Functional Groups Carboxylic Acids Esters Amides Aldehydes Ketones

Functional Groups: - Carboxylic Acids - Esters - Amides - Aldehydes - Ketones - Amines Mr. Shields Regents Chemistry U 17 L 02 1
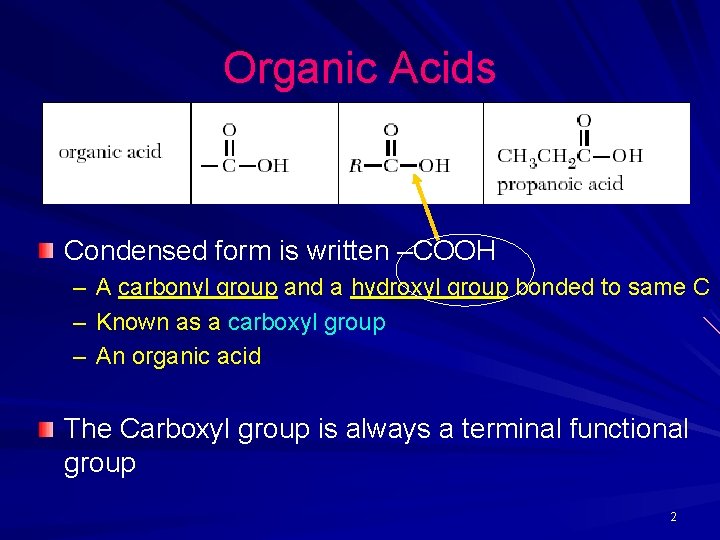
Organic Acids Condensed form is written –COOH – A carbonyl group and a hydroxyl group bonded to same C – Known as a carboxyl group – An organic acid The Carboxyl group is always a terminal functional group 2
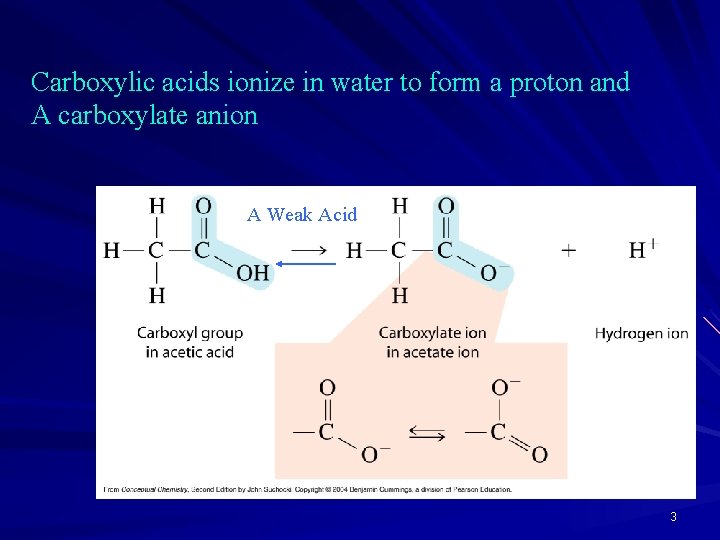
Carboxylic acids ionize in water to form a proton and A carboxylate anion A Weak Acid 3
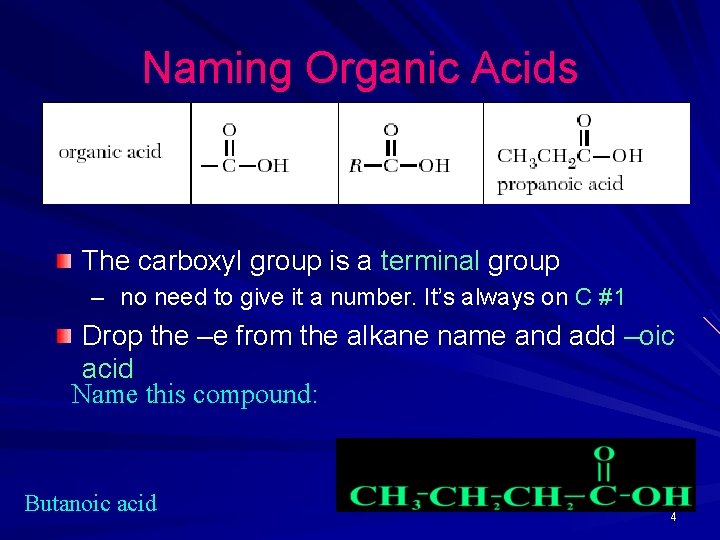
Naming Organic Acids The carboxyl group is a terminal group – no need to give it a number. It’s always on C #1 Drop the –e from the alkane name and add –oic acid Name this compound: Butanoic acid 4

Naming Organic Acids Name this compound: C-C-C-COOH hexanoic acid 5

Physical properties - Sharp or noxious smells - polar molecule that can form 2 hydrogen bonds with themselves or with water C-C=O OH HO O=C- C C-C=O OH H O H This leads to high B. P. and high solubility in water - 1 st four acids are totally miscible in water 6
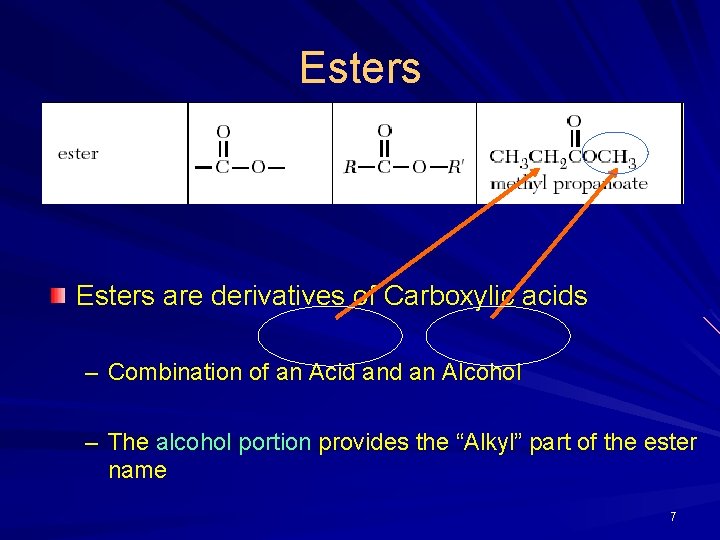
Esters are derivatives of Carboxylic acids – Combination of an Acid an Alcohol – The alcohol portion provides the “Alkyl” part of the ester name 7
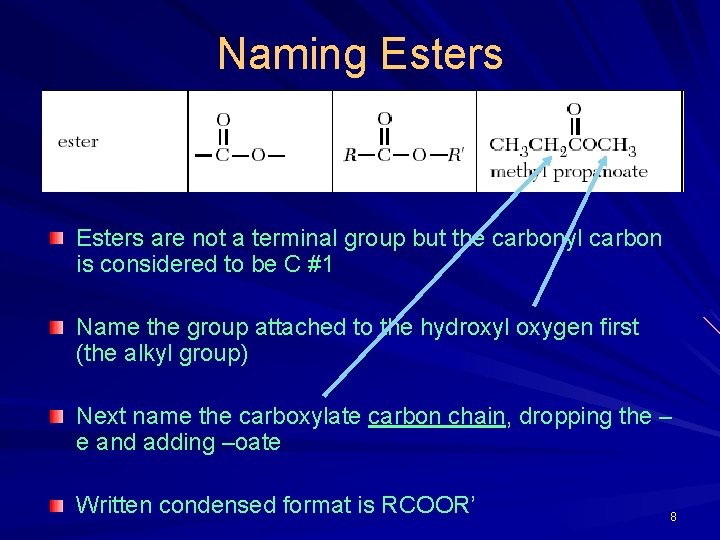
Naming Esters are not a terminal group but the carbonyl carbon is considered to be C #1 Name the group attached to the hydroxyl oxygen first (the alkyl group) Next name the carboxylate carbon chain, dropping the – e and adding –oate Written condensed format is RCOOR’ 8
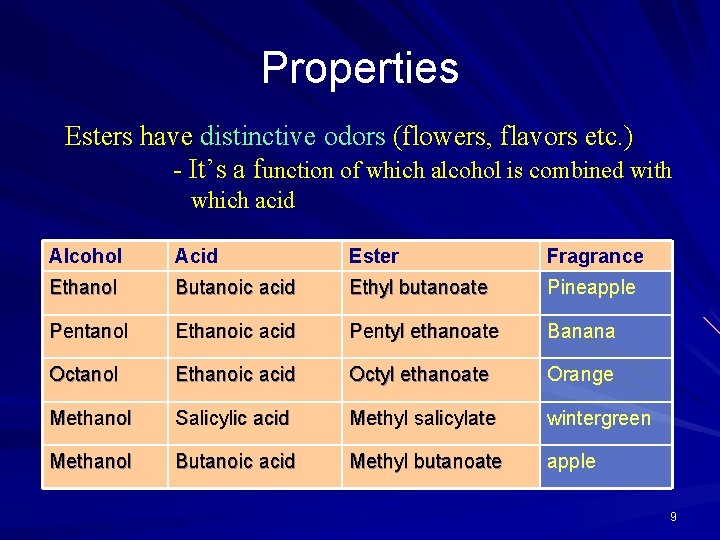
Properties Esters have distinctive odors (flowers, flavors etc. ) - It’s a function of which alcohol is combined with which acid Alcohol Acid Ester Fragrance Ethanol Butanoic acid Ethyl butanoate Pineapple Pentanol Ethanoic acid Pentyl ethanoate Banana Octanol Ethanoic acid Octyl ethanoate Orange Methanol Salicylic acid Methyl salicylate wintergreen Methanol Butanoic acid Methyl butanoate apple 9
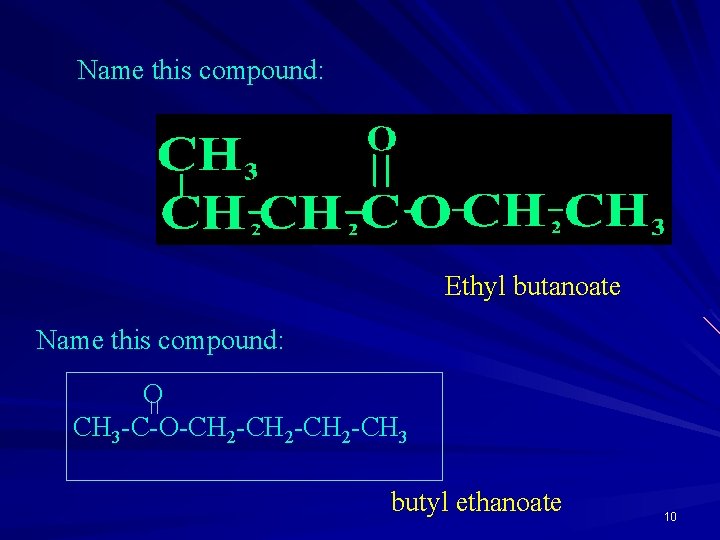
Name this compound: Ethyl butanoate Name this compound: O CH 3 -C-O-CH 2 -CH 3 butyl ethanoate 10
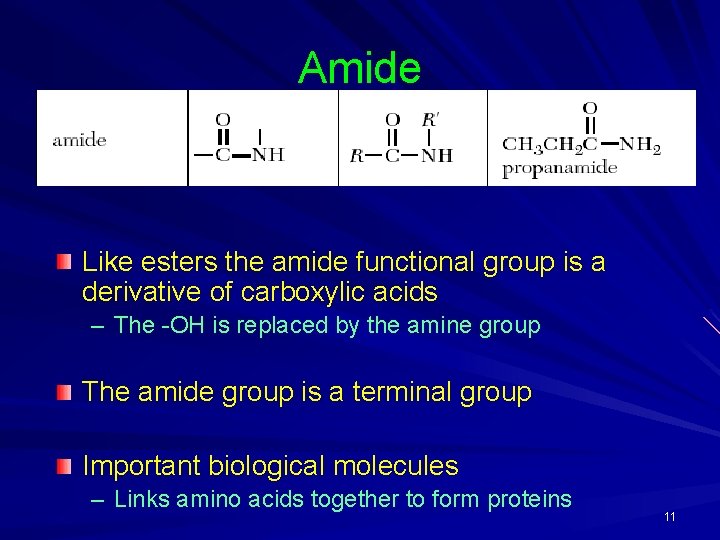
Amide Like esters the amide functional group is a derivative of carboxylic acids – The -OH is replaced by the amine group The amide group is a terminal group Important biological molecules – Links amino acids together to form proteins 11
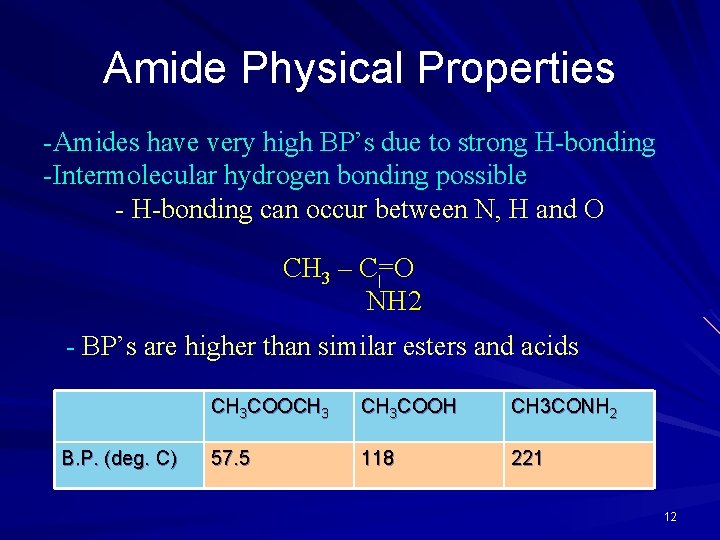
Amide Physical Properties -Amides have very high BP’s due to strong H-bonding -Intermolecular hydrogen bonding possible - H-bonding can occur between N, H and O CH 3 – C=O NH 2 - BP’s are higher than similar esters and acids B. P. (deg. C) CH 3 COOCH 3 COOH CH 3 CONH 2 57. 5 118 221 12
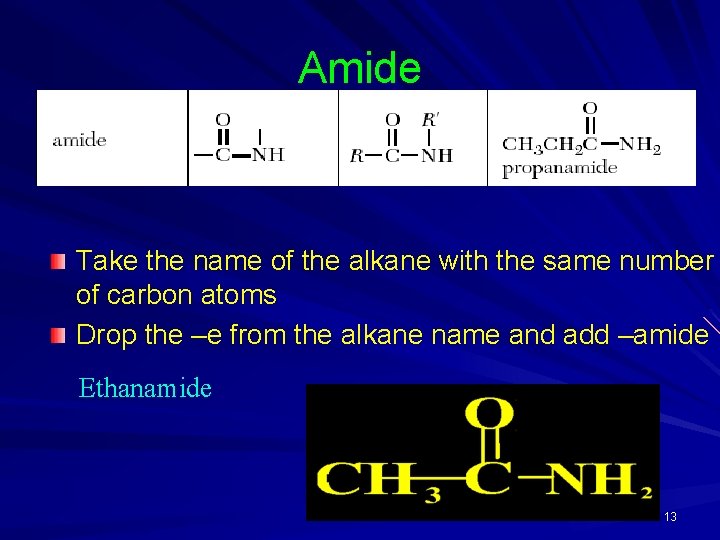
Amide Take the name of the alkane with the same number of carbon atoms Drop the –e from the alkane name and add –amide Ethanamide 13
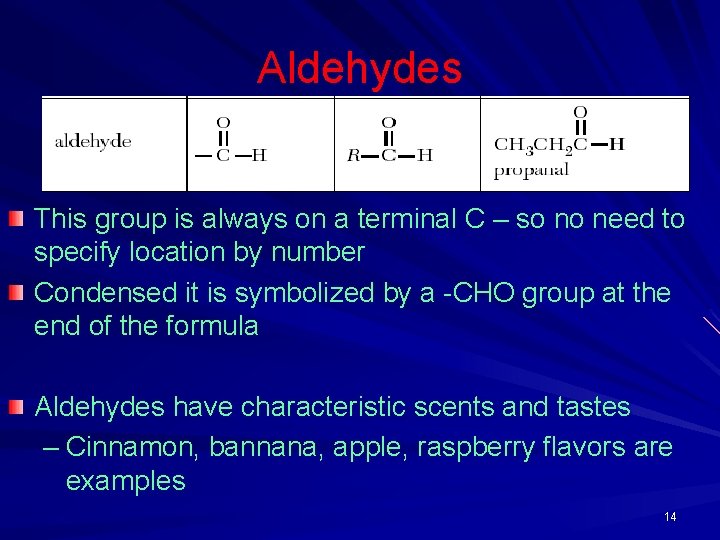
Aldehydes This group is always on a terminal C – so no need to specify location by number Condensed it is symbolized by a -CHO group at the end of the formula Aldehydes have characteristic scents and tastes – Cinnamon, bannana, apple, raspberry flavors are examples 14
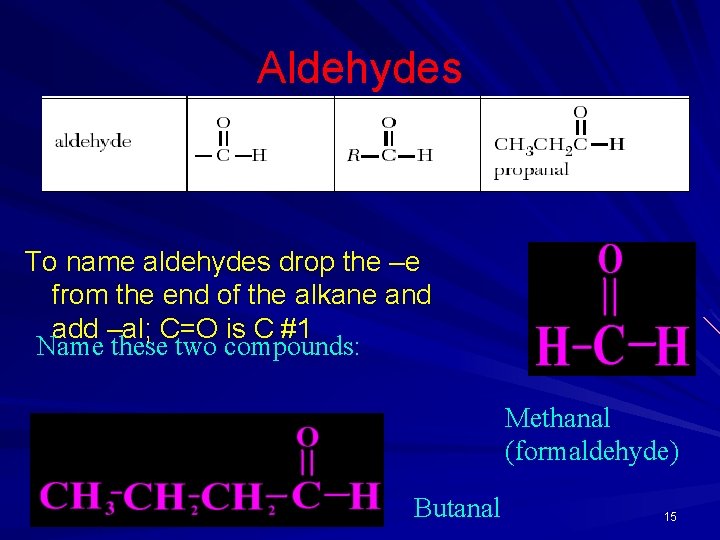
Aldehydes To name aldehydes drop the –e from the end of the alkane and add –al; C=O is C #1 Name these two compounds: Methanal (formaldehyde) Butanal 15
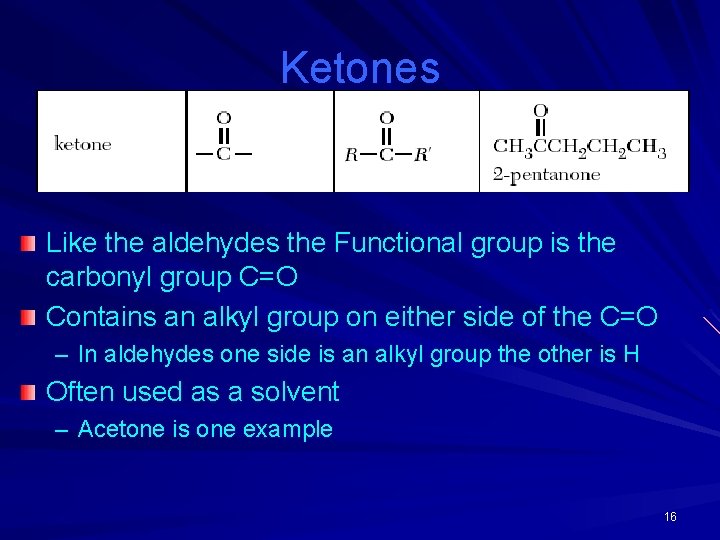
Ketones Like the aldehydes the Functional group is the carbonyl group C=O Contains an alkyl group on either side of the C=O – In aldehydes one side is an alkyl group the other is H Often used as a solvent – Acetone is one example 16
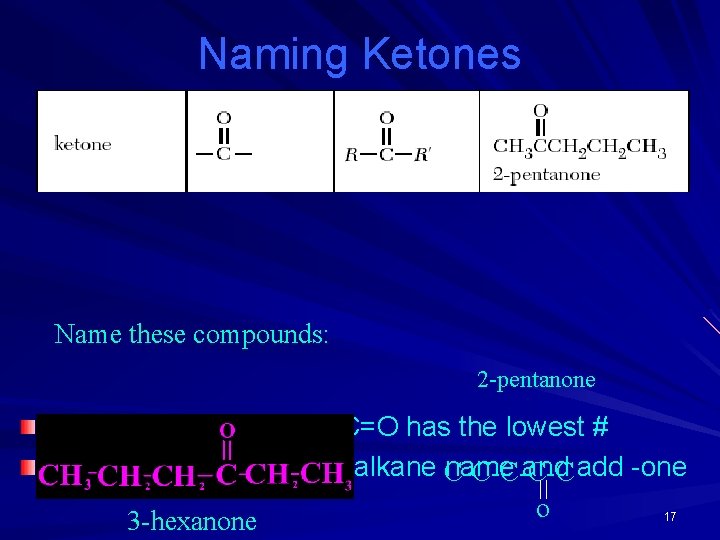
Naming Ketones Name these compounds: 2 -pentanone Number the C chain so C=O has the lowest # Drop the final e from the alkane C-C-C name and add -one o 17 3 -hexanone
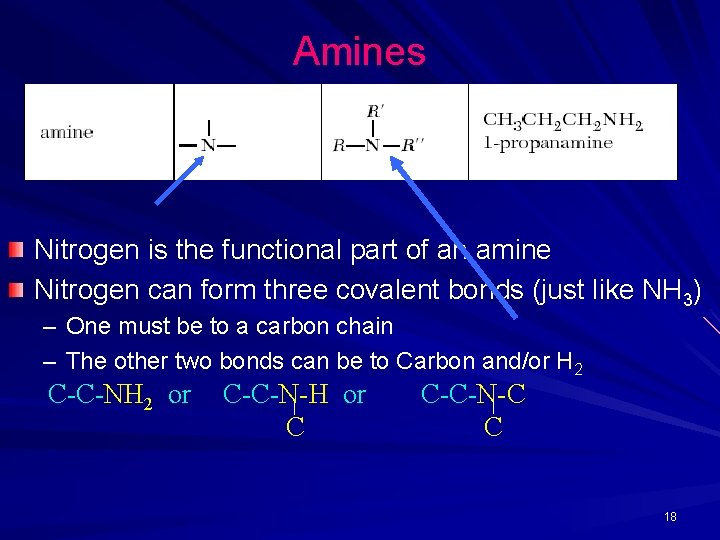
Amines Nitrogen is the functional part of an amine Nitrogen can form three covalent bonds (just like NH 3) – One must be to a carbon chain – The other two bonds can be to Carbon and/or H 2 C-C-NH 2 or C-C-N-H or C C-C-N-C C 18
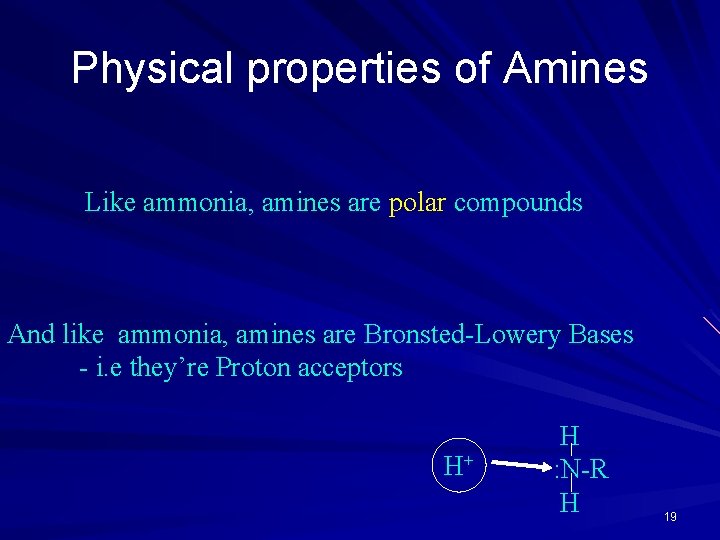
Physical properties of Amines Like ammonia, amines are polar compounds And like ammonia, amines are Bronsted-Lowery Bases - i. e they’re Proton acceptors H+ H : N-R H 19
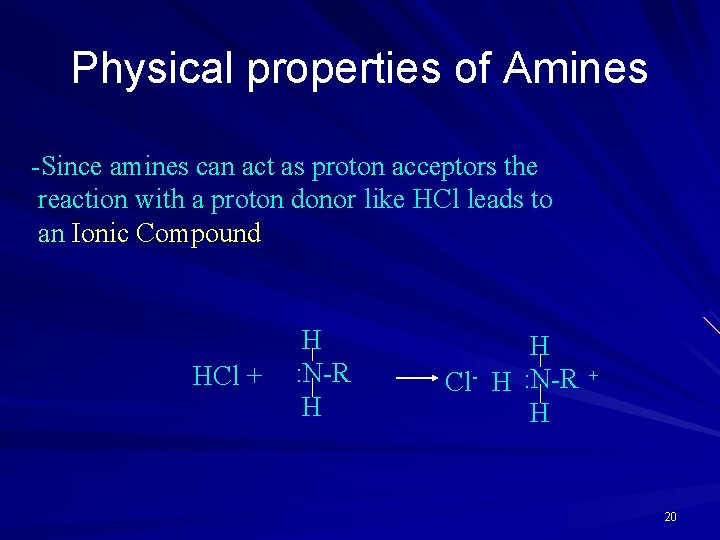
Physical properties of Amines -Since amines can act as proton acceptors the reaction with a proton donor like HCl leads to an Ionic Compound HCl + H : N-R H H Cl- H : N-R H + 20
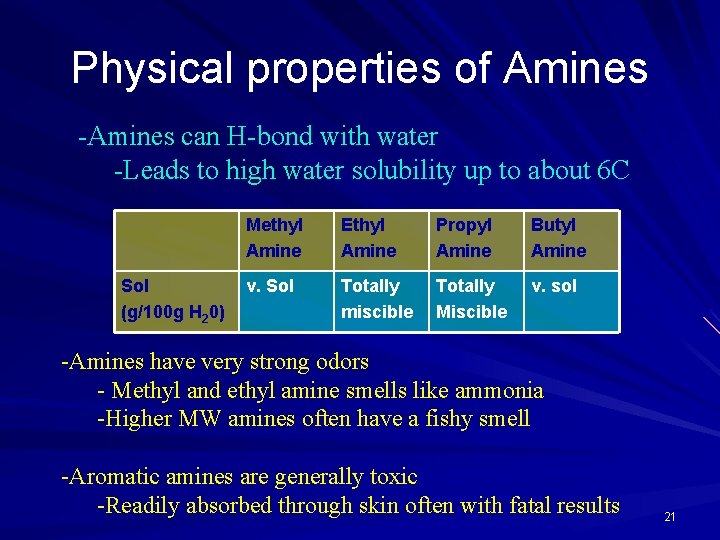
Physical properties of Amines -Amines can H-bond with water -Leads to high water solubility up to about 6 C Sol (g/100 g H 20) Methyl Amine Ethyl Amine Propyl Amine Butyl Amine v. Sol Totally miscible Totally Miscible v. sol -Amines have very strong odors - Methyl and ethyl amine smells like ammonia -Higher MW amines often have a fishy smell -Aromatic amines are generally toxic -Readily absorbed through skin often with fatal results 21
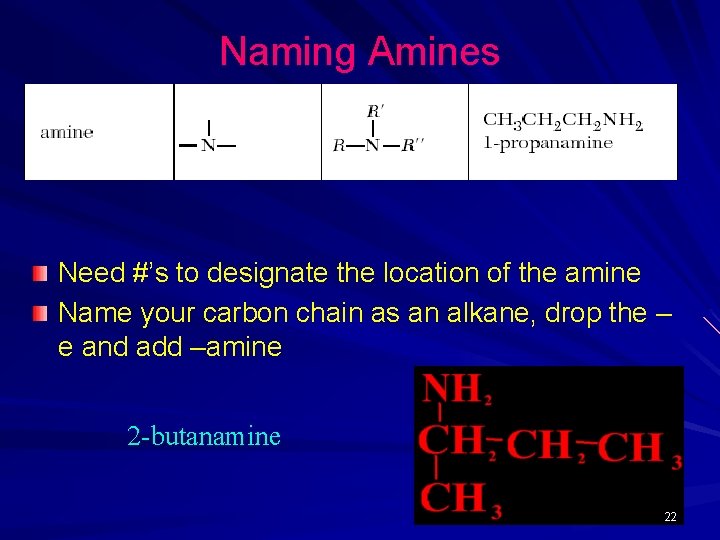
Naming Amines Need #’s to designate the location of the amine Name your carbon chain as an alkane, drop the – e and add –amine 2 -butanamine 22
Some nasty DIAMINES …Yuk! 23
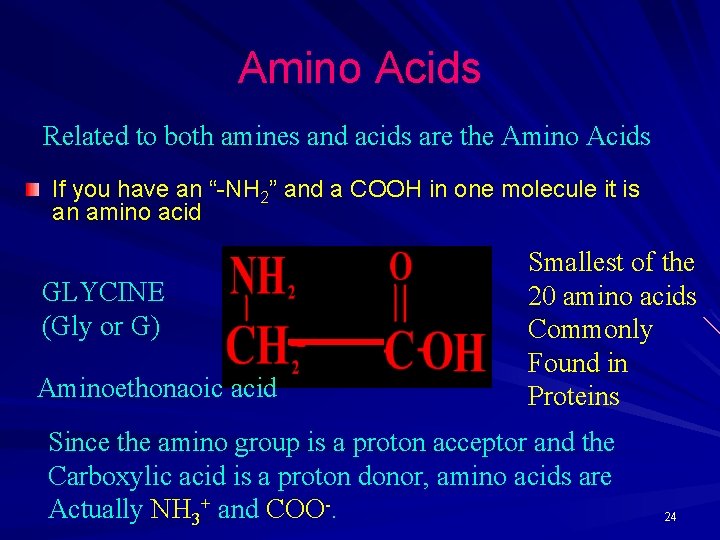
Amino Acids Related to both amines and acids are the Amino Acids If you have an “-NH 2” and a COOH in one molecule it is an amino acid GLYCINE (Gly or G) Aminoethonaoic acid Smallest of the 20 amino acids Commonly Found in Proteins Since the amino group is a proton acceptor and the Carboxylic acid is a proton donor, amino acids are Actually NH 3+ and COO-. 24
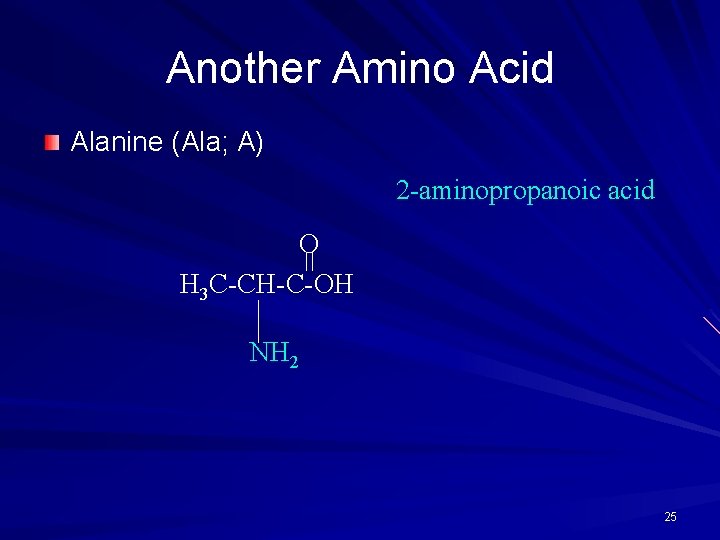
Another Amino Acid Alanine (Ala; A) 2 -aminopropanoic acid O H 3 C-CH-C-OH NH 2 25
- Slides: 25