Functional Neurosurgery Managing Parkinsons Disease with Deep Brain

Functional Neurosurgery Managing Parkinson’s Disease with Deep Brain Stimulation Ravi Vissapragada

Case Introduction • P. R: 63 yo male suffering with Parkinson’s disease for 15 years attending Neurology for worsening Parkinson’s disease and drug related dyskinesia • Seen in clinic for a Deep Brain Stimulation procedure consult

Presenting Complaint • P. R signs of parkisonism began 15 years ago • First noted in gait examination in a diminished arm swing noted on the left side • P. R started on Sinemet (carbidopa-levodopa) • Also treated with Mirapex (Pramipexole) • Needed increasing doses and tightened dose intervals, leading to prominent dyskinesia • Decreasing Sinemet and Mirapex worsened the effect of the disease and was not tolerable

History • Drugs: – Amantadine, Carbidopa-Levodopa, Oxycodone. Acetaminophen, Parsitan, Pramipexole, Simvastatin, Sotalol, Zolpidem, Aspirin, Docusate • Allergies: – Atorvastatin (Lipitor) • PMHx – Diabetes Mellitus Type II – Coronary Artery Disease – Paroxysmal Atrial Fibrillation • PSHx – Lumbar fusion • FHx – Heart disease • SHx – Retired gentleman, lives with wife
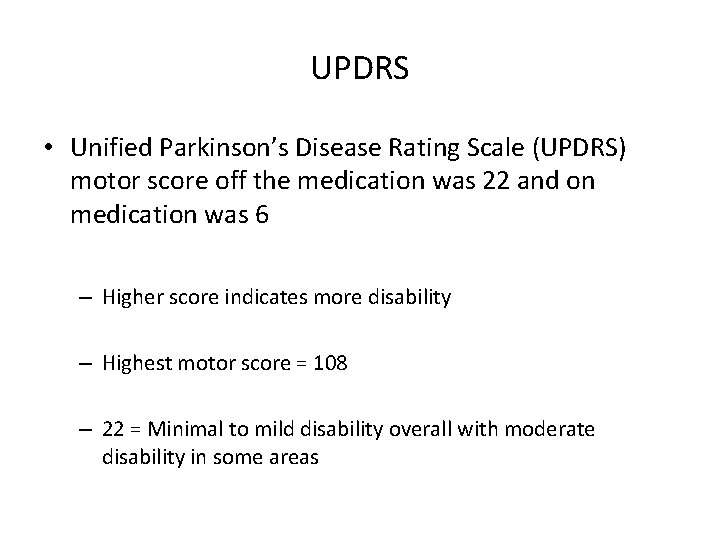
UPDRS • Unified Parkinson’s Disease Rating Scale (UPDRS) motor score off the medication was 22 and on medication was 6 – Higher score indicates more disability – Highest motor score = 108 – 22 = Minimal to mild disability overall with moderate disability in some areas
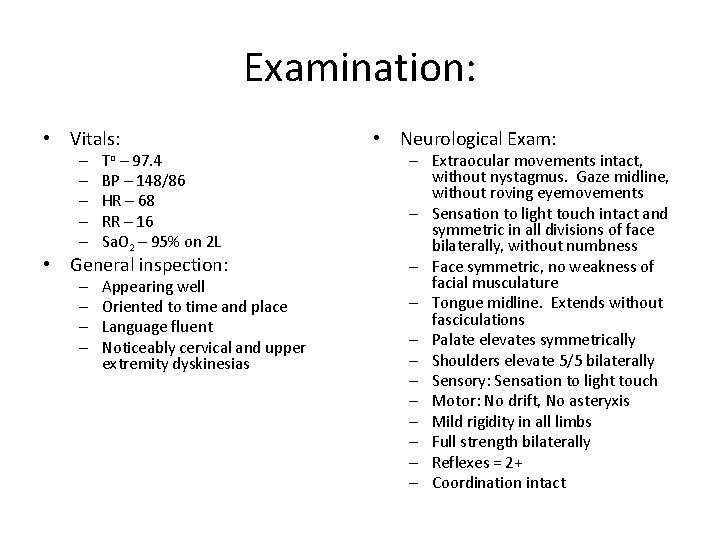
Examination: • Vitals: – – – To – 97. 4 BP – 148/86 HR – 68 RR – 16 Sa. O 2 – 95% on 2 L • General inspection: – – Appearing well Oriented to time and place Language fluent Noticeably cervical and upper extremity dyskinesias • Neurological Exam: – Extraocular movements intact, without nystagmus. Gaze midline, without roving eyemovements – Sensation to light touch intact and symmetric in all divisions of face bilaterally, without numbness – Face symmetric, no weakness of facial musculature – Tongue midline. Extends without fasciculations – Palate elevates symmetrically – Shoulders elevate 5/5 bilaterally – Sensory: Sensation to light touch – Motor: No drift, No asteryxis – Mild rigidity in all limbs – Full strength bilaterally – Reflexes = 2+ – Coordination intact
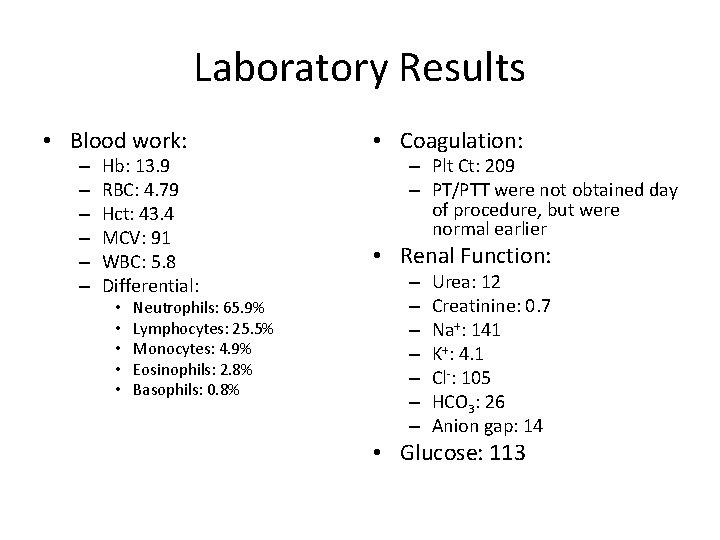
Laboratory Results • Blood work: – – – Hb: 13. 9 RBC: 4. 79 Hct: 43. 4 MCV: 91 WBC: 5. 8 Differential: • • • Neutrophils: 65. 9% Lymphocytes: 25. 5% Monocytes: 4. 9% Eosinophils: 2. 8% Basophils: 0. 8% • Coagulation: – Plt Ct: 209 – PT/PTT were not obtained day of procedure, but were normal earlier • Renal Function: – – – – Urea: 12 Creatinine: 0. 7 Na+: 141 K+: 4. 1 Cl-: 105 HCO 3: 26 Anion gap: 14 • Glucose: 113
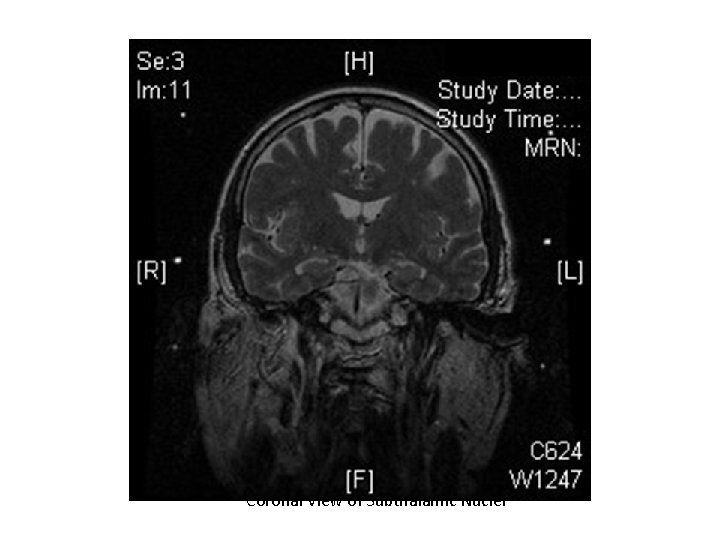
Coronal View of Subthalamic Nuclei

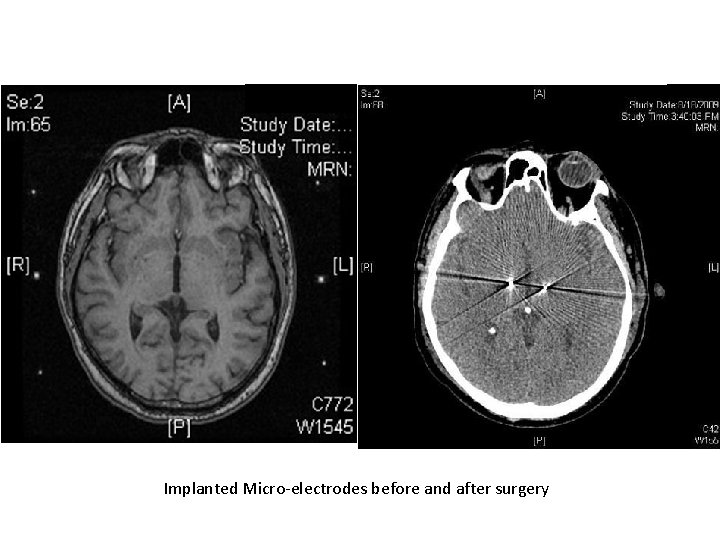
Implanted Micro-electrodes before and after surgery

Parkinson’s Disease Mohammed Ali and Micheal J. Fox http: //medicaltechnologyavenue. blogspot. com/2008/12/parkinson-twitch. html

Parkinson’s Disease • Type of movement disorder • Characterized by: – Bradykinesia – Rigidity – Tremors • Pathophysiology: – Degeneration of dominergic cells in the pars compacta of substantia nigra – Projects to the striatum to participate in the Direct/Indirect Pathway
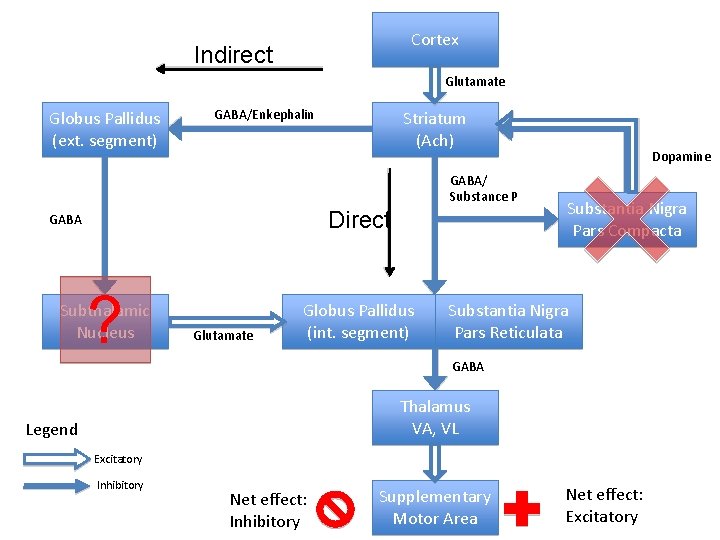
Cortex Indirect Glutamate Globus Pallidus (ext. segment) GABA/Enkephalin Striatum (Ach) GABA/ Substance P Direct GABA ? Subthalamic Nucleus Glutamate Globus Pallidus (int. segment) Dopamine Substantia Nigra Pars Compacta Substantia Nigra Pars Reticulata GABA Thalamus VA, VL Legend Excitatory Inhibitory Net effect: Inhibitory Supplementary Motor Area Net effect: Excitatory

Guidelines for Treatment of Parkinson’s Disease. Jankovic and Aguilar. • Ensure correct diagnosis • Determine level of motor, mental, sensory, autonomic, and other impairments • Educate the patient about the disease and importance of mental and motor activity • Consider putative neuroprotective agent(s) • Select the most appropriate symptomatic therapy, targeted to the most troublesome symptoms • Consider surgery (Deep Brain Stimulation) in patient who are levodopa-responsive but their levodopa-related motor complications cannot be managed adequately with medication adjustments • Therapy must be customized and tailored to the individual needs of the patient
Deep Brain Stimulation • Surgical procedure to implant device to stimulate subthalamic nucleus in the basal ganglia • Applications: – Parkinson’s disease – Dystonia – Essential Tremor – Major Depression – Tourette’s Syndrome http: //blog. bioethics. net/2009/02/dbs-for-ocd-omg/

Deep Brain Stimulation in Parkinson’s Disease • Indications: – Refractory to medical therapy after trial with multiple agents – Patients with levodopa induced dyskinesias – Patients suffering primarily from rigidity and bradykinesia • Contraindications: – Significant dementia • DBS noted to cause cognitive impairment – Increased risk of intracerebral hemorrhage • Coagulopathy, hypertension, anti-platalet therapy that cannot be withheld – Ipsilateral hemianopsia: risk of contralateral optic nerve injury – Age > 85 years – Non-idiopathic Parkinsonism • Shy-Drager, PSNP, OPCA, Arteriosclerotic Parkinsonism (lacunar infarcts)
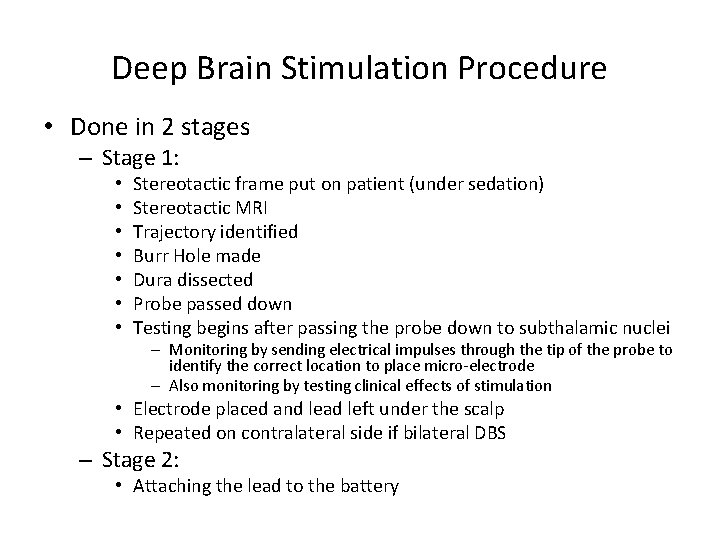
Deep Brain Stimulation Procedure • Done in 2 stages – Stage 1: • • Stereotactic frame put on patient (under sedation) Stereotactic MRI Trajectory identified Burr Hole made Dura dissected Probe passed down Testing begins after passing the probe down to subthalamic nuclei – Monitoring by sending electrical impulses through the tip of the probe to identify the correct location to place micro-electrode – Also monitoring by testing clinical effects of stimulation • Electrode placed and lead left under the scalp • Repeated on contralateral side if bilateral DBS – Stage 2: • Attaching the lead to the battery
Potential Complications • Intracerebral Hemorrhage: 1% • Wound Infection: 3 -5% • Lead Fracture • Battery Failure

Literature Overview
Bilateral Deep Brain Stimulation vs. Best Medical Therapy Weaver et al. 2009 • Compares 6 -month outcomes for patients with PD who received DBS or the best medical therapy • RCT of 255 patients with PD (Hoehn and Yahr stage ≥ 2*) stratified by age and study site** • Bilateral DBS of subthalamic nuclei or globus pallidus • Best medical therapy: actively managed by movement disorder neurologists • Measuring outcome: – Time spent in the “on” state without troubling dyskinesia – “On” state defined as “good motor control with unimpeded motor function” – Measured primarily using motor diaries, motor function, Qo. L, cognitive function, and adverse events *Hoehn and Yahr stage ≥ 2: Bilateral symptoms, minimal disability, posture and gait affected ** Patient Selection: Stage 2 or greater off medication, but have symptoms (motor fluctuations and dyskinesias) on medications, must be responsive to Levodopa, experince ≥ 3 hrs/24 hrs, were receiving medical therapy for 1 month or longer, aged > 21 yrs Exclusion criteria: atypical syndromes, previous surgery for PD, surgical contraindications, active alcohol or drug abuse, dementia, or pregnancy
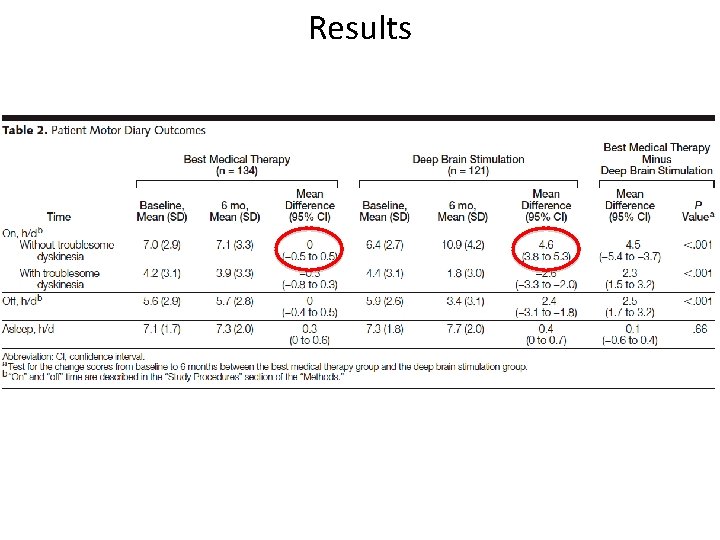
Results

A Randomized trial of Deep Brain Stimulation for Parkinson’s Disease Deuschl et al. 2006 • European study • Randomized-pairs trial of 156 patients with advanced Parkinson’s and severe motor symptoms • Deep Brain Stimulation vs. Medical Management • Measured Qo. L from baseline to six months – PDQ-39 (Parkinson’s Questionnaire) – UPDRS part III • Exclusion criteria – Age < 75, no dementia/psychiatric illnesses, symptoms must be limiting daily functioning • Optimized medical care provided by Neurologists specializing in movement disorders • Bilateral Deep brain stimulation of subthalamic nucleus using stereotactic imaging and standard microelectrode sampling techniques
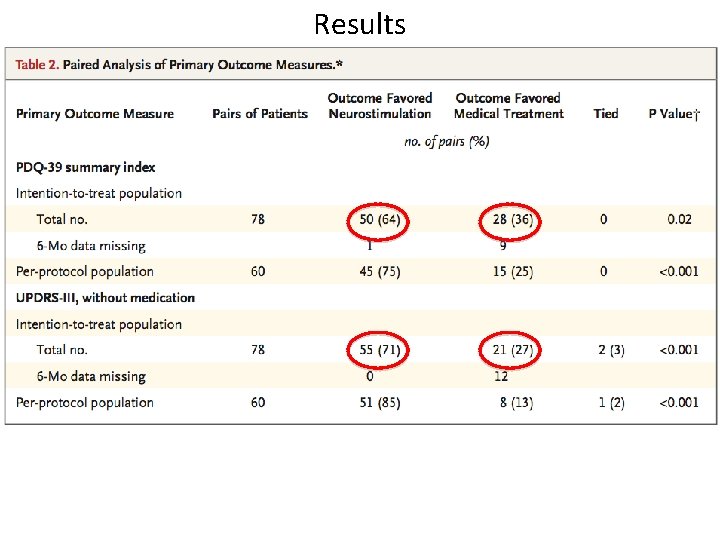
Results

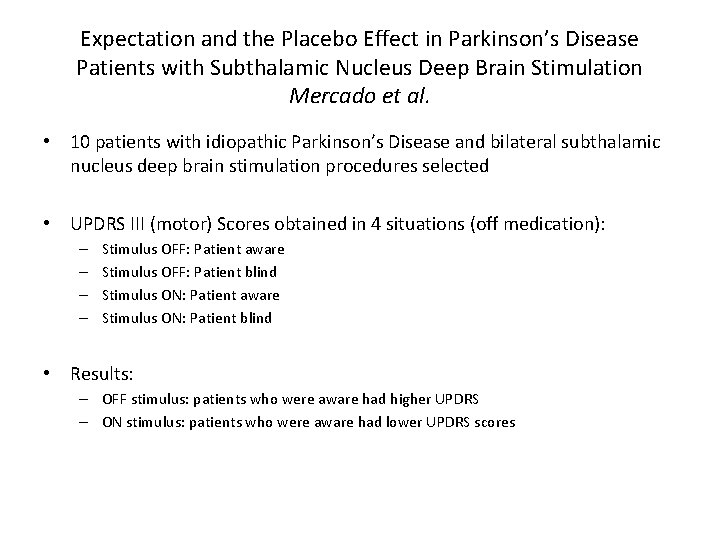
Expectation and the Placebo Effect in Parkinson’s Disease Patients with Subthalamic Nucleus Deep Brain Stimulation Mercado et al. • 10 patients with idiopathic Parkinson’s Disease and bilateral subthalamic nucleus deep brain stimulation procedures selected • UPDRS III (motor) Scores obtained in 4 situations (off medication): – – Stimulus OFF: Patient aware Stimulus OFF: Patient blind Stimulus ON: Patient aware Stimulus ON: Patient blind • Results: – OFF stimulus: patients who were aware had higher UPDRS – ON stimulus: patients who were aware had lower UPDRS scores

Five-Year Follow up of Bilateral Stimulation of the Subthalamic Nucleus in Advanced Parkinson’s Disease Krack et. al • 5 year prospective study • 49 consecutive patients with bilateral stimulation of subthalamic nucleus • Assessed at 1 year, 3 years, 5 years – On medication (levodopa) – Off medication • UPDRS III used to motor function scores • Results after 5 years: – Motor function improved greatly off medication – Dyskinesia improved markedly on medication – Worsening akinesia, speech, postural stability, freezing of gait, and cognitive function
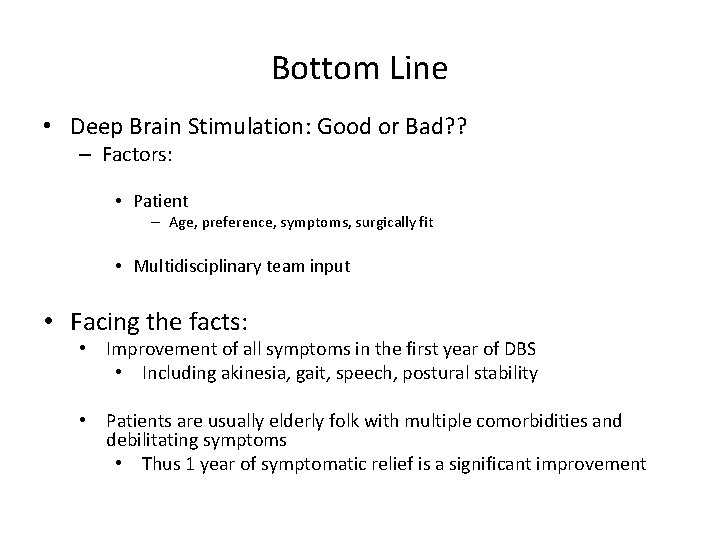
Bottom Line • Deep Brain Stimulation: Good or Bad? ? – Factors: • Patient – Age, preference, symptoms, surgically fit • Multidisciplinary team input • Facing the facts: • Improvement of all symptoms in the first year of DBS • Including akinesia, gait, speech, postural stability • Patients are usually elderly folk with multiple comorbidities and debilitating symptoms • Thus 1 year of symptomatic relief is a significant improvement

Post-Operative Details • • Surgery was well tolerated No pain except at surgical sites No confusion reported (by wife and daughter) Exam: – Awake, alert, and oriented to time and place 3. 5 hrs post-surgery – Vitals: • • • To – 97. 4 BP – 148/86 HR – 68 RR – 16 Sa. O 2 – 95% on 2 L • No neurologic deficit noted • Mild rigidity and bradykinesia noted • No dyskinesia

Thank you

Acknowledgements • • Dr. Papavassiliou Dr. Fred Lam Dr. Andrey Zinchuk Dr. Chen

Bibliography • Rodriguez RL. Pearls in Patient Selection for Deep Brain Stimulation. Neurologist. 2007 Sep; 13(5): 253 -60 • M. S. Greenberg. : Surgical Treatment of Parkinson’s Disease. Handbook of Neurosusrgery 6 th Ed. , 15. 2: 365 -6, 2006 • Deuschl et al. A Randomized Trial of Deep Brain Stimulation for Parkinson’s Disease. N Engl J Med 2006; 355: 89690 • Jankovic, Aguilar. Current approaches to the treatment of Parkinson’s Disease. Neuropsychiatric Disease and Treatment 2008: 4 (4) 743 -75 • Weaver et al. Bilateral Deep Brain Stimulation vs Best Medical Therapy for Patients With Advanced Parkinson’s Disease: A Randomized Controlled Trial. JAMA. 2009; 301(1): 63 -73 • Temel. Blokland. The functional role of the subthalamic nucleus in cognitive and limbic circuits. Progress in Neurobiology 76 (2005) 393 -413. • Mercado et al. Expectation and the Placebo Effect in Parkinson’s Disease Patients With Subthalamic Nucleus Deep Brain Stimulation. Movement Disorders. 21 (2006). 1457 -1461. • Krack et al. Five year Follow-up of Bilateral Stimulation of Subthalamic Nucleus in Advanced Parkinson’s Disease.
- Slides: 31