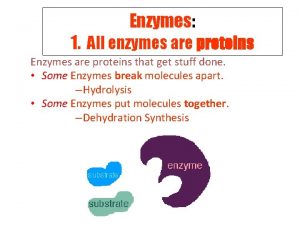
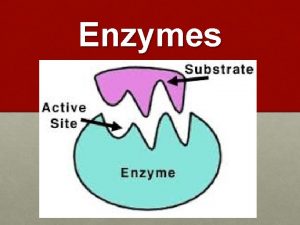
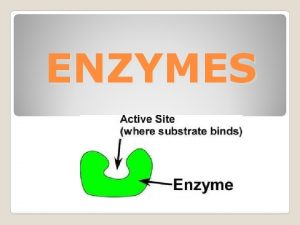
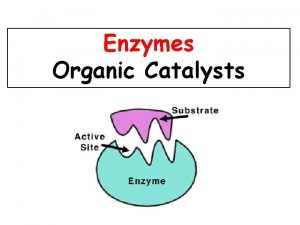
Functions of some Proteins Catalysis enzymes Structural collagen



![Acid Ionization Constant [CH 3 COO-][H+] Ka = ---------[CH 3 COOH] [CH 3 COO-] Acid Ionization Constant [CH 3 COO-][H+] Ka = ---------[CH 3 COOH] [CH 3 COO-]](https://slidetodoc.com/presentation_image_h/c344e5e07000ab702291c1c8eb3ca162/image-22.jpg)
- Slides: 83


Functions of some Proteins Catalysis (enzymes) Structural (collagen) Contractile (muscle) Transport (hemoglobin) Storage (myoglobin) Electron transport (cytochromes) Hormones (insulin) Growth factor (EGF) DNA binding (histones) Ribosomal proteins Toxins and venoms (cholera & melittin) Vision (opsins) Immunoglobins

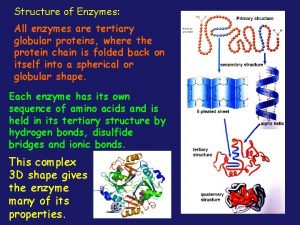
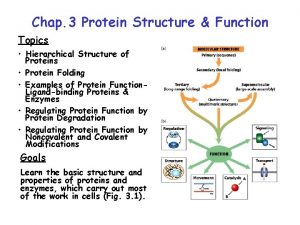
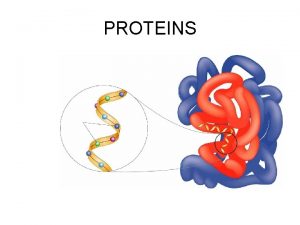
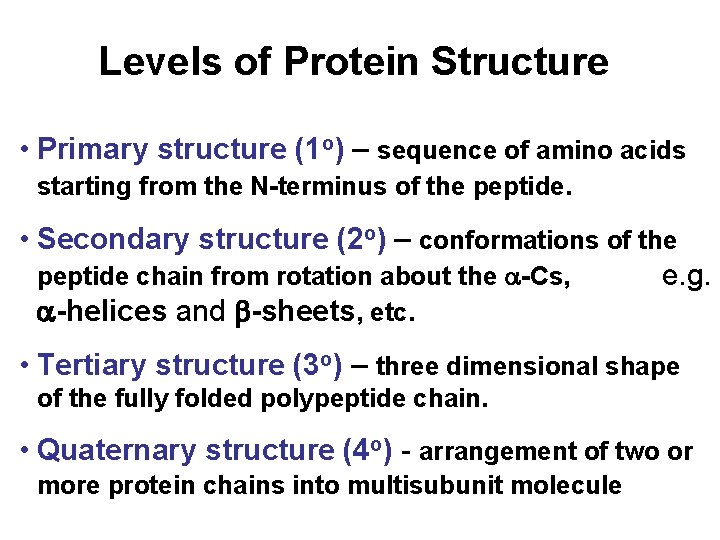
Levels of Protein Structure • Primary structure (1 o) – sequence of amino acids starting from the N-terminus of the peptide. • Secondary structure (2 o) – conformations of the peptide chain from rotation about the a-Cs, e. g. a-helices and b-sheets, etc. • Tertiary structure (3 o) – three dimensional shape of the fully folded polypeptide chain. • Quaternary structure (4 o) - arrangement of two or more protein chains into multisubunit molecule

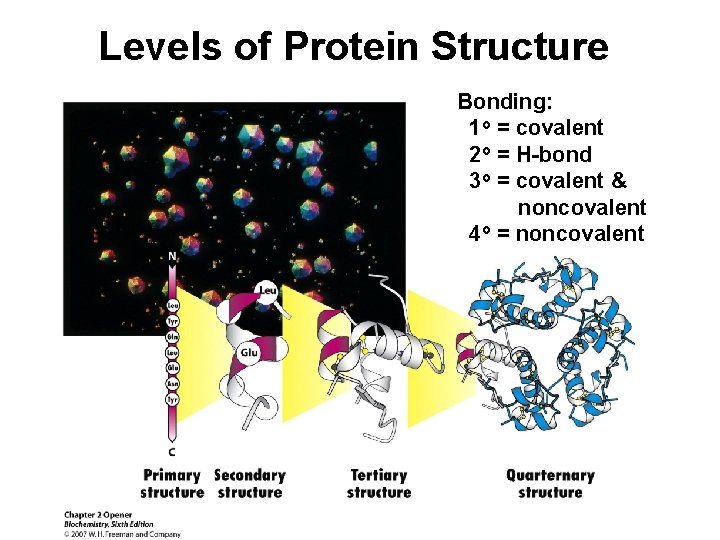
Levels of Protein Structure Bonding: 1 o = covalent 2 o = H-bond 3 o = covalent & noncovalent 4 o = noncovalent

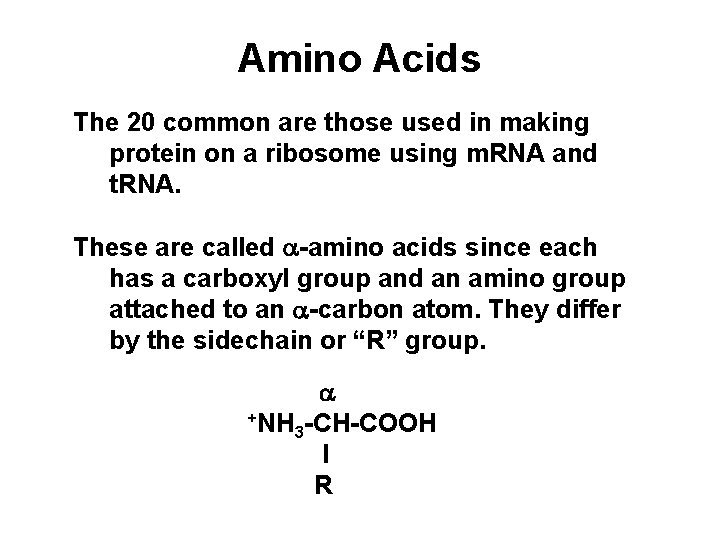
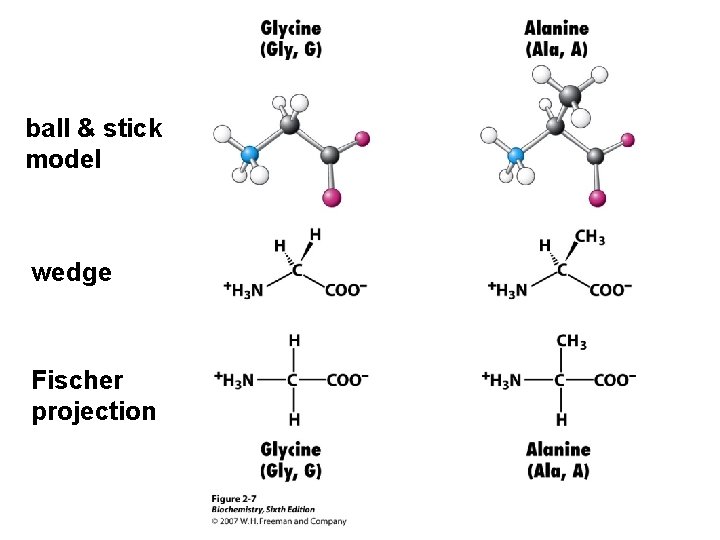
Amino Acids The 20 common are those used in making protein on a ribosome using m. RNA and t. RNA. These are called a-amino acids since each has a carboxyl group and an amino group attached to an a-carbon atom. They differ by the sidechain or “R” group. a +NH -CH-COOH 3 l R

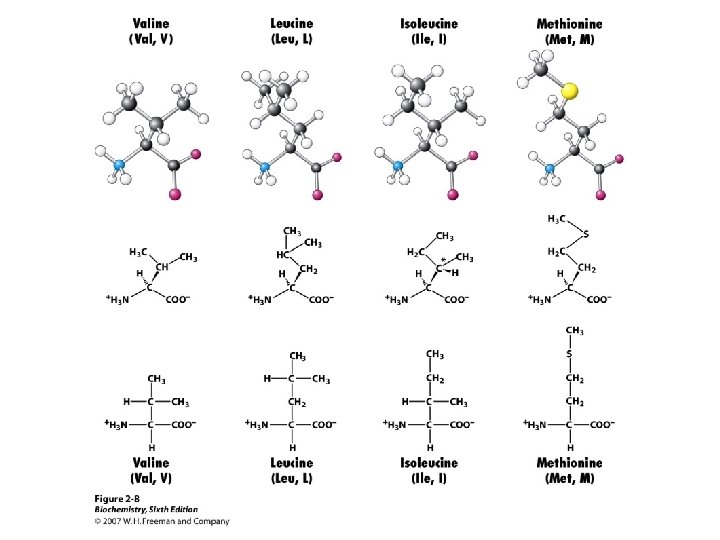
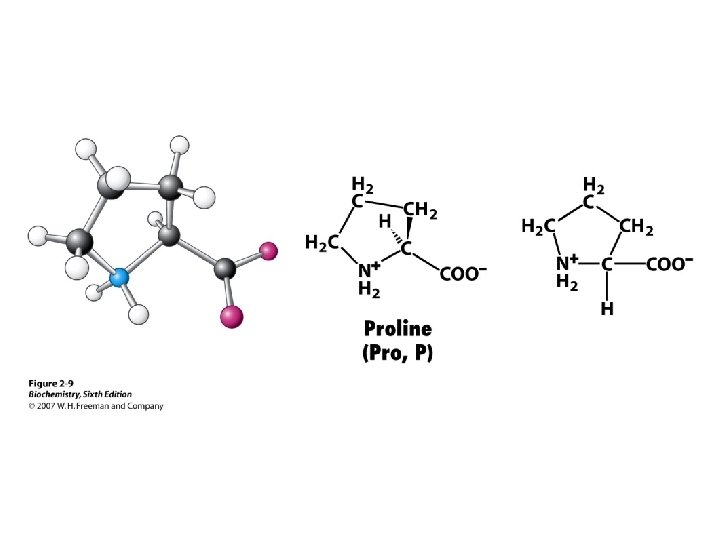
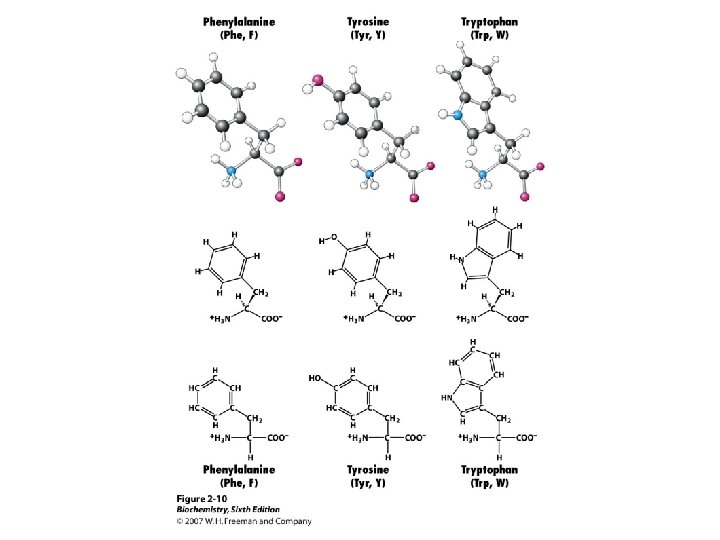
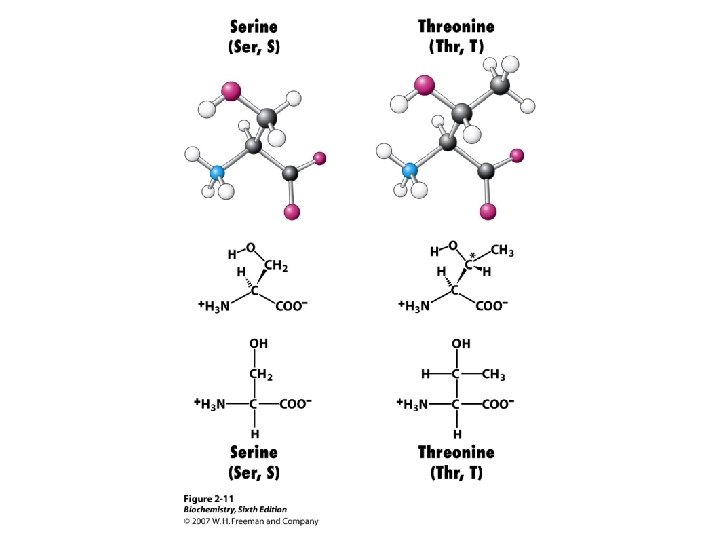
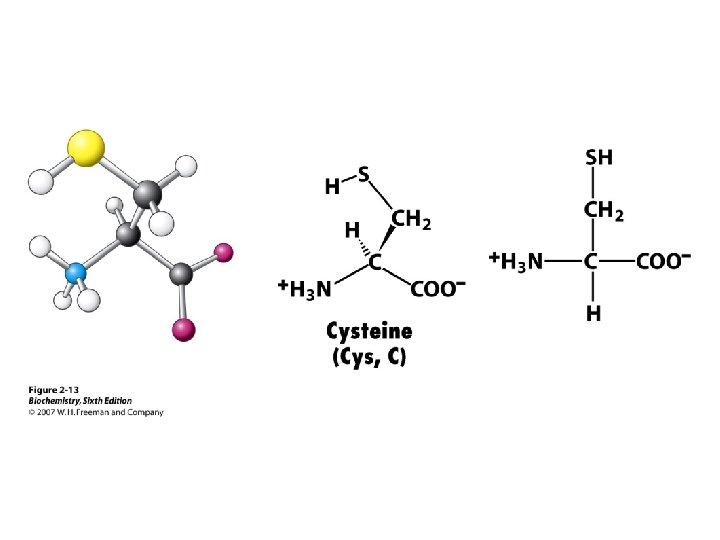
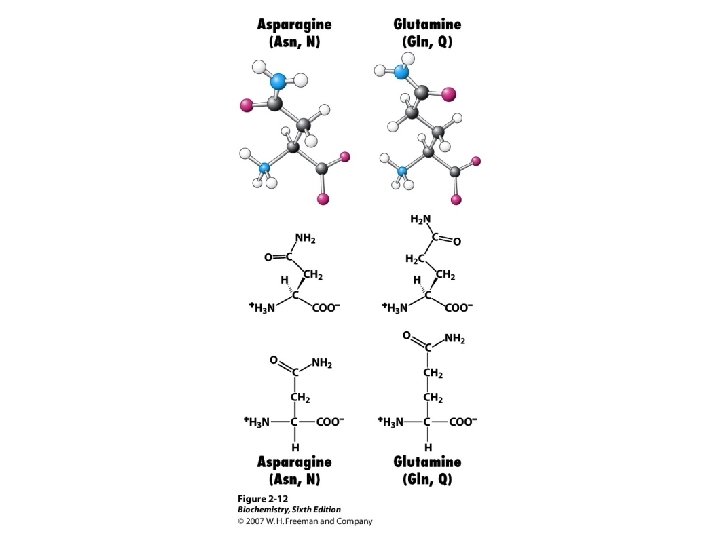
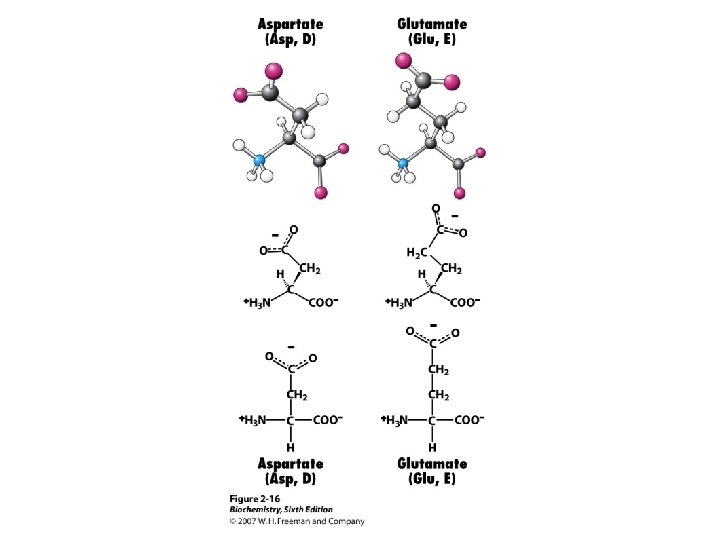
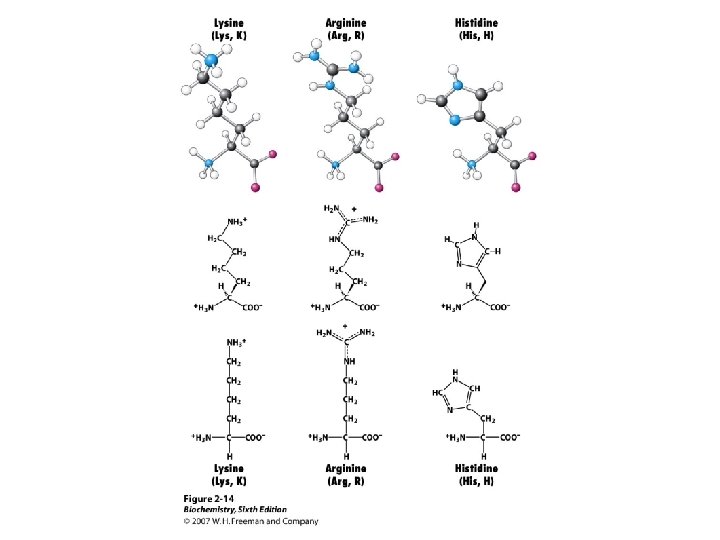
Amino Acid Classification is made using the structure of the side chain, R. (* = essential) 1. None (hydrogen): 2. Non-polar: Aliphatic: Alicyclic: 3. Aromatic: 4. Polar uncharged: 5. Thiol: 6. Acidic: 7. Basic: Gly Ala, Val*, Leu*, Ile*, Met* Pro Phe*, Tyr, Trp* Ser, Thr*, Asn, Gln Cys Asp, Glu Lys*, His*, Arg*

ball & stick model wedge Fischer projection









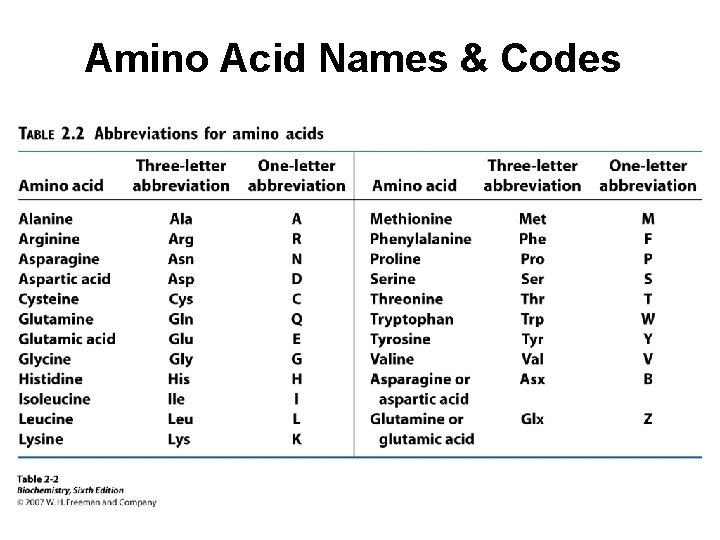
Amino Acid Names & Codes

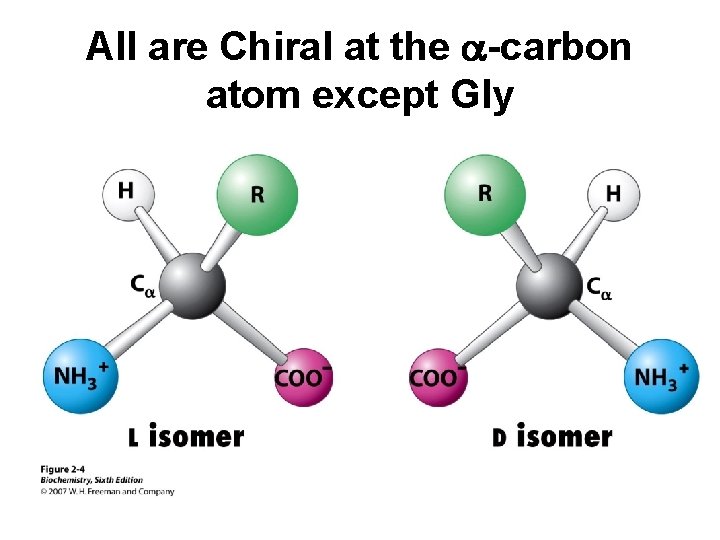
All are Chiral at the a-carbon atom except Gly

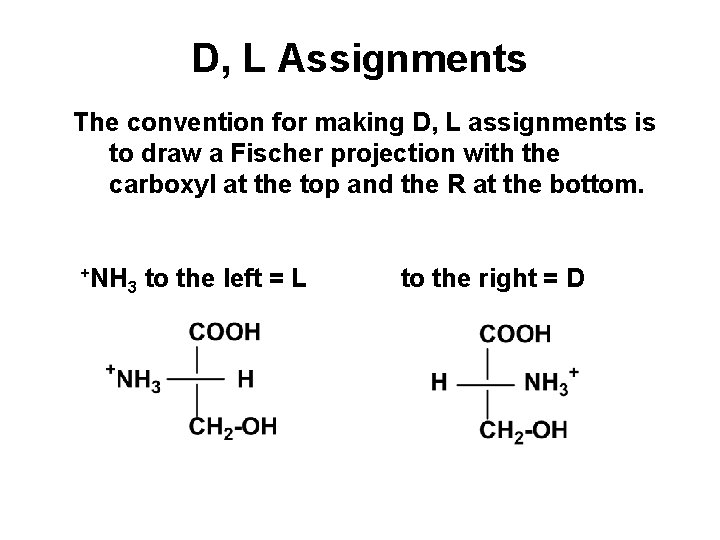
D, L Assignments The convention for making D, L assignments is to draw a Fischer projection with the carboxyl at the top and the R at the bottom. +NH 3 to the left = L to the right = D

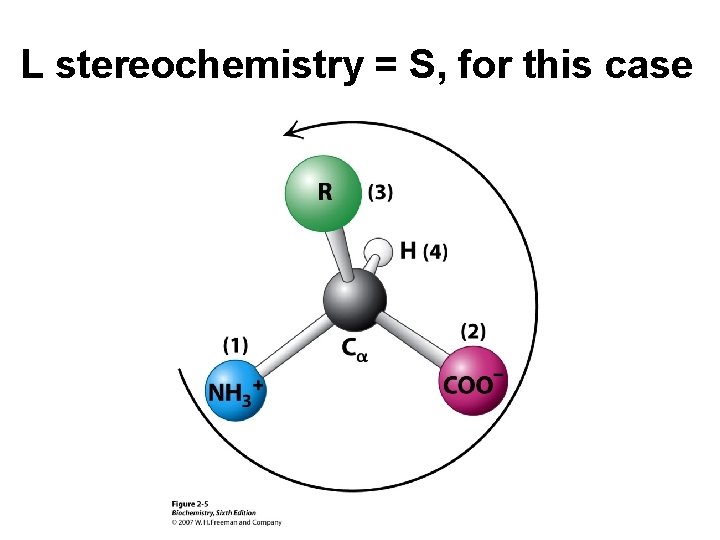
L stereochemistry = S, for this case

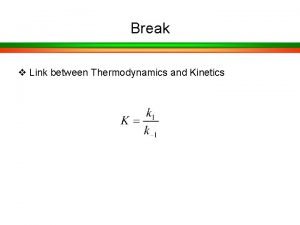
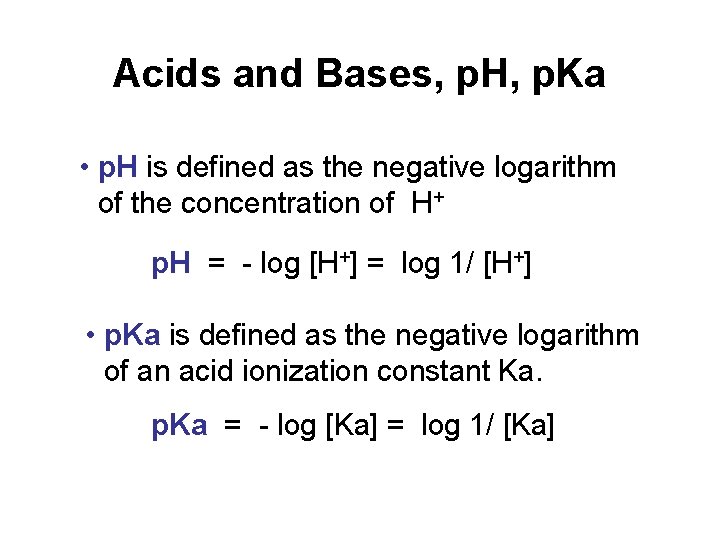
Acids and Bases, p. H, p. Ka • p. H is defined as the negative logarithm of the concentration of H+ p. H = - log [H+] = log 1/ [H+] • p. Ka is defined as the negative logarithm of an acid ionization constant Ka. p. Ka = - log [Ka] = log 1/ [Ka]

Acetic Acid is a Weak Acid • Weak acids and bases do not ionize (dissociate) completely in H 2 O. CH 3 COOH <==> CH 3 COO- + H+ acetic acid acetate anion conjugate acid form conjugate base form
![Acid Ionization Constant CH 3 COOH Ka CH 3 COOH CH 3 COO Acid Ionization Constant [CH 3 COO-][H+] Ka = ---------[CH 3 COOH] [CH 3 COO-]](https://slidetodoc.com/presentation_image_h/c344e5e07000ab702291c1c8eb3ca162/image-22.jpg)
Acid Ionization Constant [CH 3 COO-][H+] Ka = ---------[CH 3 COOH] [CH 3 COO-] -log Ka = -log [H+] + -log --------[CH 3 COOH] [CH 3 COO-] p. H = p. Ka + log --------[CH 3 COOH]

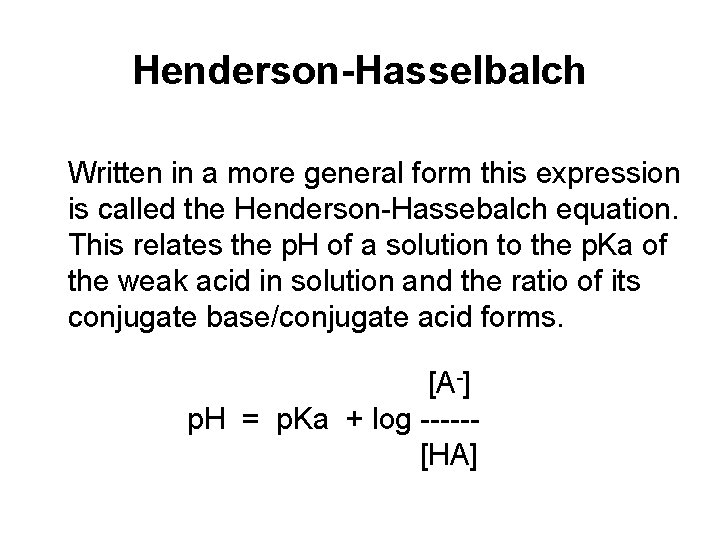
Henderson-Hasselbalch Written in a more general form this expression is called the Henderson-Hassebalch equation. This relates the p. H of a solution to the p. Ka of the weak acid in solution and the ratio of its conjugate base/conjugate acid forms. [A-] p. H = p. Ka + log -----[HA]

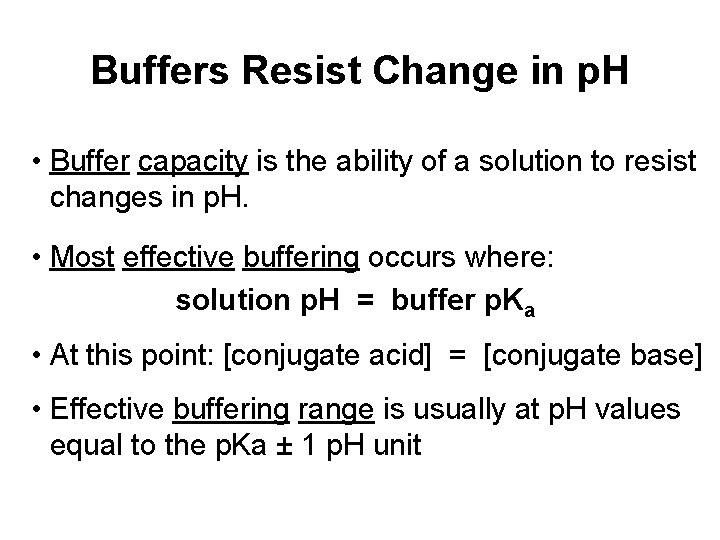
Buffers Resist Change in p. H • Buffer capacity is the ability of a solution to resist changes in p. H. • Most effective buffering occurs where: solution p. H = buffer p. Ka • At this point: [conjugate acid] = [conjugate base] • Effective buffering range is usually at p. H values equal to the p. Ka ± 1 p. H unit

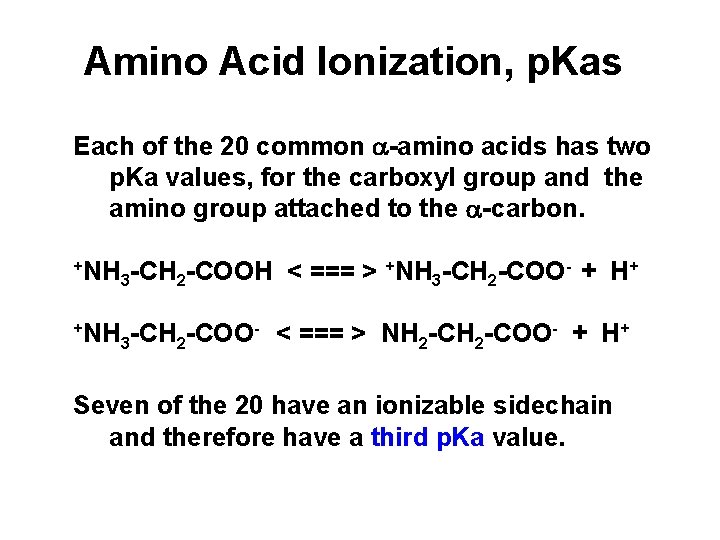
Amino Acid Ionization, p. Kas Each of the 20 common a-amino acids has two p. Ka values, for the carboxyl group and the amino group attached to the a-carbon. +NH -CH -COO- + H+ -CH -COOH < === > 3 2 +NH - < === > NH -COO- + H+ -CH -COO 3 2 2 2 Seven of the 20 have an ionizable sidechain and therefore have a third p. Ka value.

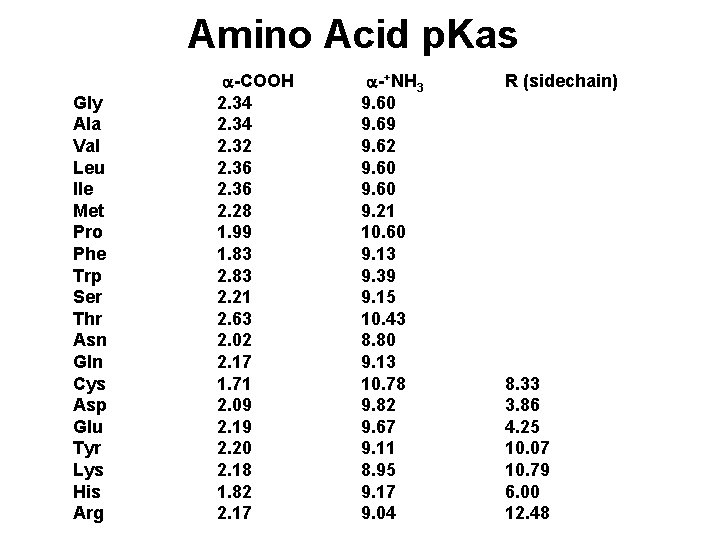
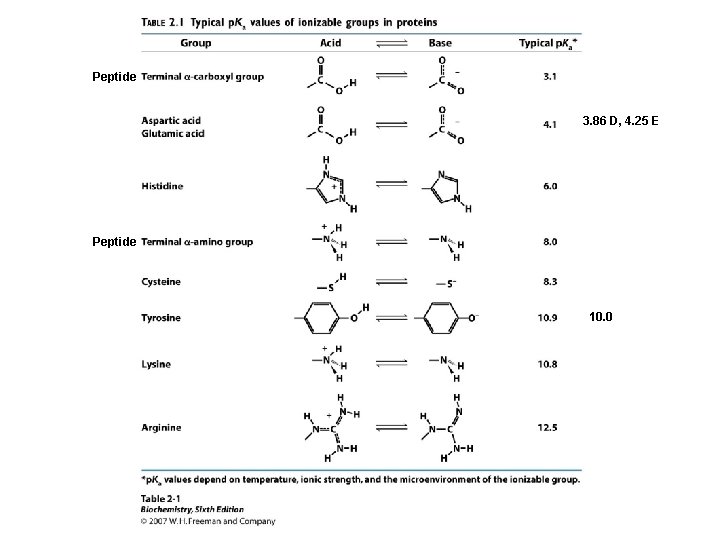
Amino Acid p. Kas Gly Ala Val Leu Ile Met Pro Phe Trp Ser Thr Asn Gln Cys Asp Glu Tyr Lys His Arg a-COOH 2. 34 2. 32 2. 36 2. 28 1. 99 1. 83 2. 21 2. 63 2. 02 2. 17 1. 71 2. 09 2. 19 2. 20 2. 18 1. 82 2. 17 a-+NH 3 9. 60 9. 69 9. 62 9. 60 9. 21 10. 60 9. 13 9. 39 9. 15 10. 43 8. 80 9. 13 10. 78 9. 82 9. 67 9. 11 8. 95 9. 17 9. 04 R (sidechain) 8. 33 3. 86 4. 25 10. 07 10. 79 6. 00 12. 48

Peptide 3. 86 D, 4. 25 E Peptide 10. 0

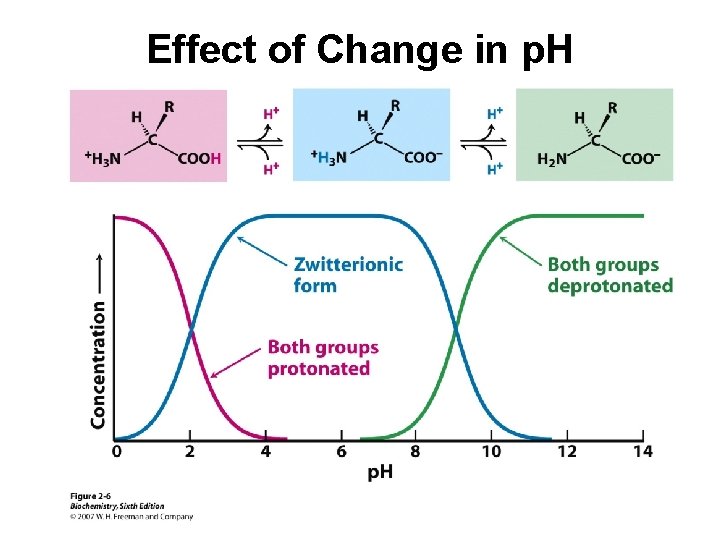
Effect of Change in p. H

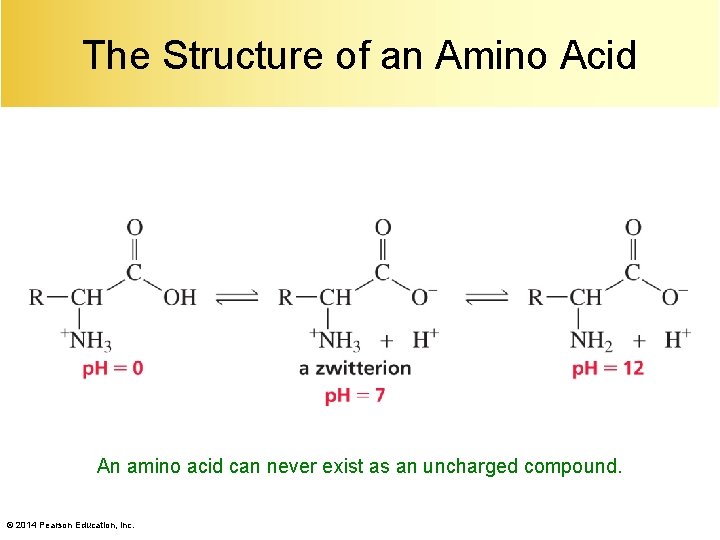
The Structure of an Amino Acid An amino acid can never exist as an uncharged compound. © 2014 Pearson Education, Inc.

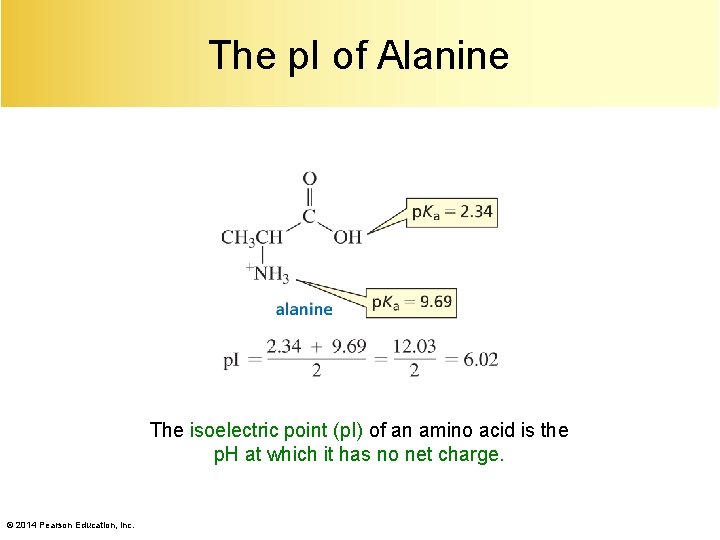
The p. I of Alanine The isoelectric point (p. I) of an amino acid is the p. H at which it has no net charge. © 2014 Pearson Education, Inc.

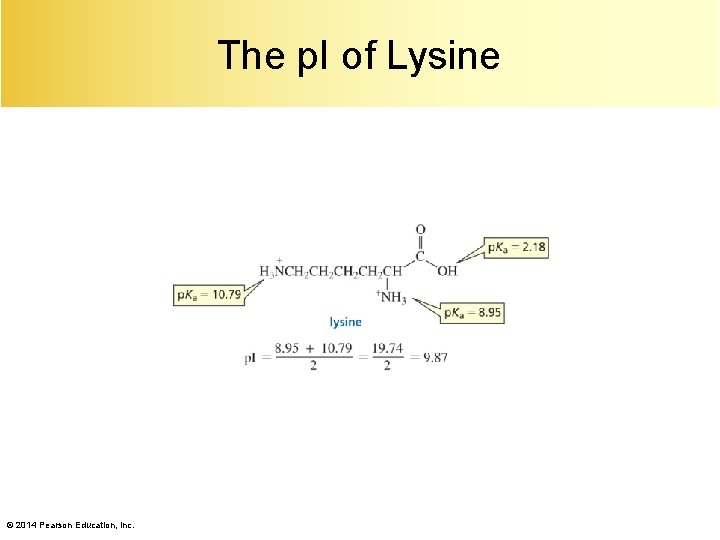
The p. I of Lysine © 2014 Pearson Education, Inc.

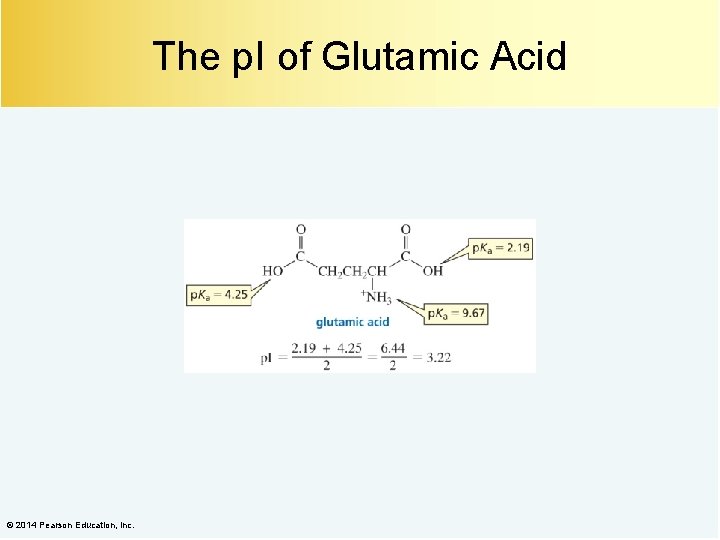
The p. I of Glutamic Acid © 2014 Pearson Education, Inc.

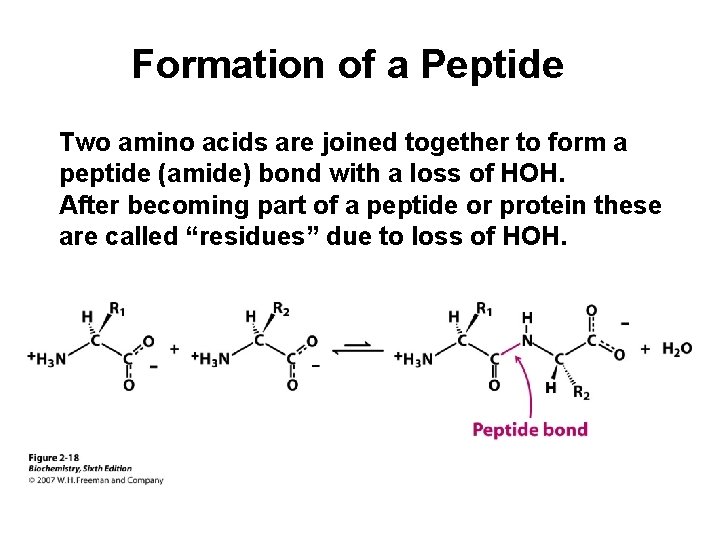
Formation of a Peptide Two amino acids are joined together to form a peptide (amide) bond with a loss of HOH. After becoming part of a peptide or protein these are called “residues” due to loss of HOH.

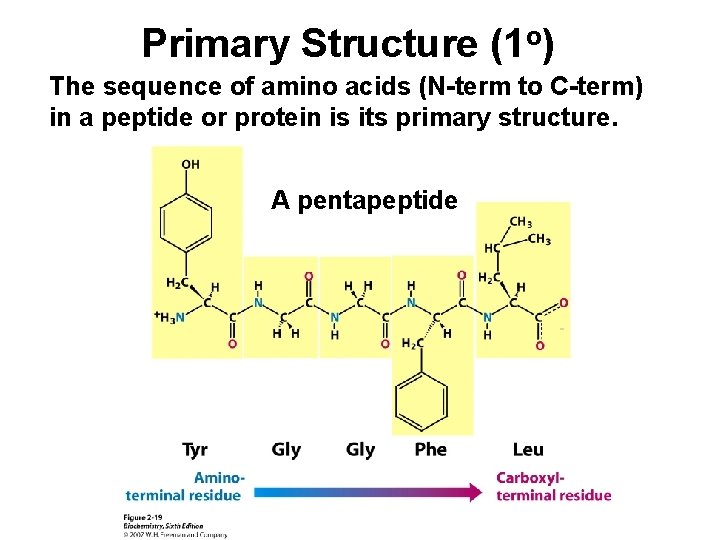
Primary Structure (1 o) The sequence of amino acids (N-term to C-term) in a peptide or protein is its primary structure. A pentapeptide

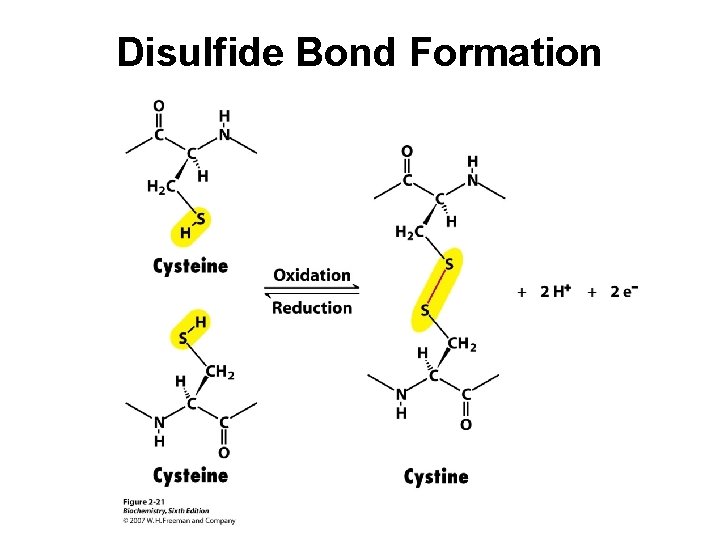
Disulfide Bond Formation

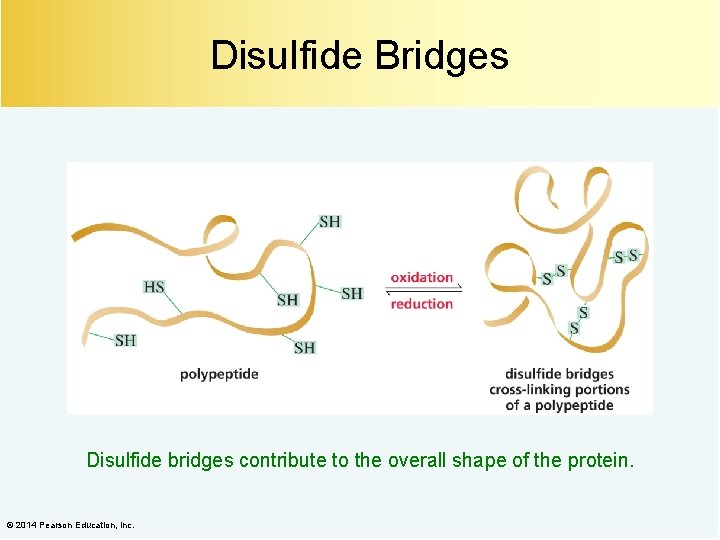
Disulfide Bridges Disulfide bridges contribute to the overall shape of the protein. © 2014 Pearson Education, Inc.

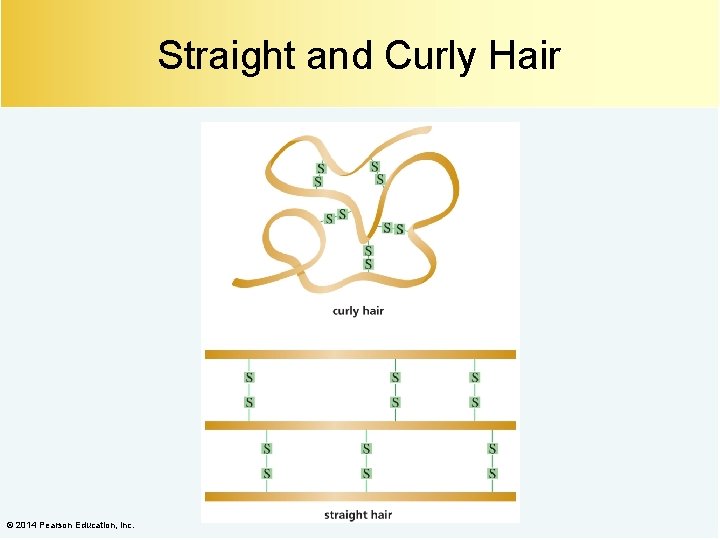
Straight and Curly Hair © 2014 Pearson Education, Inc.

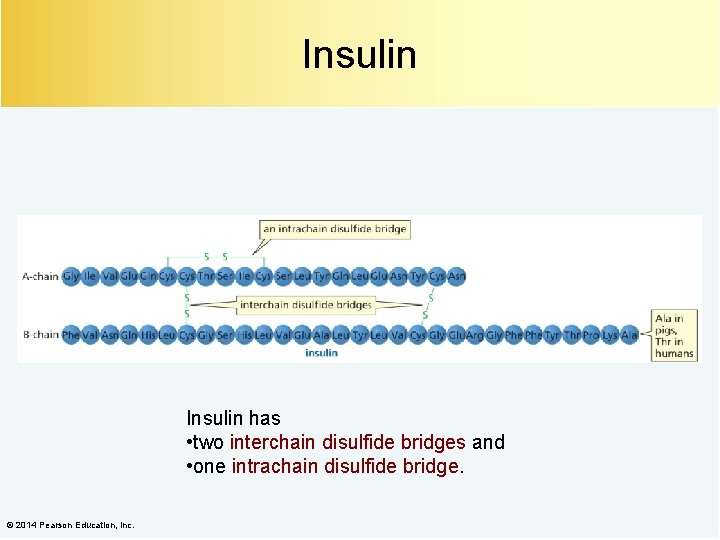
Insulin has • two interchain disulfide bridges and • one intrachain disulfide bridge. © 2014 Pearson Education, Inc.

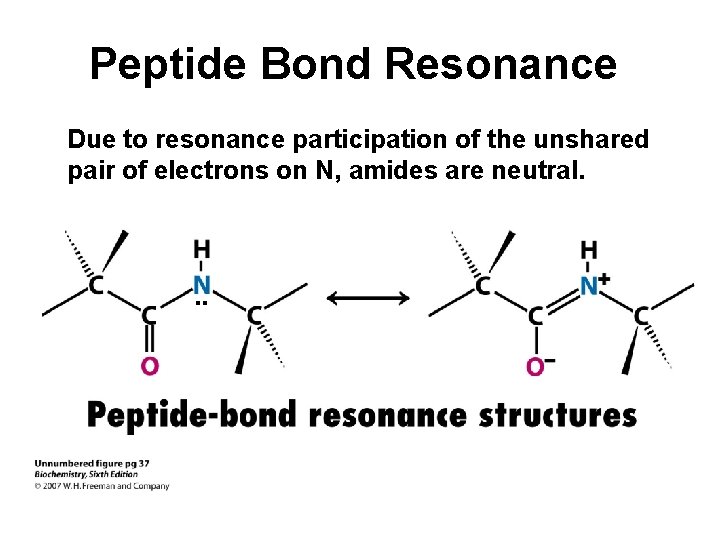
Peptide Bond Resonance Due to resonance participation of the unshared pair of electrons on N, amides are neutral. . .

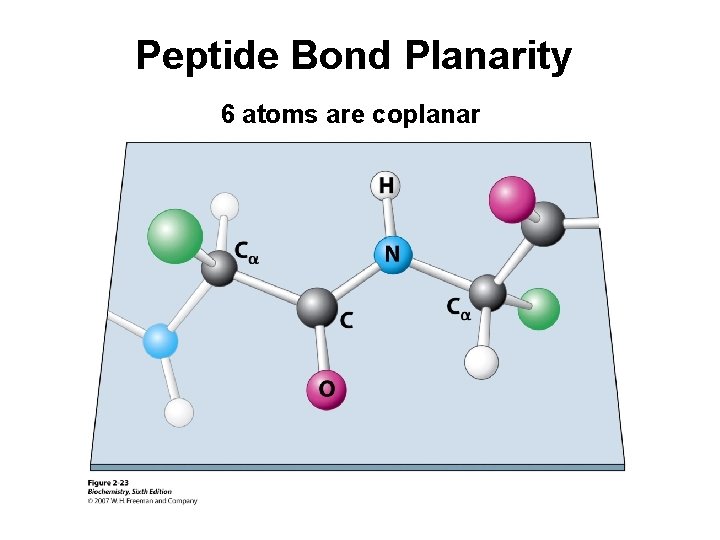
Peptide Bond Planarity 6 atoms are coplanar

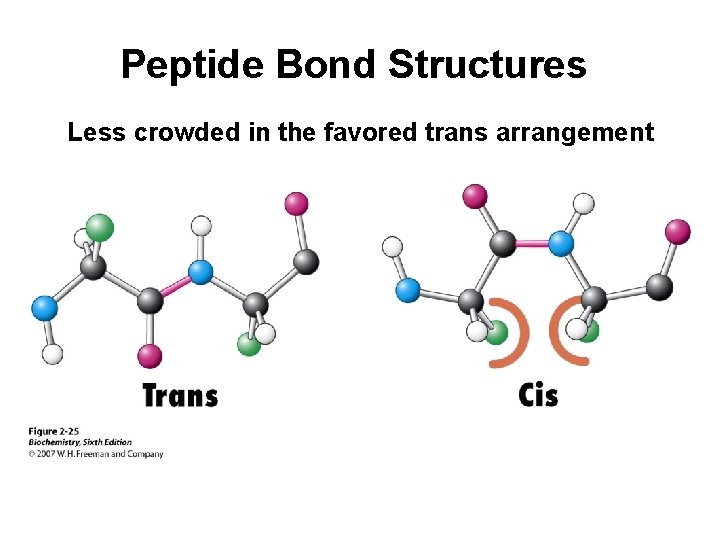
Peptide Bond Structures Less crowded in the favored trans arrangement

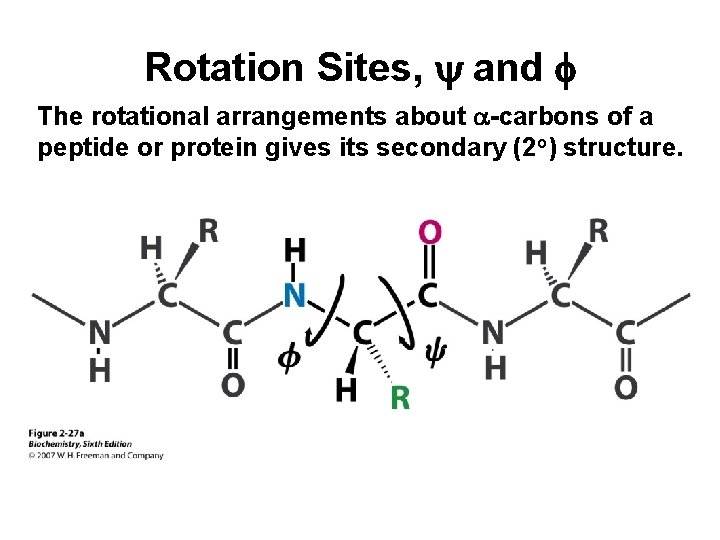
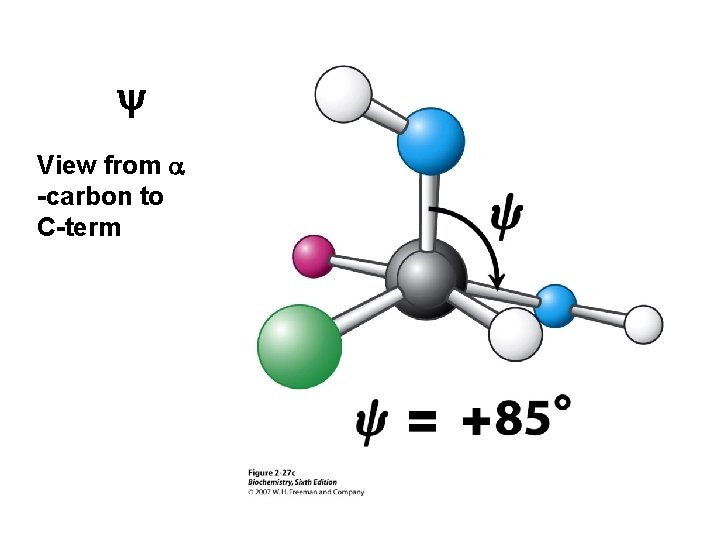
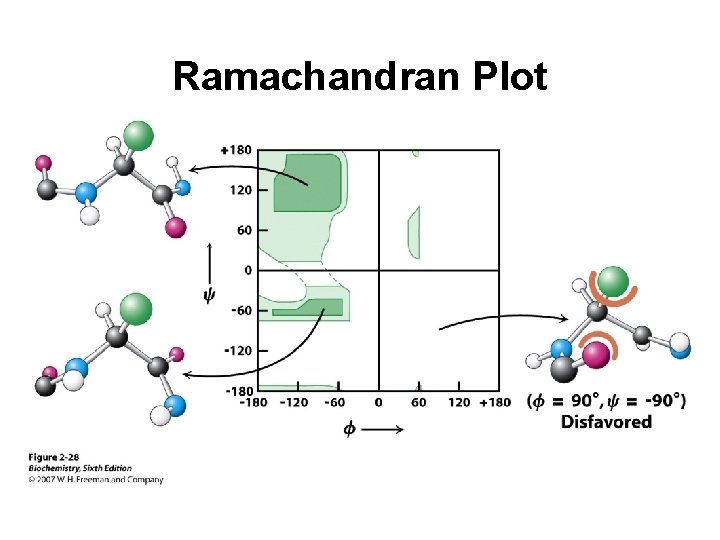
Rotation Sites, y and f The rotational arrangements about a-carbons of a peptide or protein gives its secondary (2 o) structure.

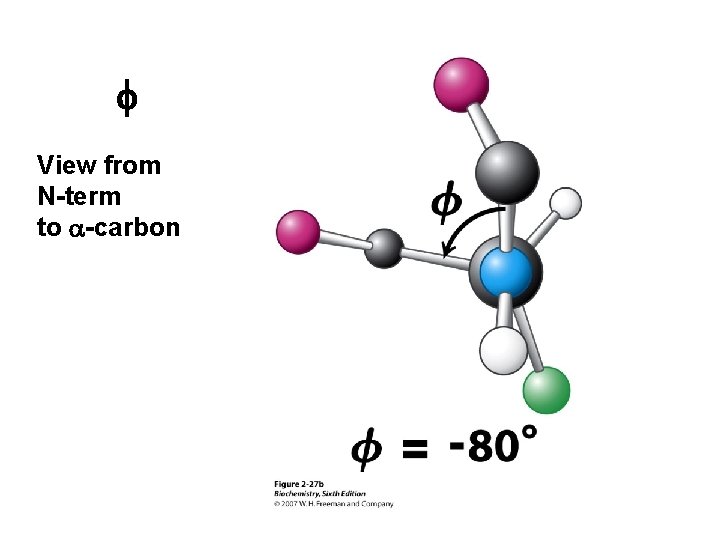
f View from N-term to a-carbon

y View from a -carbon to C-term

Ramachandran Plot

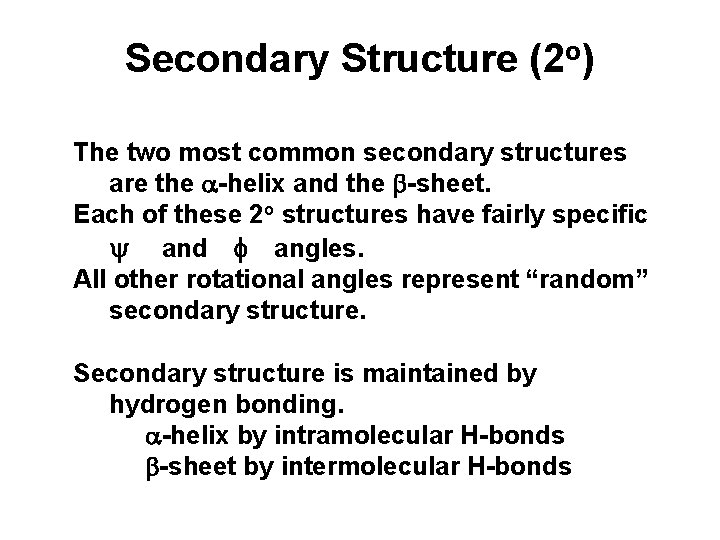
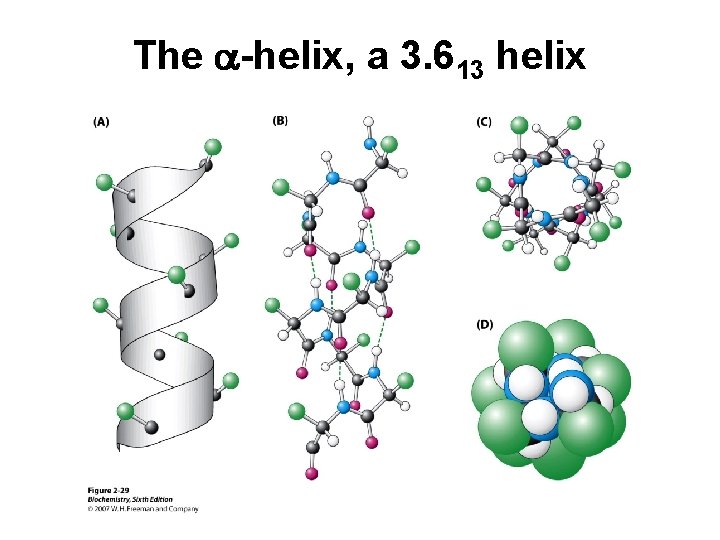
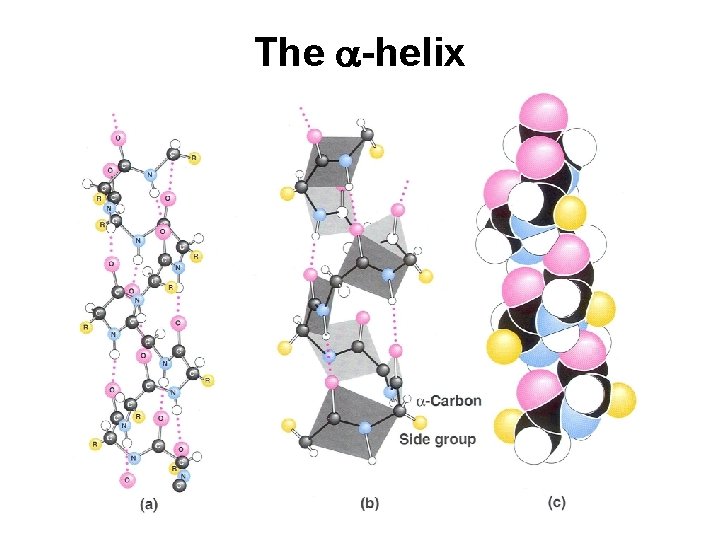
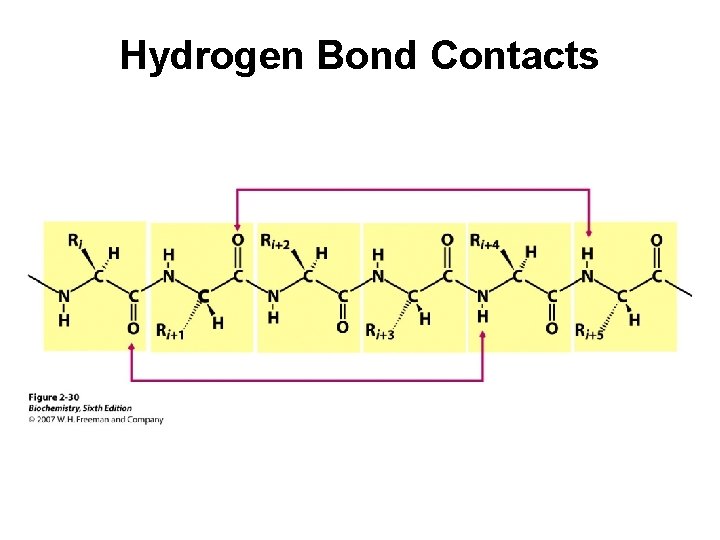
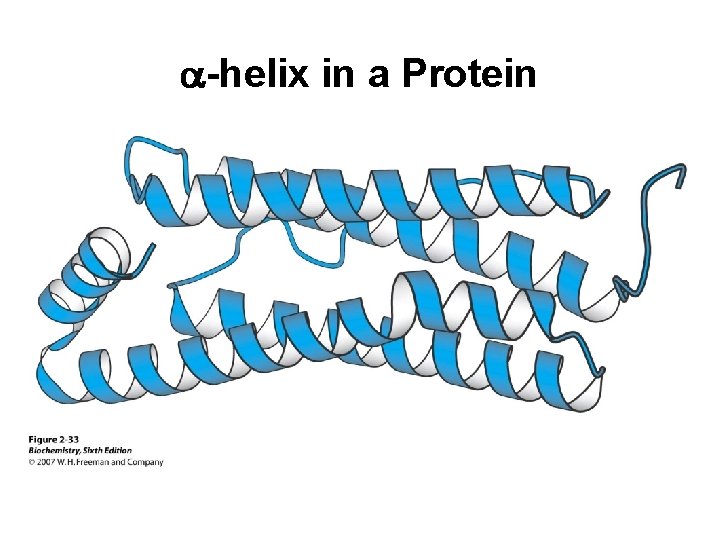
Secondary Structure (2 o) The two most common secondary structures are the a-helix and the b-sheet. Each of these 2 o structures have fairly specific y and f angles. All other rotational angles represent “random” secondary structure. Secondary structure is maintained by hydrogen bonding. a-helix by intramolecular H-bonds b-sheet by intermolecular H-bonds

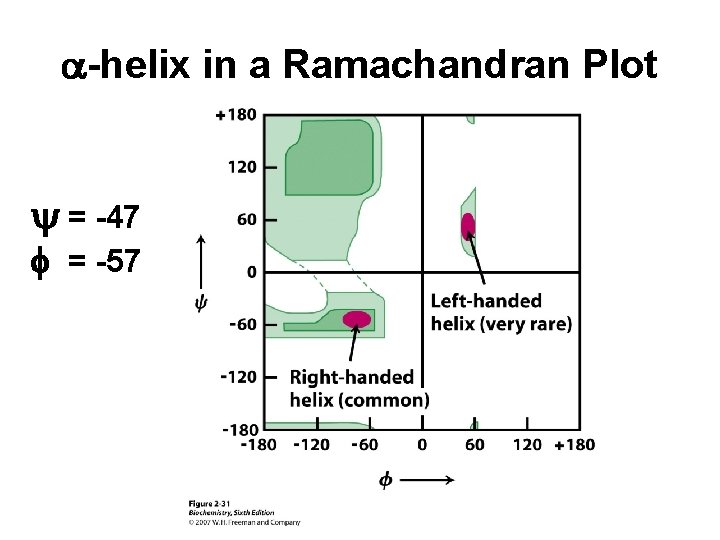
a-helix in a Ramachandran Plot y = -47 f = -57

The a-helix, a 3. 613 helix

The a-helix

Hydrogen Bond Contacts

a-helix in a Protein

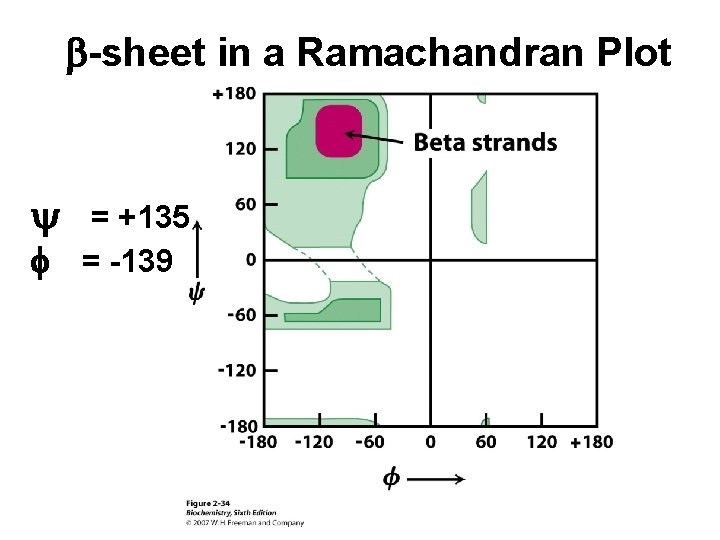
b-sheet in a Ramachandran Plot y f = +135 = -139

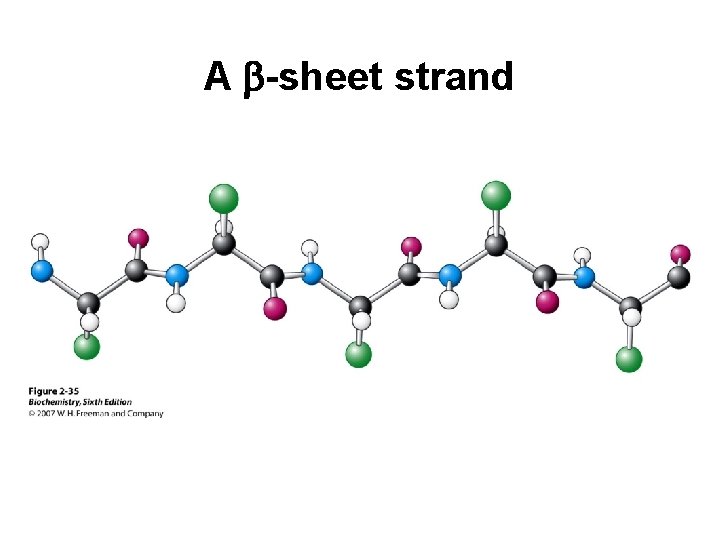
A b-sheet strand

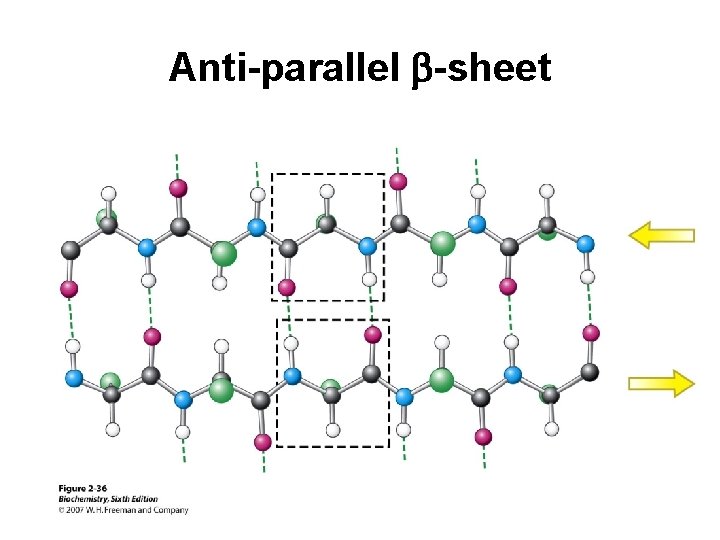
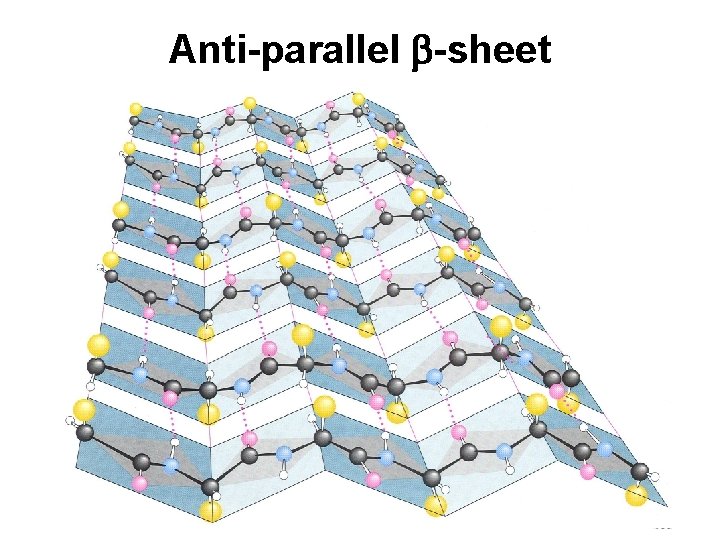
Anti-parallel b-sheet

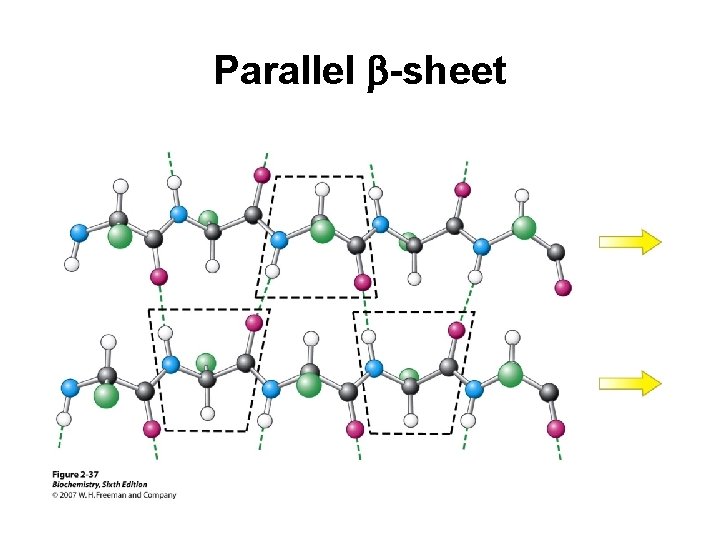
Parallel b-sheet

Anti-parallel b-sheet

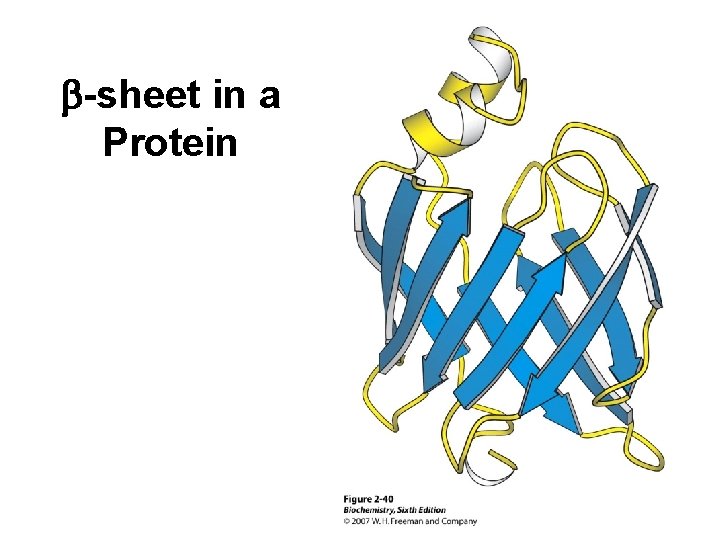
b-sheet in a Protein

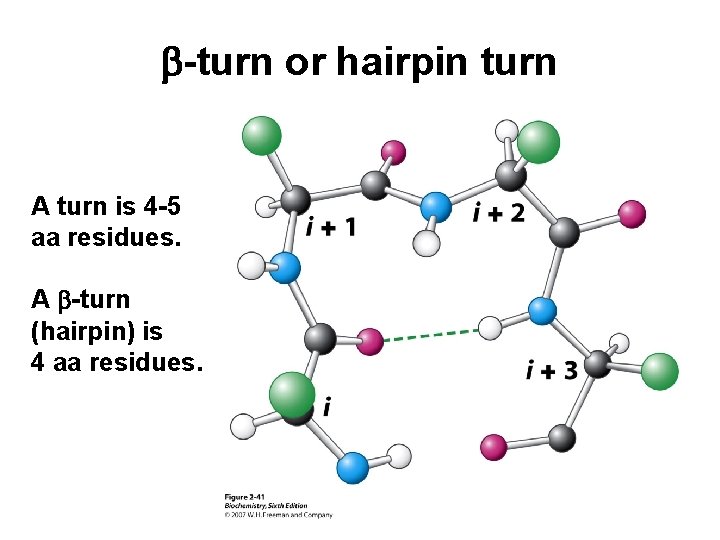
b-turn or hairpin turn A turn is 4 -5 aa residues. A b-turn (hairpin) is 4 aa residues.

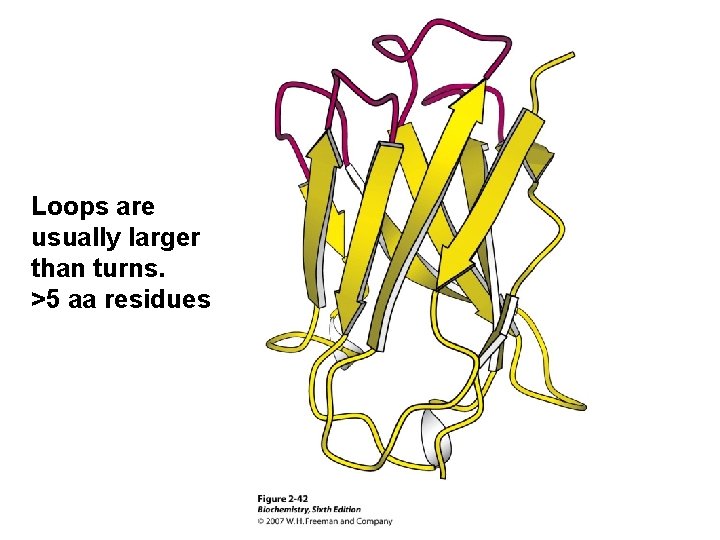
Loops are usually larger than turns. >5 aa residues

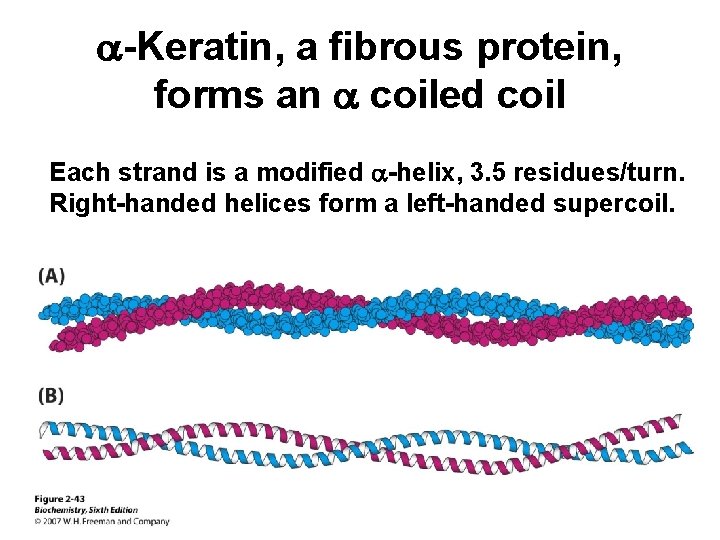
a-Keratin, a fibrous protein, forms an a coiled coil Each strand is a modified a-helix, 3. 5 residues/turn. Right-handed helices form a left-handed supercoil.

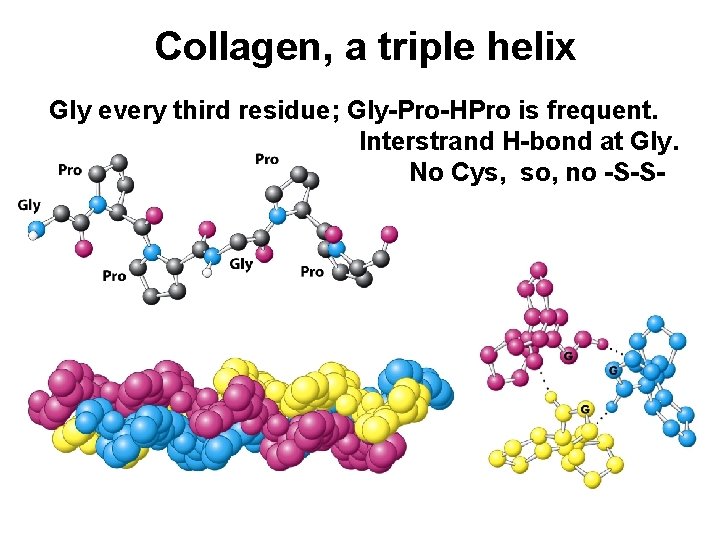
Collagen, a triple helix Gly every third residue; Gly-Pro-HPro is frequent. Interstrand H-bond at Gly. No Cys, so, no -S-S-

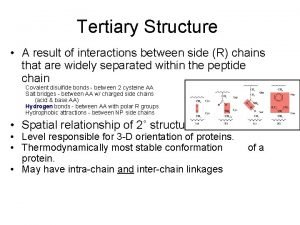
Tertiary Structure (3 o) The three dimensional folding of a polypeptide is its tertiary structure. Both the a-helix and b-sheet may exist within the tertiary structure. Generally the distribution of amino acid sidechains in a globular protein finds mostly nonpolar residues in the interior of the protein and polar residues on the surface. Tertiary structure is maintained by noncovalent interactions and disulfide bonding.

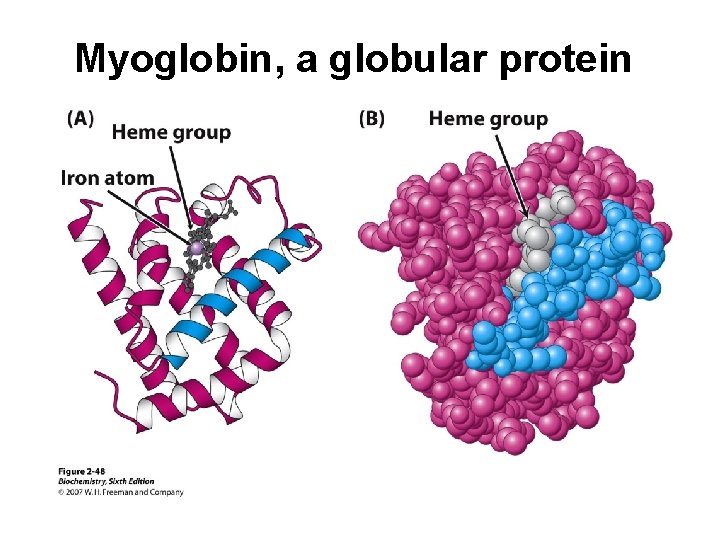
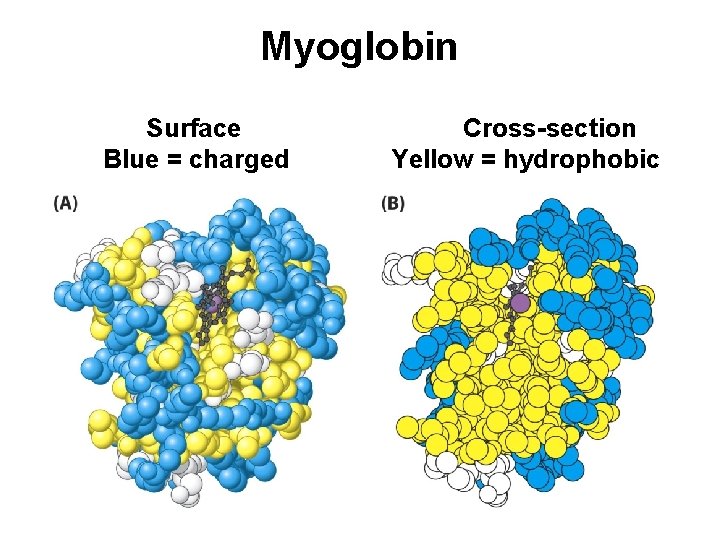
Myoglobin, a globular protein

Myoglobin Surface Blue = charged Cross-section Yellow = hydrophobic

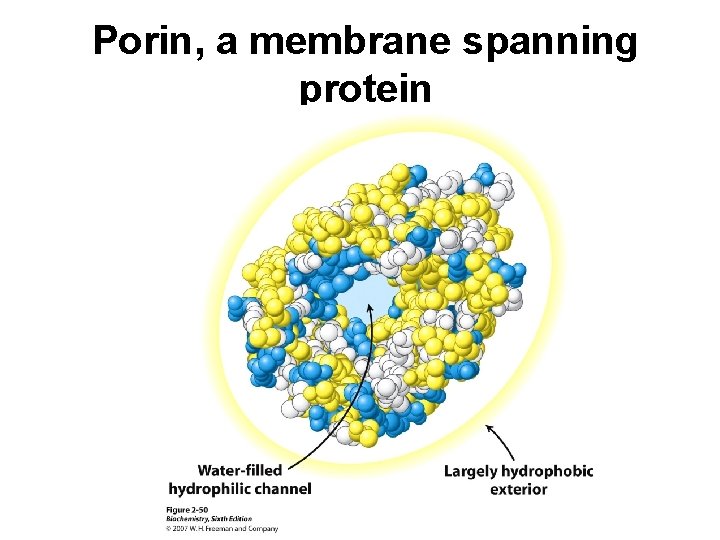
Porin, a membrane spanning protein

Domain A domain is a discrete globular area within protein. There are four general types: All a All b Mixed a/b a+b only a- helices and loops only b- sheet & loops or turns alternating abab cluster of a then cluster of b

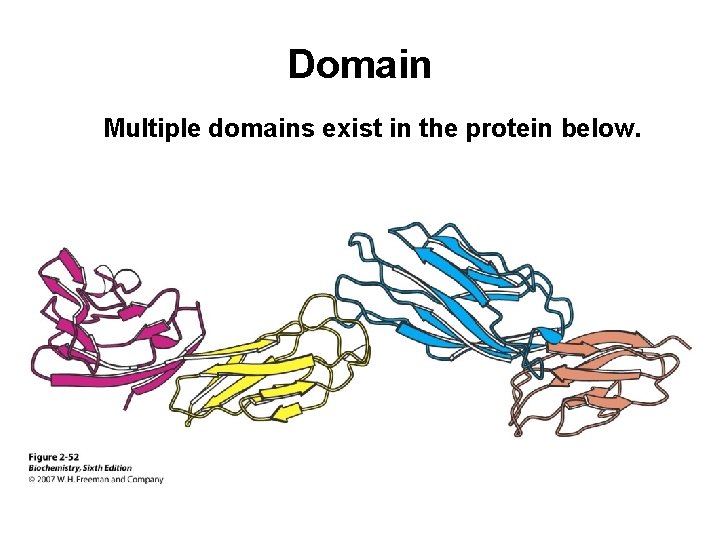
Domain Multiple domains exist in the protein below.

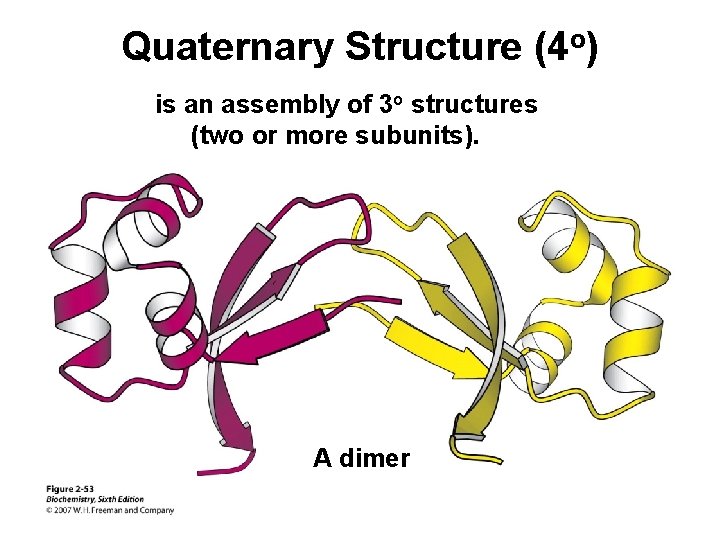
Quaternary Structure (4 o) is an assembly of 3 o structures (two or more subunits). A dimer

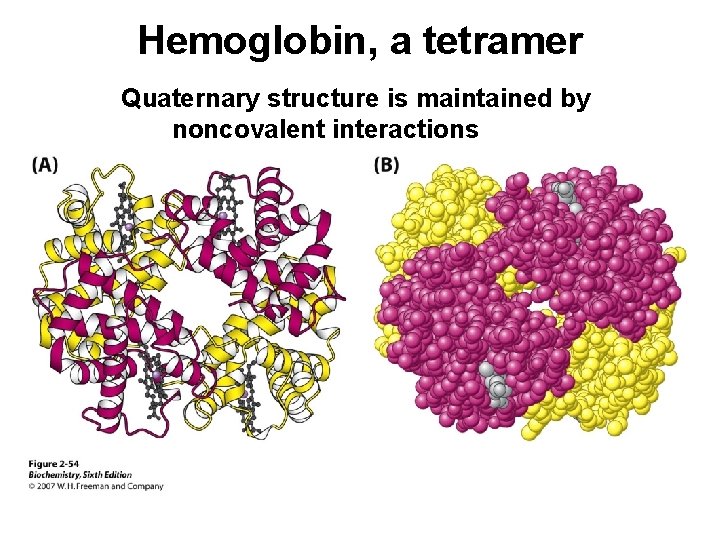
Hemoglobin, a tetramer Quaternary structure is maintained by noncovalent interactions

Denaturing Proteins Denaturing agents destroy the protein 3 o structure (causes the protein to unravel). Methods: Heat; Extremes of p. H; Detergents; Mechanical agitation; Mercaptoethanol – breaks -S-S- bonds; 6 M guanidine HCl or 10 M urea: these are chaotropic agents that break up noncovalent interactions.

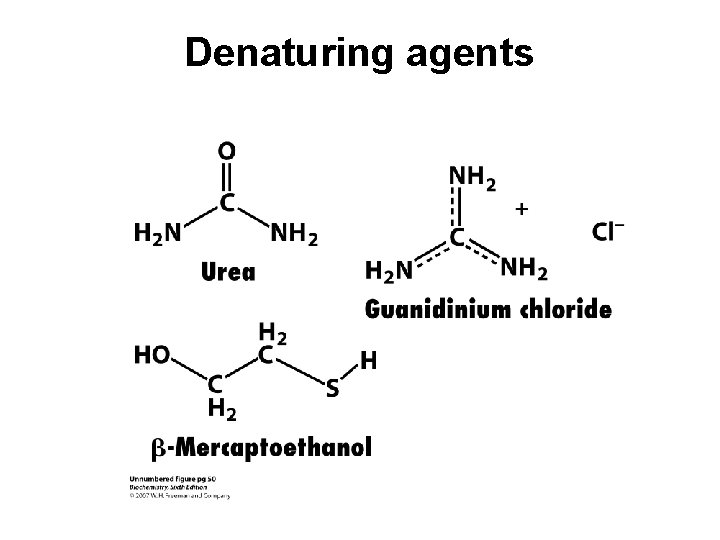
Denaturing agents

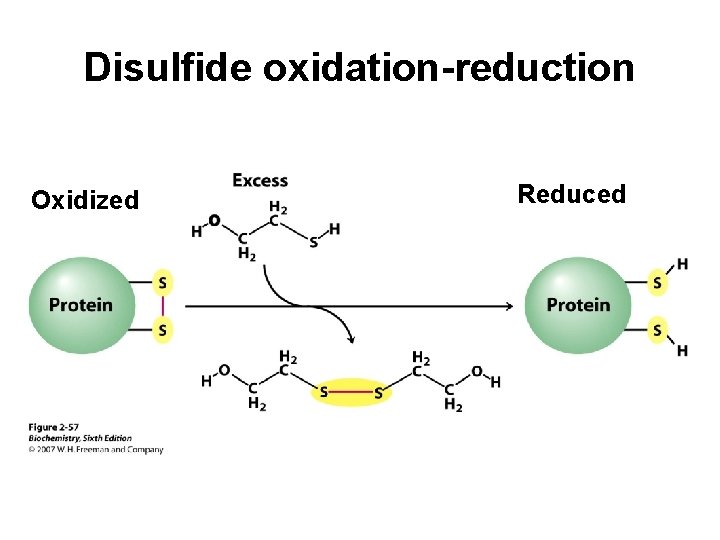
Disulfide oxidation-reduction Oxidized Reduced

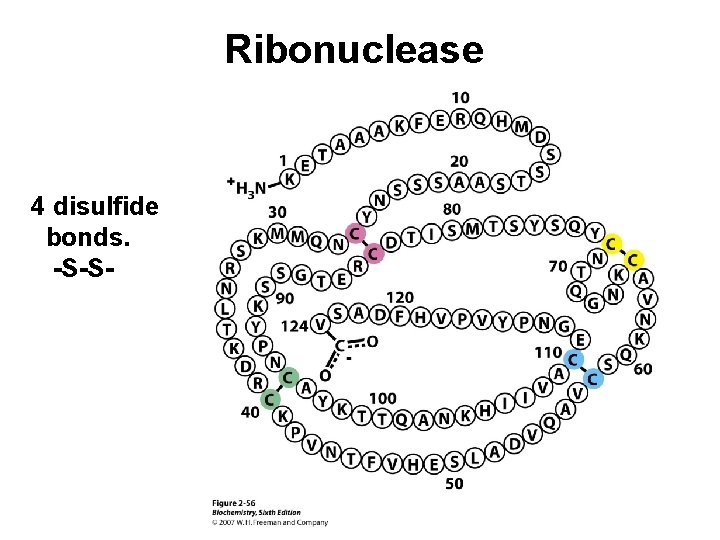
Ribonuclease 4 disulfide bonds. -S-S-

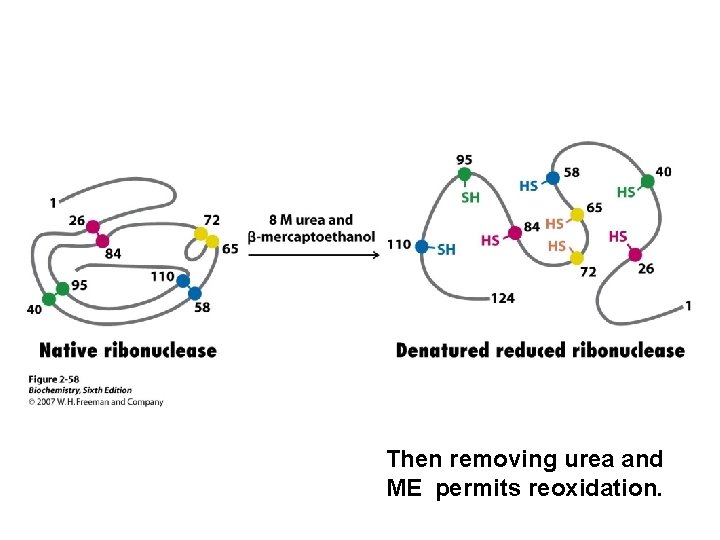
Then removing urea and ME permits reoxidation.

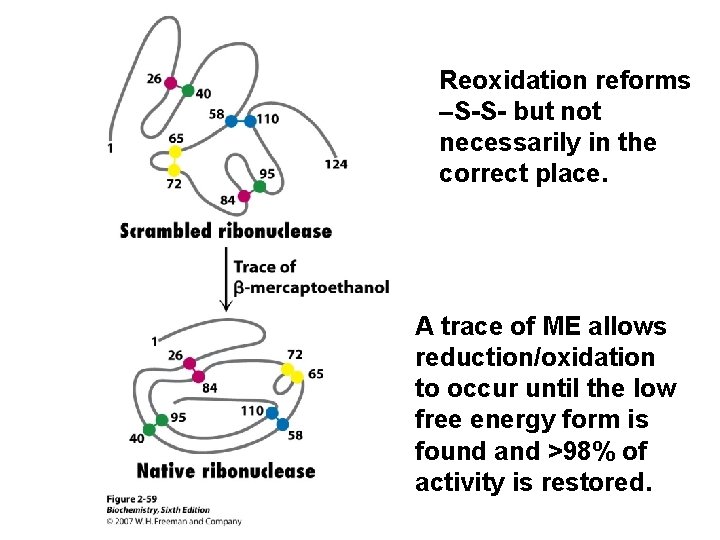
Reoxidation reforms –S-S- but not necessarily in the correct place. A trace of ME allows reduction/oxidation to occur until the low free energy form is found and >98% of activity is restored.

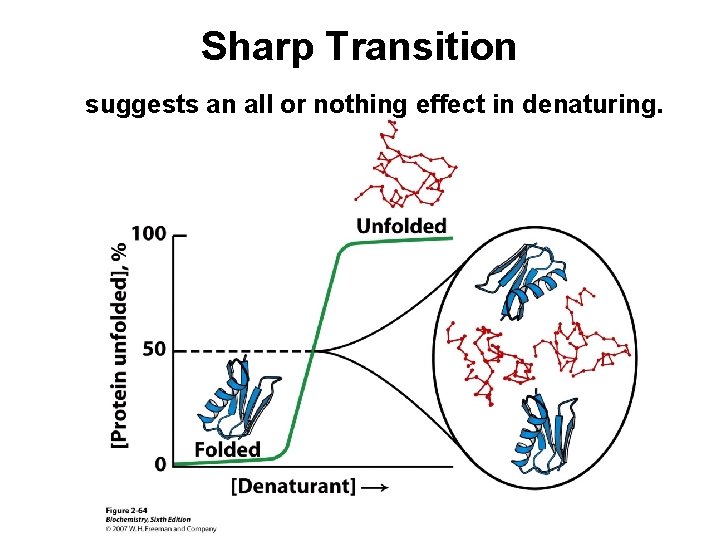
Sharp Transition suggests an all or nothing effect in denaturing.

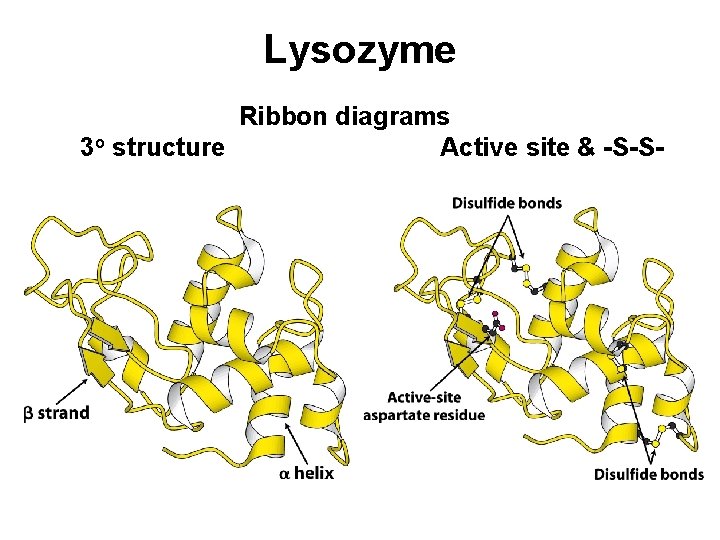
Lysozyme Ribbon diagrams 3 o structure Active site & -S-S-

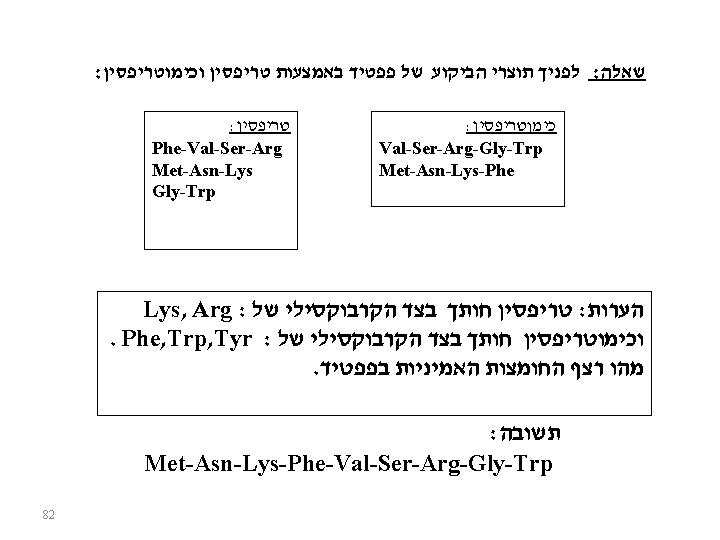
Protein Cleavage Protein sequencing is most manageable with small polypeptides. Therefore, in order to sequence a large protein, it must be cleaved into smaller pieces. Cleavage is conducted using either chemical or enzymatic methods. The pieces must be separated and purified before sequencing.

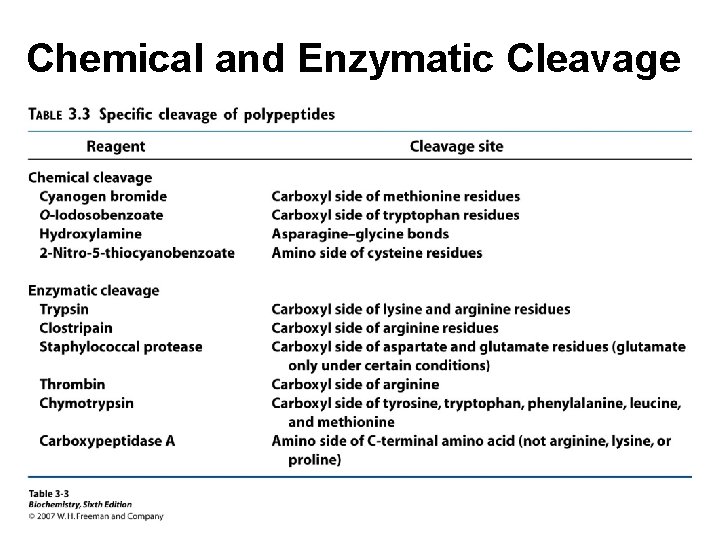
Chemical and Enzymatic Cleavage

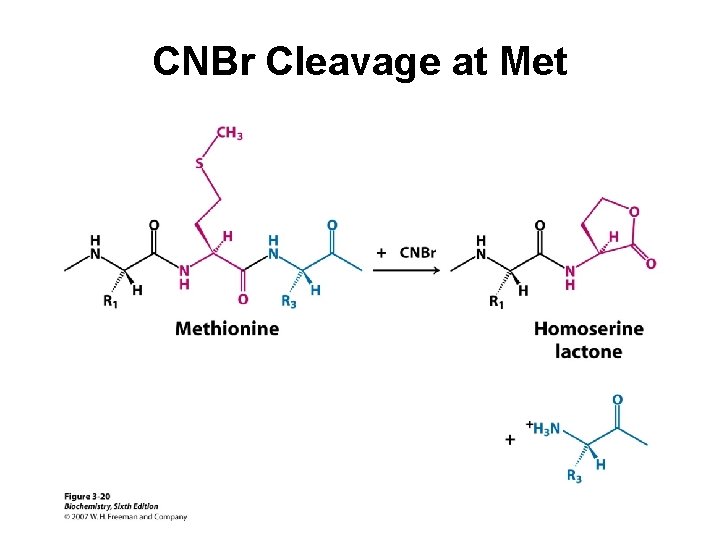
CNBr Cleavage at Met

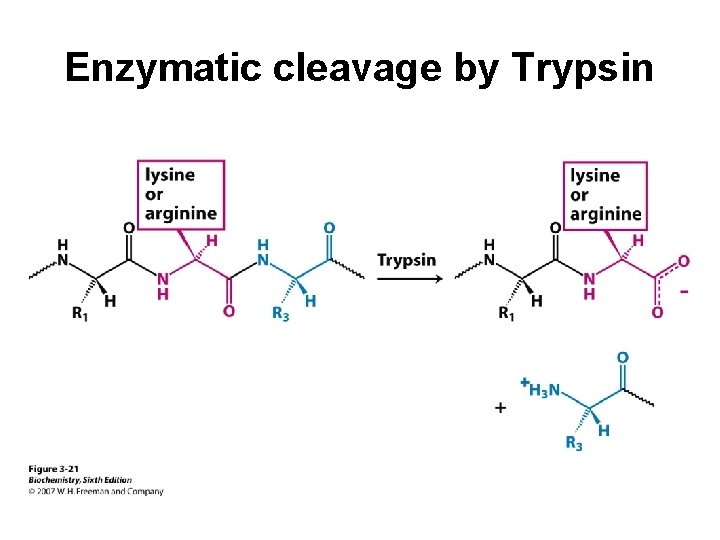
Enzymatic cleavage by Trypsin


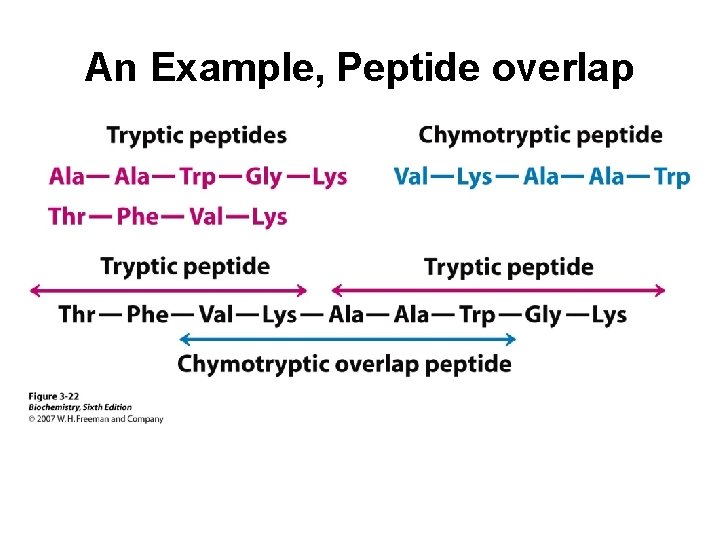
An Example, Peptide overlap
 All enzymes are globular proteins
All enzymes are globular proteins Not all enzymes are proteins
Not all enzymes are proteins Structural proteins function
Structural proteins function Thin
Thin Structural role of proteins
Structural role of proteins What is the difference between hbv and lbv
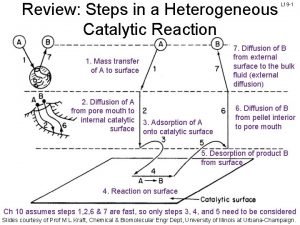
What is the difference between hbv and lbv 7 steps of heterogeneous catalysis
7 steps of heterogeneous catalysis Enzymatic catalysis
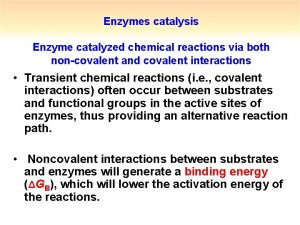
Enzymatic catalysis Catalysis by approximation
Catalysis by approximation What is covalent catalysis
What is covalent catalysis What is covalent catalysis
What is covalent catalysis Specific acid base catalysis
Specific acid base catalysis Specific acid base catalysis
Specific acid base catalysis Specific acid base catalysis
Specific acid base catalysis Mixed inhibitor km and vmax
Mixed inhibitor km and vmax Langmuir-hinshelwood mechanism heterogeneous catalysis
Langmuir-hinshelwood mechanism heterogeneous catalysis Erzeng
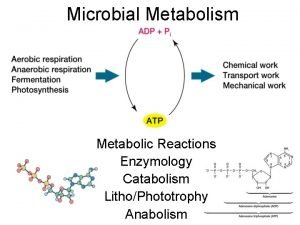
Erzeng Energy catalysis and biosynthesis
Energy catalysis and biosynthesis Catalytic combustion
Catalytic combustion Sodium hydroxide iupac id sodium oxidanide
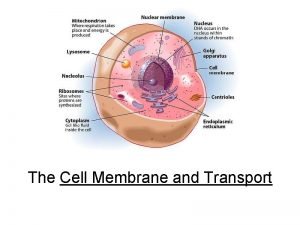
Sodium hydroxide iupac id sodium oxidanide Structure of protein
Structure of protein Glycoprotein vs glycolipid
Glycoprotein vs glycolipid Transport
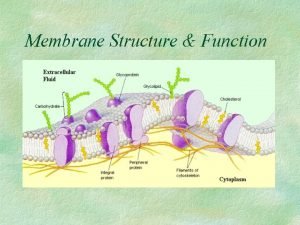
Transport Functions of membrane proteins
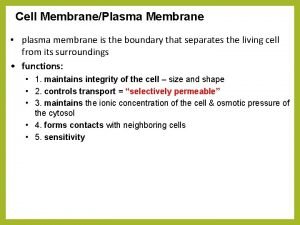
Functions of membrane proteins Cell membrane selectively permeable
Cell membrane selectively permeable Functions and importance of proteins
Functions and importance of proteins Function of plasma protein
Function of plasma protein Types of globulin
Types of globulin Name the enzymes secreted by small intestine
Name the enzymes secreted by small intestine Digestive enzymes and their functions table
Digestive enzymes and their functions table Examples of enzymes
Examples of enzymes Cocoa collagen sendayu tinggi
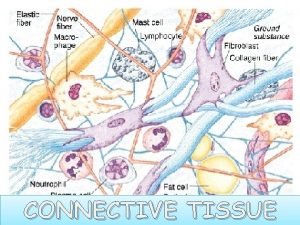
Cocoa collagen sendayu tinggi Classification of connective tissue
Classification of connective tissue Collagen is a major component of
Collagen is a major component of Matrix vesicle theory of mineralization
Matrix vesicle theory of mineralization Connective tissue description
Connective tissue description Totally derma collagen
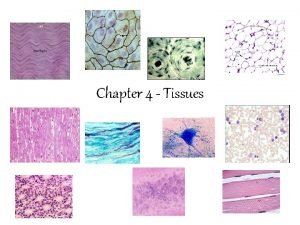
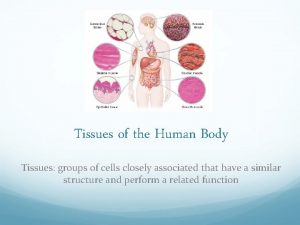
Totally derma collagen Types of tissues
Types of tissues Totally derma tub
Totally derma tub Fibrous proteins
Fibrous proteins Collagen fibre structure
Collagen fibre structure Parkinson's disease
Parkinson's disease Dr. alireza peyman
Dr. alireza peyman Unicellular organisms
Unicellular organisms They say sometimes you win some
They say sometimes you win some Sometimes you win some
Sometimes you win some Ice cream is countable or uncountable
Ice cream is countable or uncountable Force and motion
Force and motion Fire and ice diamante poem
Fire and ice diamante poem Some say the world will end in fire some say in ice
Some say the world will end in fire some say in ice Some trust in chariots and some in horses song
Some trust in chariots and some in horses song Intensity transformation functions
Intensity transformation functions Function of a chromosomes
Function of a chromosomes Carrier vs channel proteins
Carrier vs channel proteins Globular vs fibrous proteins
Globular vs fibrous proteins Section 8-1 carbohydrates fats and proteins answer key
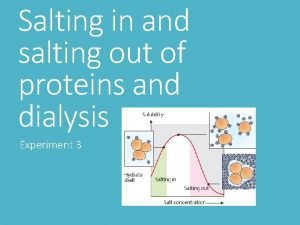
Section 8-1 carbohydrates fats and proteins answer key Salting in salting out
Salting in salting out Complementary proteins
Complementary proteins Example of denatured protein
Example of denatured protein Primary derived proteins
Primary derived proteins Monomers protein
Monomers protein Protein digestion
Protein digestion Protein folding
Protein folding Proteins database
Proteins database Bradford method
Bradford method Dr nutrition
Dr nutrition Motifs and domains of proteins
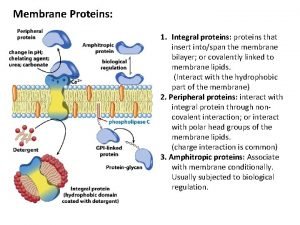
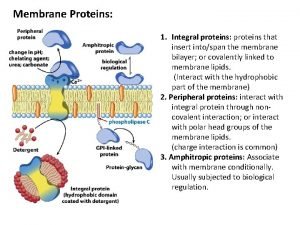
Motifs and domains of proteins Peripheral vs integral proteins
Peripheral vs integral proteins Precipitation of proteins by strong mineral acids
Precipitation of proteins by strong mineral acids Salting out proteins
Salting out proteins Salting out proteins
Salting out proteins Salt bridges in proteins
Salt bridges in proteins Higher order structure of proteins
Higher order structure of proteins Carrier vs channel proteins
Carrier vs channel proteins Salting in
Salting in Biomedical importance of proteins
Biomedical importance of proteins Organic compounds such as proteins and starches are too
Organic compounds such as proteins and starches are too Three peptide chains woven like a rope
Three peptide chains woven like a rope Three main types of rna
Three main types of rna Steroid hormones include
Steroid hormones include Section 8-1 summary carbohydrates fats and proteins
Section 8-1 summary carbohydrates fats and proteins Protein elements
Protein elements Integral proteins hydrophobic
Integral proteins hydrophobic Dna rna and proteins study guide answers
Dna rna and proteins study guide answers