Physiology of sport and exercise 5th edition Physiology of respiration
Physiology of respiration Tattoo anatomy and physiology
Tattoo anatomy and physiology Anatomy science olympiad
Anatomy science olympiad Perfect vs imperfect flower
Perfect vs imperfect flower Bone metabolism
Bone metabolism Anatomy and physiology of peptic ulcer ppt
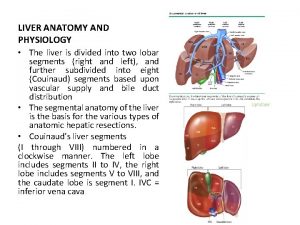
Anatomy and physiology of peptic ulcer ppt Liver anatomy
Liver anatomy Podbřišek
Podbřišek Hypogastric region
Hypogastric region Straw colored fluid
Straw colored fluid Http://anatomy and physiology
Http://anatomy and physiology Anatomy and physiology of appendicitis
Anatomy and physiology of appendicitis Aohs foundations of anatomy and physiology 1
Aohs foundations of anatomy and physiology 1 Aohs foundations of anatomy and physiology 2
Aohs foundations of anatomy and physiology 2 Anatomy and physiology of swine
Anatomy and physiology of swine Unit 26 agriscience
Unit 26 agriscience Science olympiad anatomy and physiology 2020 cheat sheet
Science olympiad anatomy and physiology 2020 cheat sheet Anatomy and physiology of stomach ppt
Anatomy and physiology of stomach ppt Anatomy and physiology of pancreas
Anatomy and physiology of pancreas Aohs foundations of anatomy and physiology 1
Aohs foundations of anatomy and physiology 1 Aohs foundations of anatomy and physiology 1
Aohs foundations of anatomy and physiology 1 What produces bile
What produces bile Cornell notes for anatomy and physiology
Cornell notes for anatomy and physiology Holes essential of human anatomy and physiology
Holes essential of human anatomy and physiology Anatomy and physiology unit 7 cardiovascular system
Anatomy and physiology unit 7 cardiovascular system Anatomy and physiology
Anatomy and physiology The speed at which the body consumes energy
The speed at which the body consumes energy Aohs foundations of anatomy and physiology 1
Aohs foundations of anatomy and physiology 1 Animal physiology exam 1
Animal physiology exam 1 Welcome to anatomy and physiology
Welcome to anatomy and physiology Physiology of the foot and ankle
Physiology of the foot and ankle Integumentary system psoriasis
Integumentary system psoriasis Physiology vs anatomy
Physiology vs anatomy Pancreas histology slide
Pancreas histology slide Anatomy and physiology vocabulary
Anatomy and physiology vocabulary Anatomy and physiology
Anatomy and physiology Biceps muscle names
Biceps muscle names Anatomy and physiology
Anatomy and physiology Organ orientation
Organ orientation Anatomy and physiology
Anatomy and physiology Anatomy and physiology
Anatomy and physiology Anatomy and physiology
Anatomy and physiology Dorsifelxion
Dorsifelxion Anatomy and physiology
Anatomy and physiology Anatomy and physiology
Anatomy and physiology Anatomy and physiology
Anatomy and physiology Figure 10-1 blood
Figure 10-1 blood Label o
Label o Anatomy and physiology of eye
Anatomy and physiology of eye Cranial nuclei
Cranial nuclei Irn.org anatomy and physiology
Irn.org anatomy and physiology Anatomy and physiology body parts
Anatomy and physiology body parts Unit 26 animal anatomy physiology and nutrition
Unit 26 animal anatomy physiology and nutrition Figure 14-1 digestive system
Figure 14-1 digestive system Anatomy and physiology of the retina
Anatomy and physiology of the retina Mucous connective tissue
Mucous connective tissue Anatomy and physiology of meningitis ppt
Anatomy and physiology of meningitis ppt Jeopardy anatomy and physiology game
Jeopardy anatomy and physiology game Short note on homeostasis
Short note on homeostasis Anatomy and physiology
Anatomy and physiology Types of respiration in human
Types of respiration in human Anatomy and physiology trivia
Anatomy and physiology trivia Superior mouth fish
Superior mouth fish Brain anatomy and physiology
Brain anatomy and physiology Anatomy and physiology
Anatomy and physiology Long axis of body
Long axis of body 2012 pearson education inc anatomy and physiology
2012 pearson education inc anatomy and physiology Unit 5 anatomy and physiology
Unit 5 anatomy and physiology Fish anatomy and physiology
Fish anatomy and physiology Cengage anatomy and physiology
Cengage anatomy and physiology Fundamentals of information systems 9th edition
Fundamentals of information systems 9th edition Fundamentals of information systems 9th edition
Fundamentals of information systems 9th edition Fluid mechanics fundamentals and applications 3rd edition
Fluid mechanics fundamentals and applications 3rd edition Digital fundamentals floyd
Digital fundamentals floyd Machining fundamentals 10th edition
Machining fundamentals 10th edition Fundamentals of organizational communication 9th edition
Fundamentals of organizational communication 9th edition Fundamentals of organizational communication 9th edition
Fundamentals of organizational communication 9th edition Fundamentals of corporate finance, third canadian edition
Fundamentals of corporate finance, third canadian edition Floyd digital fundamentals ppt
Floyd digital fundamentals ppt Digital fundamentals by floyd
Digital fundamentals by floyd Electronics fundamentals a systems approach
Electronics fundamentals a systems approach Computer security fundamentals 4th edition
Computer security fundamentals 4th edition Management fundamentals 8th edition
Management fundamentals 8th edition Fundamentals of information systems
Fundamentals of information systems Fundamentals of corporate finance third canadian edition
Fundamentals of corporate finance third canadian edition Fundamentals of corporate finance fifth edition
Fundamentals of corporate finance fifth edition Fundamentals of corporate finance 6th edition
Fundamentals of corporate finance 6th edition